Abstract
Introduction
Postmortem studies suggest that fibrillar brain amyloid places people at higher risk for hazardous driving in the preclinical stage of Alzheimer's disease (AD).
Methods
We administered driving questionnaires to 104 older drivers (19 AD, 24 mild cognitive impairment, and 61 cognitive normal) who had a recent 18F-florbetapir positron emission tomography scan. We examined associations of amyloid standardized uptake value ratios with driving behaviors: traffic violations or accidents in the past 3 years.
Results
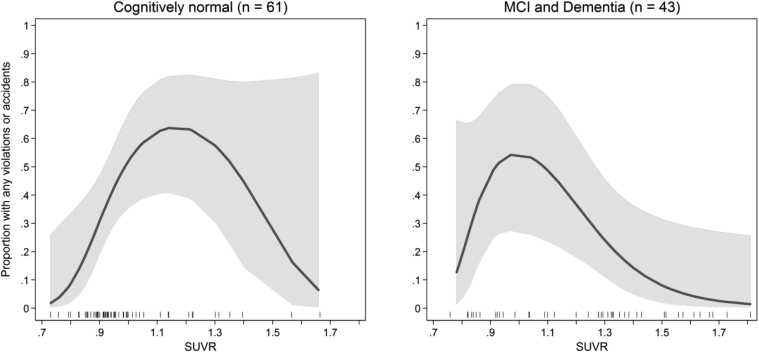
The frequency of violations or accidents was curvilinear with respect to standardized uptake value ratios, peaking around a value of 1.1 (model r2 = 0.10, P = .002); moreover, this relationship was evident for the cognitively normal participants.
Discussion
We found that driving risk is strongly related to accumulating amyloid on positron emission tomography, and that this trend is evident in the preclinical stage of AD. Brain amyloid burden may in part explain the increased crash risk reported in older adults.
Keywords: Alzheimer's disease, Cognitive aging, Assessment of cognitive disorders, Dementia, MCI (mild cognitive impairment), Biomarkers, Driving
1. Introduction
Postmortem studies of the brains of older drivers who were killed in motor vehicle accidents (MVAs) have found that many had the neuropathologic changes of Alzheimer's disease (AD), although they may have never been diagnosed to have the disease [1], [2], [3], [4]. Since then, advances in biomarker technology have fostered new research criteria for the “preclinical” stage of AD [5] preceding the intermediate stage of mild cognitive impairment (MCI) or “prodromal” AD [6], during which time amyloid pathology may be present before any noticeable symptoms of cognitive or functional impairments exist. These criteria are leading to an increasing body of knowledge about the very earliest signs and symptoms of AD as well as an impetus to identify sensitive clinical markers of underlying AD pathology such as amyloid plaque deposition.
Abnormal levels of AD biomarkers using the Pittsburgh compound amyloid positron emission tomography (PET) ligand were recently reported to predict performance on a standardized road test by cognitively normal older individuals, raising concern that amyloid deposition during the preclinical phase of AD could indicate an increased risk for hazardous driving [7]. Therefore, we examined whether the presence of increased amyloid on PET places an older person at risk for unsafe driving as evidenced by actual traffic violations or MVAs in the preclinical and the symptomatic stages of the disease. Because recent models of AD pathophysiology suggest that amyloid pathology rises during the preclinical stage and then plateaus in the symptomatic stage [8], we hypothesized that this relationship, if it exists, may be more evident in preclinical disease than in AD or MCI.
2. Methods
2.1. Standard protocol approvals, registrations, and patient consents
The institutional review board of Rhode Island Hospital approved the research protocol, and all participants or their legally authorized representative provided informed consent to participate.
2.2. Study design and participants
We selected a convenience sample of people attending an outpatient memory clinic and included 19 AD, 24 MCI, and 61 cognitively normal elders. Inclusion criteria included attendance to the memory clinic and amyloid PET done as part of other observational or clinical trial research studies within the past 6 months. Clinical diagnoses of AD [9] and MCI [6] were made by a neurologist based on research criteria of the National Institute on Aging and the Alzheimer's Association. Cognitively normal elders all had a Clinical Dementia Rating [10] of 0 (see Table 1). The age range for participants was 51 to 85 years, standardized uptake value ratio (SUVR) range was 0.7 to 1.8, driving avoidance range of ratings was 1 to 5, and the number of miles driven per week range was 5 to 600.
