Abstract
Ferric citrate (FC) has demonstrated efficacy as a phosphate binder and reduces the requirements for erythropoiesis-stimulating agents (ESAs) and intravenous (IV) iron in dialysis patients. We developed a net budgetary impact model to evaluate FC vs. other phosphate binders from the vantage of a large dialysis provider. We used a Markov microsimulation model to simulate mutually referential longitudinal effects between serum phosphate and phosphate binder dose; categories of these defined health states. Health states probabilistically determined treatment attendance and utilization of ESA and IV iron. We derived model inputs from a retrospective analysis of incident phosphate binder users from a large dialysis organization (January 2011–June 2013) and incorporated treatment effects of FC from a phase III trial. The model was run over a 1-year time horizon. We considered fixed costs of providing dialysis; costs of administering ESA and IV iron; and payment rates for dialysis, ESAs, and IV iron. In the base-case model, FC had a net budgetary impact (savings) of +US$213,223/year per 100 patients treated vs. standard of care. One-way sensitivity analyses showed a net budgetary impact of up to +US$316,296/year per 100 patients treated when higher hemoglobin levels observed with FC translated into a 30% additional ESA dose reduction, and up to +US$223,281/year per 100 patients treated when effects on missed treatment rates were varied. Two-way sensitivity analyses in which acquisition costs for ESA and IV iron were varied showed a net budgetary impact of +US$104,840 to +US$213,223/year per 100 patients treated. FC as a first-line phosphate binder would likely yield substantive savings vs. standard of care under current reimbursement.
Electronic supplementary material
The online version of this article (doi:10.1007/s40268-016-0163-7) contains supplementary material, which is available to authorized users.
Key Points
| Ferric citrate has been shown to be efficacious as a phosphate binder in end-stage renal disease patients receiving hemodialysis and also results in reduced utilization of erythropoiesis-stimulating agents and intravenous iron, higher hemoglobin levels, and lower hospitalization rates. |
| Using a Markov microsimulation model, we show that under the current reimbursement paradigm, use of ferric citrate as a first-line phosphate binder is associated with a base-case cost savings of approximately US$213,223/year per 100 patients treated compared with standard of care. |
| Cost savings arise principally from reductions in erythropoiesis-stimulating agent and intravenous iron utilization and in the number of hemodialysis sessions missed as a result of hospitalizations. |
Introduction
Hyperphosphatemia is a nearly ubiquitous consequence of end-stage renal disease (ESRD) and is associated with increased risks of mortality and hospitalization [1–6]. The Kidney Disease Outcomes Quality Initiative guidelines recommend maintaining serum phosphate levels within the range of 3.5–5.5 mg/dL. Phosphate homeostasis in patients receiving dialysis generally requires dietary phosphate restriction and, frequently, the use of phosphate binders to prevent systemic absorption [7].
Anemia is another common complication of ESRD [8, 9]. Patients receiving maintenance dialysis are frequently treated with erythropoiesis-stimulating agents (ESAs) to stimulate red blood cell production. Iron is required to support erythropoiesis and patients typically also receive intravenous (IV) iron [10]. However, the use of IV iron is associated with hepcidin-mediated iron sequestration, as well as oxidative stress [11]. Since the restructuring of dialysis remuneration by the Centers for Medicare and Medicaid Services Prospective Payment System, there has been a national shift toward lower ESA utilization and higher IV iron provision [12, 13]. However, this therapeutic approach, whereby ESA and iron are used as alternatives, is not well aligned with the complementary manner in which these agents act biologically.
The iron-based phosphate binder ferric citrate (Auryxia™; Keryx Biopharmaceuticals, Inc., New York, NY, USA) has recently completed phase III clinical trials in the US (NCT01191255, NCT01554982) and Japan (CTI-111433) and has been approved by the US Food and Drug Administration. In addition to being safe and efficacious as a phosphate binder, clinical trials have shown that ferric citrate results in a stable and sustained increase in serum ferritin levels and transferrin saturation, without evidence of iron overload. Ferric citrate use also resulted in reduced utilization of ESA and IV iron, with higher hemoglobin (Hb) levels and lower hospitalization rates [14–16].