Table 1.
Participant characteristics by cognitive impairment group
| Characteristic | Total (N = 104) | Normal (N = 61) | MCI (N = 24) | Dementia (N = 19) |
|---|---|---|---|---|
| Sex [N (%)] | ||||
| Women | 69 (66) | 43 (71) | 13 (54) | 13 (68) |
| Men | 35 (34) | 18 (30) | 11 (46) | 6 (32) |
| Age [M (SD)] | 67 (8) | 64 (7) | 72 (8) | 68 (11) |
| SUVR [M (SD)] | 1.1 (0.3) | 1.0 (0.2) | 1.2 (0.3) | 1.3 (0.3) |
| SUVR class [N (%)] | ||||
| <1.1; Negative | 64 (62) | 49 (80) | 9 (38) | 6 (32) |
| 1.1–1.2; Intermediate | 5 (5) | 3 (5) | 1 (4) | 1 (5) |
| >1.2; Positive | 35 (34) | 9 (15) | 14 (58) | 12 (63) |
| Consensus reading [N (%)] | ||||
| Negative | 68 (65) | 50 (82) | 11 (46) | 7 (37) |
| Positive | 36 (35) | 11 (18) | 13 (54) | 12 (63) |
| No violation or accident | ||||
| In past 3 years [N (%)] | 70 (67) | 38 (62) | 17 (71) | 15 (79) |
| Any violation or accident | ||||
| In past 3 years [N (%)] | 34 (33) | 23 (38) | 7 (29) | 4 (21) |
| Accident, family report | 21 (20) | 11 (21) | 6 (26) | 4 (21) |
| Violation, self report | 17 (16) | 14 (23) | 2 (9) | 1 (6) |
| Accident, self-report | 17 (16) | 11 (18) | 4 (17) | 2 (12) |
| Violation, family report | 9 (9) | 6 (11) | 2 (9) | 1 (5) |
| Driving avoidance [M (SD)] | ||||
| Self-report | 1.5 (0.8) | 1.4 (0.8) | 1.5 (0.9) | 1.7 (1.0) |
| Family report | 1.7 (0.9) | 1.4 (0.8) | 1.9 (1.1) | 2.3 (0.9) |
| Miles driven per week [M (SD)] | 118 (111) | 147 (119) | 92 (104) | 58 (49) |
Abbreviations: M, mean; MCI, mild cognitive impairment; SD, standard deviation; SUVR, standard uptake value ratio.
NOTE. The mean of reports of limiting the amount of nighttime, rain, and busy traffic driving are based on a scale of 1 (strongly agree) to 5 (strongly disagree).
2.3. Driving behavior
We administered a driving questionnaire, developed by the American Academy of Neurology as part of its most recent practice parameter on driving and dementia [11]. Questionnaire versions are available for drivers and family informants. The questionnaire includes three initial questions: (1) “How many times have you been stopped or ticketed for a traffic violation in the last three years?” (2) “How many accidents have you been in, or caused, within the last three years?” and (3) “In how many accidents were you at fault in the last three years?” The answers were circled on paper with the choices being 1, 2, 3, or 4 or more. We defined a positive response to any violation or accident in the past 3 years from the participant or their family informant as our primary outcome measure (see Table 2). Ten additional questions were about current driving behaviors that were generally regarded as risk factors for MVAs. These were anchored by a 5-point Likert scale ranging from “strongly disagree” to “strongly agree.” We defined a driving avoidance outcome variable as the mean across four of these items: limited amount of time driving, avoiding driving at night, avoiding driving in the rain, and avoiding driving in busy traffic. We used the maximum of the mean rating from driver and family informant in analytic models. Finally, at the end of the questionnaire, the respondent was asked “How many miles a week do you drive?” and we used this response as an outcome variable for driving avoidance. The driver and a family member informant each completed the questionnaire independently without knowledge of the other's responses.
Table 2.
Association of standard uptake value ratio (SUVR) and driving behavior within regions of SUVR
| Driving behavior | SUVR |
Model |
|
|---|---|---|---|
| ≤1.1 | >1.1 | r2 | |
| Any violation or accident | 0.56∗ | −0.57 † | 0.38 |
| Miles driven | −0.07 | 0.02 | 0.22 |
| Driving avoidance ratings | 0.13 | −0.29 † | 0.20 |
NOTE. Table entries are standardized regression coefficients (on the scale of correlation coefficients) describing the linear relationship of driving behavior and SUVR within two regions of SUVR. The model includes adjustment for age, sex, and diagnostic group. Driving avoidance ratings refer to the maximum of self- and family-reported mean ratings on four driving avoidance patterns (amount, nighttime, rain, and busy traffic). Items are rated on a 1 to 5 scale, where higher ratings indicate greater agreement with avoidance patterns. A negative coefficient between SUVR and driving avoidance ratings implies that the more Alzheimer's disease–like amyloid burden, driving avoidance behaviors are less or fewer.
P < .01.
P < .05.
2.4. Aβ PET imaging
The radiotracer 18F-florbetapir was used in all studies. A 370-MBq (10 mCi) bolus injection of F-18 florbetapir was administered intravenously. Approximately 50 minutes after injection, a 20-minute PET scan was performed with head computed tomography (CT) scan for attenuation correction purposes. The scans were all performed on a Philips Gemini GXL 16 PET/CT with the CT scan performed for attenuation purposes. PET standardized uptake value (SUV) data for six regions in the cerebral cortex were summed and normalized to the whole cerebellum SUV, resulting in a cerebral cortex-to-cerebellum ratio termed SUVR. This calculation was performed using MIMneuro software [12]. We used an SUVR threshold of 1.2 or greater to discriminate between Aβ positive and negative, as recommended previously to indicate the presence of moderate to frequent amyloid plaques [13], [14]. SUVR of 1.1 to 1.2 was regarded as intermediate positivity based on a previous report showing that SUVR of 1.08 to −1.17 represents an intermediate band representing the presence of any amount of amyloid deposits [15]. Aβ positivity was also determined by consensus qualitative visual reading by two board-certified radiologists specializing in nuclear medicine.
2.5. Statistical analysis
We evaluated nonlinear trends in the relationship between driving behaviors and SUVR using two-degree fractional polynomial functions [16]. Logistic regression was used for the binary accidents or violations' outcome, linear regression was used for driving avoidance and miles driven models. Fractional polynomial model parameter estimates were obtained with Stata software (version 14.1; Stata Corporation, College Station, TX). Multivariate models were estimated with Mplus software (version 7.4; Muthén & Muthén, Los Angeles, CA).
3. Results
Participant characteristics are shown in Table 1. Consensus reading by visual interpretation yielded 68 negative scans and 36 positive scans. Classification by SUVR yielded 64 negative scans and 35 positive scans, with the mean SUVR of 1.1 and range 0.7 to 1.8. Seventeen subjects self-reported being ticketed and 17 subjects self-reported being in an MVA in the past 3 years, whereas family member reports for the same items were 9 and 21, respectively. For descriptive analyses we used a composite outcome that represents the occurrence of any violation or MVA by either self or family member report. This composite variable defined 70 negatively involved subjects, that is, no retrospective history of recent adverse driving events, and 34 positively involved subjects. The average reported number of miles driven per week was 118 ± 111 (range 5–600).
To assess the safety index of drivers in each subgroup, we examined the number of reported accidents per reported miles driven. The mean (standard error) number of accidents per 1000 miles driven was 2.5 (2.4), 9.5 (3.6), and 9.2 (4.0) for the normal, MCI, and dementia groups, respectively.
We found a nonlinear relationship between the risk of a violation or MVA (either from self or family member report) and average SUVR as determined from a fractional polynomial model. At an SUVR of about less than 1.1, risk for violations or accidents increased linearly with increasing amyloid. The model using just nonlinear functions of SUVR to account for the occurrence of violations or accidents has a pseudo-r2 of 0.10 and is highly significant versus a null model (P = .002). Using multivariate regression, we determined that at an SUVR level of less than 1.1, the biserial correlation of SUVR and violations or accidents was r = 0.56 (P = .007). At an SUVR of about greater than 1.1, risk for violations or accidents decreased with increasing SUVR (r = −0.57, P = .014).