The objective of the current study was to evaluate the budgetary impact of ferric citrate as a first-line phosphate binder from the perspective of a dialysis provider within the context of the current reimbursement paradigm and accounting for the effects of ferric citrate on ESA and IV iron utilization, Hb levels, and the potential for missed hemodialysis sessions resulting from hospitalizations. To this end, we constructed a Markov microsimulation model drawing input data from a retrospective evaluation of incident users of phosphate binders at a large dialysis organization (LDO) in the US as well as primary results from a phase III clinical trial.
Methods
Model Structure Overview
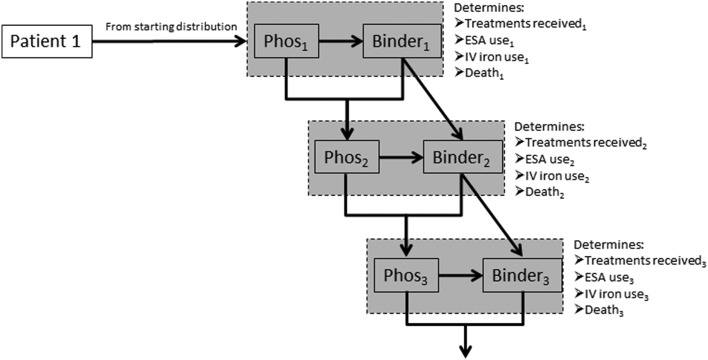
We constructed a Markov microsimulation model using TreeAge Pro 2013 (TreeAge Software Inc., Williamston, MA, USA). The model considered 21 health states: 20 based on permuted categories of serum phosphate (<3.5, 3.5–5.5, 5.6–6.5, >6.5 mg/dL) and phosphate binder dose strength (0, >0 to <2, ≥2 to <3, ≥3 to <4, ≥4); the 21st state was death, which was an absorbing state. We modeled serum phosphate and phosphate binder dose strength as continuous tracer variables and modeled the mutually referential longitudinal effects between the two as follows (Fig. 1):
For each patient, baseline serum phosphate was assigned probabilisitically.
Starting phosphate binder dose strength was then probabilistically assigned conditional on baseline phosphate.
Interval change in serum phosphate was probabilistically assigned conditional on preceding serum phosphate and phosphate binder dose strength; serum phosphate in the subsequent interval was defined by prior value plus interval change.
Interval change in phosphate binder dose strength was probabilistically assigned conditional on preceding phosphate binder dose strength and serum phosphate; phosphate binder dose strength in the subsequent interval was defined by the prior dose strength plus interval change.
Fig. 1.
Schematic of the microsimulation model. At study start, the patient is probabilistically assigned a starting serum phosphate level (Phos1) based on the empiric distribution of phosphate levels that immediately preceded phosphate binder initiation. The phosphate binder dose strength for cycle 1 (Binder1) is assigned probabilistically conditional on Phos1. Phos1 and Binder1 determine the patient’s health state for cycle 1, which probabilistically determines the number of dialysis treatments received during the period (Treatments received1), the total dose of ESA and IV iron received during cycle 1 (ESA use1 and IV iron use1) and the probability of death during cycle 1 (Death1). For patients who survive cycle 1, a change in phosphate between cycle 1 and cycle 2 is probabilistically sampled based on Phos1 and Binder1; this is added to Phos1 to give serum phosphate in cycle 2 (Phos2). A change in phosphate binder strength at the start of cycle 2 is probabilistically sampled conditional on Phos2 and Binder1; this is added to Binder1 to give binder strength in cycle 2 (Binder2). Phos2 and Binder2 then define the health state for cycle 2. This process iterates forward. ESA erythropoiesis-stimulating agent, IV intravenous
For each interval, probability of death, hemodialysis treatment attendance, ESA utilization, and IV iron utilization were probabilistically assigned conditional upon health state. We defined variable revenues and costs by monetizing these utilization parameters (see below).
We ran the model over a 1-year time horizon and considered a 1-month cycle length. We used combined first- and second-order Monte Carlo simulation to fit models. Each model considered 1000 second-order trials. The number of random walks (representing patients) was set to 100 to loosely correspond to the number of patients who might be treated with phosphate binders at a single dialysis facility. To assess the validity of the model, the modeled course of serum phosphate, phosphate binder utilization and mortality were compared with actual values and found to be comparable.