The nonlinear relationship was similar across the diagnostic group and is illustrated for the cognitively normal subgroup and the MCI or dementia subgroups in Fig. 1. Because of the low frequency of events, the relationship between SUVR and violations or accidents in the normal subgroup was relatively flat across the confidence levels beyond an SUVR of 1.1 and not statistically significant. In post hoc analysis, we tested for possible modification of the effect of SUVR in prior violations or accidents. We accomplished this using a multivariable logistic regression model, the fractional polynomial terms for SUVR, and their interaction with the diagnostic group. We found no evidence (P = .24) that the nonlinear relationship between SUVR and the frequency of violations or accidents varies across the diagnostic group, including statistical adjustment for age, sex, and the main effect of diagnostic group.
Fig. 1.
Relationship between the proportion of subjects in the cognitively normal group (left) and the MCI and dementia subgroup (right) with any violation or accident and SUVR. The smoothed function is an estimated fractional polynomial logistic function and 95% confidence interval fit to the observed SUVR data. The one-way distribution of SUVR within the group is illustrated with the rug at the bottom of the plot area. Abbreviations: MCI, mild cognitive impairment; SUVR, standard uptake value ratio.
The optimally fitting two-degree fractional polynomial function for the relationship of driving avoidance and SUVR suggested a curvilinear pattern as seen with violations or accidents, and for driving avoidance neither a simple linear nor a curvilinear model fit better than a null model (P values .52 and .86, respectively). Thus, these data cannot be used to support a meaningful relationship between driving avoidance and SUVR. The optimally fitting two-degree fractional polynomial function for the relationship of miles driven and SUVR suggested a curvilinear pattern as seen with violations or accidents, and although a simple linear model was significant (P = .04), an optimally fitting two-degree fractional polynomial offered no additional significant improvement in fit (P = .70).
3.1. Multivariate models
We used multivariate piecewise regression modeling to estimate general trends in driving behavior outcomes as a function of SUVR. We used a piecewise effect for SUVR, estimating one linear slope for SUVR values increasing up to 1.1, and a second linear slope from values 1.1 and higher. Models included statistical adjustment for the effects of age, sex, and diagnostic group. Results are summarized in Table 2 in the form of standardized regression coefficients, which are on the scale of correlation coefficients and useful effect size measures. Results show large effects of SUVR in the propensity to have had any violation or accident, increasing with increasing SUVR less than 1.1 (r = 0.56, P = .007) and decreasing greater than 1.1 (r = 0.57, P = .01). Driving avoidance behaviors were slightly positively (r = 0.13, P = .26) correlated with increasing SUVR if SUVR was less than 1.1, and moderately inversely correlated with SUVR greater than 1.1 (r = −0.29, P = .03). That is, after controlling for age and sex and diagnostic group, SUVR values greater than 1.1 were associated with less or fewer driving avoidance behaviors. Miles driven was essentially uncorrelated with SUVR values both less than 1.1 (r = −0.07, P = .68) and greater than 1.1 (r = 0.02, P = .86).
In the same multivariate models estimated and reported in Table 2, we also included dummy variables for the diagnostic group. The results of the baseline complete sample standardized mean differences (d) for MCI and dementia groups for each of the driving behavior outcomes are summarized in Table 3. Here, we see that although the diagnostic group does not relate significantly to risk of accidents or violations for more than the past 3 years (however, MCI participants have a slightly increased propensity [d = 0.11, P = .70] and dementia participants a slightly diminished propensity [d = −0.17, P = .64]), we see strong relationships between the diagnostic group and driving behavior outcomes. Relative to cognitive normal participants, those with MCI had moderately lower mean miles driven (d = −0.65, P = .01) and more driving avoidance behaviors (d = 0.62, P = .006). Also relative to cognitively normal participants, those with dementia had large effects in terms of miles driven (d = −1.1, P < .001) and driving avoidance behaviors (d = 1.2, P < .001).
Table 3.
Effect size for difference in mean driving outcomes relative to control subjects for mild cognitive impairment (MCI) and dementia groups
| Driving behavior | MCI | Dementia |
|---|---|---|
| Any violation or accident | 0.11 | −0.17 |
| Miles driven | −0.65∗ | −1.07† |
| Driving avoidance ratings | 0.62‡ | 1.17† |
NOTE. Table entries describe standardized mean differences in driving outcome attributable to MCI or dementia group membership. Any violations or accidents' outcome is binary, and the standardized mean difference refers to the underlying latent response variable for violations or accidents. Driving avoidance ratings refer to the maximum of self- and family-reported mean ratings on four driving avoidance patterns (amount, nighttime, rain, and busy traffic). Items are rated on a 1 to 5 scale, where higher ratings indicate greater agreement with avoidance patterns. Model r2 are reported in Table 2.
P < .05.
P < .001.
P < .01.
4. Discussion
The decline in driving ability among older adults may be associated with multiple age-related phenomena, including changes in vision, motor function, and reaction time, as well as comorbid conditions such as cataracts, sleep apnea, arthritis, medication adverse effects, metabolic disorders [17], [18], and cognitive changes because of AD [19]. The observational data from our study based on retrospective questionnaire information suggest that driving is an important and highly complex daily living function that provides a sensitive indicator of declining cognition and may be an important safety consideration for older people who are in the preclinical stage of AD [20].
Before discussing our results in the context of prior research, it is important to acknowledge the limitations of our research. Most importantly, our results derive from a descriptive analysis of an observational cohort. We used a retrospective collection of driving outcomes for more than the past 3 years and were not able to examine those events that occurred in a more temporally proximate time to the PET scan, which reduces the strength of cause and effect inferences. The significant effects we observe may be false positive research findings and require replication. This possibility is exacerbated by a relatively small sample size, which has the result of requiring effects larger than might be considered clinically or practically relevant to infer statistical significance, and also raises the possibility that detected effects represent false positives. Another limitation is that our outcome data on traffic incidents are based on self and proxy report.
Two large longitudinal driving studies of normal and cognitively impaired elders have previously shown decrements in driving performance on road test performance even among control subjects with normal cognition at baseline, raising important questions as to what may be the underlying cause or causes for such decline [21], [22]. In a 2-year longitudinal study of more than 1051 older adults, future development of dementia was the only factor found to be significantly associated with self-reported crashes at baseline, and it was not associated with driving cessation [23].
Severity of driving impairment in people with AD has been shown to correlate with cerebral blood flow changes in the temporo-occipital regions in early stage disease and with frontal deficits in more advanced impairment, particularly in the right hemisphere, using single photon emission CT [24]. Recent advances in PET now permit investigators to examine brain behavior relationships with actual amyloid brain deposits during life. Several studies have demonstrated a significant correlation between amyloid PET results and postmortem examinations [14], [25], [26], [27]. Use of this technology allows investigators to examine whether the observations described in postmortem studies of older drivers can be supported by studies of well-described living people defined by standard diagnostic criteria who have been involved in MVAs or traffic violations.
As we expected, a history of accidents and violations was predictive of higher levels of amyloid deposition on PET imaging. This observation supports previous case reports and postmortem series that found a higher than expected frequency of AD pathology in older drivers who had died in car MVAs [1], [2], [3], [4]. Among 98 older drivers aged more than 64 years who died in MVAs, 33% had neuritic plaque scores indicating probable AD and another 20% possible AD [2], [4]. A more recent postmortem study of 27 drivers older than 64 years found that 52% of autopsied drivers who died in an MVA had mild AD pathology compared with 25% of control subjects who died of other causes. None had a history of AD on medical records [1]. In a detailed case report of a 75-year-old woman who died in an MVA, she had no diagnosis of dementia before death, but an interview with her son suggested that she may have had MCI symptoms for 6 months before the MVA [3]. AD pathology has also been described as a risk factor for older pedestrians dying in traffic accidents [28], [29]. Although such research raises the intriguing possibility that driving impairment in the elderly may signify a preclinical or prodromal stage of AD, a major limitation of these postmortem studies is that there is little if any information regarding diagnosis, so some of these cases may have actually had unrecognized dementia at the time of death.
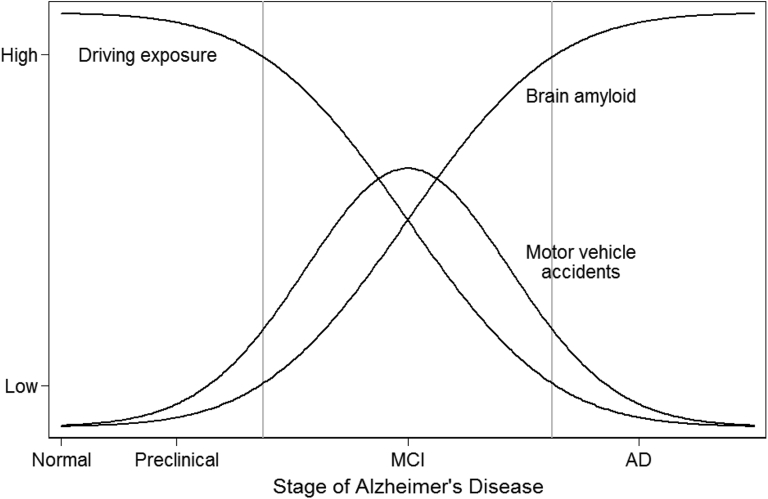
We also found that moderation of driving behaviors that might mitigate crash risk seems to be dependent on the presence of clinically manifest symptoms of cognitive impairment and dementia, rather than solely on the presence of increased amyloid levels in the brain. The attenuation of driving behaviors and crash risk at high levels of SUVR can be explained by moderation of driving behavior among those with diagnosed MCI and AD dementia. Among those in the preclinical stage of the disease, where cognitive deficits are minimal or unrecognized as a clinical problem, driving restriction probably does not occur, leaving those with increasing amyloid burden in their brains exposed to increased risk in more frequent and complex hazardous driving situations. An illustration of this proposed model of interaction among brain amyloid, driving exposure, and accident occurrence is provided in Fig. 2.
Fig. 2.
Conceptual diagram illustrating idealized relationships among driving exposure, amyloid burden, and risks for violations or accidents as a function of AD stage. Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; SUVR, standard uptake value ratio.
Additional support for amyloid being a risk factor for hazardous driving comes from our recent report showing that amyloid biomarkers measured using Pittsburgh Compound B with PET as well as cerebrospinal fluid (CSF) biomarkers of amyloid were associated with impaired road test performance in people with preclinical AD [7]. In this study, data from129 cognitively normal elders who had amyloid PET (N = 113) and/or CSF biomarker (N = 123) found that higher ratios of CSF tau/Aβ42 and ptau181/Aβ42, in addition to higher Pittsburgh compound B (PIB) mean cortical binding potentials, were associated with increased number of driving errors. Lower levels (the direction of biomarker abnormality for CSF Aβ42) of CSF Aβ42 were also associated with increases in the number of driving errors, but this did not reach statistical significance (P = .06), indicating overall that cognitively normal older adults with more AD-like biomarker values have worse driving performance.
Perhaps the most interesting and provocative finding in our study is the clinical relevance of increasing levels of amyloid at levels less than what is typically considered indicative of significant Alzheimer amyloid pathology. At this level of SUVR there are sparse if any well-developed amyloid plaques, because florbetapir binds primarily if not solely to fibrillar amyloid. However, at low levels of florbetapir binding, more soluble Aβ oligomers may still be present and represent the more directly neurotoxic forms of the protein, as suggested by recent studies [30], [31], [32], [33]. If this is the case, further studies using radiologic ligands that tag more soluble amyloid peptide species may yield more robust associations with driving risk than the present study. Our results also support a recent observation that many patients with MCI experience cognitive and functional decline associated with “prethreshold” levels of CSF Aβ42 [34].
These results highlight how potentially important the early recognition and accurate identification of preclinical AD might be for personal and public safety. They also suggest that clinically meaningful changes in function may occur in the preclinical stage when amyloid burden in the brain is lower than SUVR thresholds on PET typically felt to indicate moderate to extensive amyloid pathology.
Future studies using larger sample sizes may be able to focus solely on MVAs, as this is the major safety concern for older drivers, who have an exceptionally high rate of fatal car crashes compared with younger drivers because of their frailty [35]. Also of note, one might expect that a Stage III level of preclinical AD, as defined by the presence of cognitive impairment in addition to biomarkers of disease [5], might be associated with higher levels of driving risk. In this study, we did not have uniform psychometric test scores available across participants to enable such an analysis, but this would be an interesting future focus of study.
In summary, the results from this retrospective observational study of older drivers suggest that driving problems in the preclinical stage of AD may be related to increasing levels of amyloid deposition in the brain. If so, then assessment of driving may provide important information about the effectiveness of antiamyloid prevention therapies and their potential to improve the safety of older people who remain active drivers. Prospective longitudinal research studies should help to further clarify the magnitude of the safety risk posed by amyloid deposits in the brains of older drivers.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional sources, including PubMed search of relevant articles during the past 20 years. There have been a small number of postmortem publications reporting a higher than expected prevalence of Alzheimer pathology and two recent studies examining epidemiologic and road test correlates in preclinical Alzheimer's disease.
-
2.
Interpretation: Our findings provide additional support for these earlier observations, indicating that driving impairment may be evident in older people without apparent signs of cognitive decline although they have accumulating Alzheimer pathology.
-
3.
Future directions: The correlation between rising levels of brain amyloid on positron emission tomography with accidents and traffic violations at a standardized uptake value ratio level lower than that typically associated with moderate to severe Alzheimer pathology suggests that lower standardized uptake value ratio cutoff levels than currently being used to define subjects for Alzheimer's disease prevention trials should be considered.
Acknowledgments
These results were presented at the Alzheimer's Association International Conference in Toronto, Canada, July 27, 2016. This research was supported in part by grant R03-AG046472 to B.R.O. from the National Institute on Aging.
References
- 1.Gorrie C.A., Rodriguez M., Sachdev P., Duflou J., Waite P.M. Mild neuritic changes are increased in the brains of fatally injured older motor vehicle drivers. Accid Anal Prev. 2007;39:1114–1120. doi: 10.1016/j.aap.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Johansson K., Bogdanovic N., Kalimo H., Winblad B., Viitanen M. Alzheimer's disease and apolipoprotein E epsilon 4 allele in older drivers who died in automobile accidents. Lancet. 1997;349:1143–1144. doi: 10.1016/S0140-6736(97)24016-X. [DOI] [PubMed] [Google Scholar]
- 3.Kibayashi K., Shojo H. Incipient Alzheimer's disease as the underlying cause of a motor vehicle crash. Med Sci Law. 2002;42:233–236. doi: 10.1177/002580240204200307. [DOI] [PubMed] [Google Scholar]
- 4.Viitanen M., Johansson K., Bogdanovic N., Berkowicz A., Druid H., Eriksson A. Alzheimer changes are common in aged drivers killed in single car crashes and at intersections. Forensic Sci Int. 1998;96:115–127. doi: 10.1016/s0379-0738(98)00114-5. [DOI] [PubMed] [Google Scholar]
- 5.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roe C.M., Barco P.P., Head D.M., Ghoshal N., Selsor N., Babulal G.M. Amyloid imaging, cerebrospinal fluid biomarkers predict driving performance among cognitively normal individuals. Alzheimer Dis Assoc Disord. 2016 doi: 10.1097/WAD.0000000000000154. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack C.R., Jr., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris J.C. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9 Suppl 1:173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 11.Iverson D.J., Gronseth G.S., Reger M.A., Classen S., Dubinsky R.M., Rizzo M. Practice parameter update: evaluation and management of driving risk in dementia: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:1316–1324. doi: 10.1212/WNL.0b013e3181da3b0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MIMneuro user guide [computer program]. Version 6.1. MIM Software, Inc.; Cleveland, OH: 2013. [Google Scholar]
- 13.Johnson K.A., Sperling R.A., Gidicsin C.M., Carmasin J.S., Maye J.E., Coleman R.E. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–S83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark C.M., Pontecorvo M.J., Beach T.G., Bedell B.J., Coleman R.E., Doraiswamy P.M. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 15.Fleisher A.S., Chen K., Liu X., Roontiva A., Thiyyagura P., Ayutyanont N. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–1411. doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 16.Royston P., Altman D. Regression using fractional polynomials of continuous covariates: parsimonious parametric modeling. Appl Stat. 1994;43:425–467. [Google Scholar]
- 17.American Medical Association. Clinician's guide to assessing and counseling older drivers. Report No DOT HS 812 228 2016 [cited 2016 Mar 4];3:1–313. Available at: http://www.nhtsa.gov/staticfiles/nti/older_drivers/pdf/812228_CliniciansGuideToOlderDrivers.pdf. Accessed December 2, 2016.
- 18.Rizzo M. Impaired driving from medical conditions: a 70-year-old man trying to decide if he should continue driving. JAMA. 2011;305:1018–1026. doi: 10.1001/jama.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr D.B., Ott B.R. The older adult driver with cognitive impairment: “It's a very frustrating life”. JAMA. 2010;303:1632–1641. doi: 10.1001/jama.2010.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverberg N.B., Ryan L.M., Carrillo M.C., Sperling R., Petersen R.C., Posner H.B. Assessment of cognition in early dementia. Alzheimers Dement. 2011;7:e60–e76. doi: 10.1016/j.jalz.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duchek J.M., Carr D.B., Hunt L., Roe C.M., Xiong C., Shah K. Longitudinal driving performance in early-stage dementia of the Alzheimer type. J Am Geriatr Soc. 2003;51:1342–1347. doi: 10.1046/j.1532-5415.2003.51481.x. [DOI] [PubMed] [Google Scholar]
- 22.Ott B.R., Heindel W.C., Papandonatos G.D., Festa E.K., Davis J.D., Daiello L.A. A longitudinal study of drivers with Alzheimer disease. Neurology. 2008;70:1171–1178. doi: 10.1212/01.wnl.0000294469.27156.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lafont S., Laumon B., Helmer C., Dartigues J.F., Fabrigoule C. Driving cessation and self-reported car crashes in older drivers: the impact of cognitive impairment and dementia in a population-based study. J Geriatr Psychiatry Neurol. 2008;21:171–182. doi: 10.1177/0891988708316861. [DOI] [PubMed] [Google Scholar]
- 24.Ott B.R., Heindel W.C., Whelihan W.M., Caron M.D., Piatt A.L., Noto R.B. A single-photon emission computed tomography imaging study of driving impairment in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2000;11:153–160. doi: 10.1159/000017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabri O., Sabbagh M.N., Seibyl J., Barthel H., Akatsu H., Ouchi Y. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer's disease: phase 3 study. Alzheimers Dement. 2015;11:964–974. doi: 10.1016/j.jalz.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Thal D.R., Beach T.G., Zanette M., Heurling K., Chakrabarty A., Ismail A. [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer's disease: specific detection of advanced phases of amyloid-beta pathology. Alzheimers Dement. 2015;11:975–985. doi: 10.1016/j.jalz.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Clark C.M., Schneider J.A., Bedell B.J., Beach T.G., Bilker W.B., Mintun M.A. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorrie C.A., Rodriguez M., Sachdev P., Duflou J., Waite P.M. Increased neurofibrillary tangles in the brains of older pedestrians killed in traffic accidents. Dement Geriatr Cogn Disord. 2006;22:20–26. doi: 10.1159/000093066. [DOI] [PubMed] [Google Scholar]
- 29.Gorrie C.A., Brown J., Waite P.M. Crash characteristics of older pedestrian fatalities: dementia pathology may be related to ‘at risk’ traffic situations. Accid Anal Prev. 2008;40:912–919. doi: 10.1016/j.aap.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Benilova I., Karran E., De S.B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 31.Goure W.F., Krafft G.A., Jerecic J., Hefti F. Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer's disease immunotherapeutics. Alzheimers Res Ther. 2014;6:42. doi: 10.1186/alzrt272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harte N.P., Klyubin I., McCarthy E.K., Min S., Garrahy S.A., Xie Y. Amyloid oligomers and mature fibrils prepared from an innocuous protein cause diverging cellular death mechanisms. J Biol Chem. 2015;290:28343–28352. doi: 10.1074/jbc.M115.676072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hefti F., Goure W.F., Jerecic J., Iverson K.S., Walicke P.A., Krafft G.A. The case for soluble Abeta oligomers as a drug target in Alzheimer's disease. Trends Pharmacol Sci. 2013;34:261–266. doi: 10.1016/j.tips.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Insel P.S., Mattsson N., Mackin R.S., Scholl M., Nosheny R.L., Tosun D. Accelerating rates of cognitive decline and imaging markers associated with beta-amyloid pathology. Neurology. 2016;86:1887–1896. doi: 10.1212/WNL.0000000000002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kent R., Henary B., Matsuoka F. On the fatal crash experience of older drivers. Annu Proc Assoc Adv Automot Med. 2005;49:371–391. [PMC free article] [PubMed] [Google Scholar]