Source Data
We derived model input data from a retrospective analysis of new phosphate binder users from an LDO. Patients considered in the source cohort were those who received in-center hemodialysis at the LDO between January 2011 and June 2013, were enrolled in the LDO’s pharmacy management program (to ensure visibility to phosphate binder use), and were incident users of phosphate binders, defined as receiving an index fill of phosphate binder after 90+ days without any phosphate binder supply. We considered patients forward in time for a maximum of 24 months or until censoring (death, transfer, modality change, transplant, end of study period). The specific parameters we estimated for the source cohort were: distribution of payer mix (Medicare vs. private insurance) as of index phosphate binder fill; distribution of starting serum phosphate, estimated from the most recent serum phosphate measurement preceding the index phosphate binder fill (within 30 days); frequency distribution of starting phosphate binder dose strength within each category of starting phosphate, where dose strength for individual phosphate binders was assigned as described in Table 1 (which accounts for diminishing incremental efficacy at higher doses) and each simulated patient’s total phosphate binder strength was determined by summing strength for each individual agent; distribution of change in serum phosphate (current interval minus prior interval) within each category of prior serum phosphate and phosphate binder dose strength; frequency distribution of change in phosphate binder dose strength (current interval minus prior interval) within each category of prior phosphate binder dose strength and concurrent serum phosphate; probability of death during the cycle based on categories of concurrent serum phosphate and phosphate binder dose strength; distributions of ESA dose, IV iron dose, number of IV iron administrations, and number of dialysis sessions attended per cycle based on categories of concurrent serum phosphate and phosphate binder dose strength. Analytically, ESA dose and IV iron dose were log transformed and back transformed to the native scale prior to cost conversion.
Table 1.
Phosphate binder dose strength categories
| Phosphate binder type | Dose (mg) | Strengtha |
|---|---|---|
| Calcium acetate | 1–2001 2002–4002 >4002 |
+1 +1.5 +2 |
| Sevelamer | 1–2300 2401–4800 >4800 |
+1 +1.5 +2 |
| Lanthanum | 1–3000 3001–6000 >6000 |
+1 +1.5 +2 |
aEmpirically, very few patients (<2%) started at dose strengths >2. Starting dose strength was therefore truncated. At later times, simulated patients could transition to higher dose strengths commensurate with transitions observed in the empiric data
We incorporated the effects of ferric citrate from the results of a phase III clinical trial [14, 15] and applied these to the ferric citrate group except when patients were probabilistically determined to have a phosphate binder dose strength of zero (i.e., were not on a phosphate binder). Given the new user design, all patients were on a phosphate binder at study start, but at later times patients could transition to no binder use, commensurate with observations made in the empiric data. Additional information on model inputs and source data is provided in Online Resource 1.
Costing
Costs and revenues considered were: revenue for attended treatments (payer specific), fixed costs of providing dialysis, cost of ESA, remuneration for ESA (commercial payer only), cost of IV iron (drug and peripherals/disposables associated with drug administration), and payment for IV iron (commercial payer only). Acquisition costs for IV iron and ESA were based on REDBOOK™ average wholesale prices (AWP) (accessed 31 March, 2015). Phosphate binder costs were not included in the model as these costs are not borne by dialysis facilities under current reimbursement policies [17]. Given the short time horizon, we did not apply discounting.
Results
Model Inputs, Assumptions, Transitions, and Costs
Retrospective analysis of the LDO database yielded 25,950 patients who met all criteria for inclusion and 321,543 patient-months of observation were assessed. The mean starting serum phosphate level was 5.4 mg/dL and the distribution of payer type was 85% Medicare, 15% private insurance. The distribution of phosphate binder types prescribed at index fill was 47% sevelamer (carbonate or hydrochloride), 45% calcium acetate, and 7% lanthanum carbonate. Higher starting serum phosphate level was associated with higher starting phosphate binder strength: 69% of patients in the highest serum phosphate category (>6.5 mg/dL) started with a phosphate binder dose strength of 2 vs. 52% of patients in the lowest serum phosphate category (<3.5 mg/dL). Almost no patients started on a phosphate binder dose strength of >2; therefore only strengths of 1, 1.5, and 2 were modeled for initial phosphate binder dose strength. Thereafter, modeled phosphate binder dose strength could exceed 2, commensurate with patterns observed in the source data.
We estimated the reduction in ESA dose (36%), iron dose (55%), and number of IV iron administrations (59%) based on primary study data from a phase III trial [14]. The number of hospitalizations was reduced by 24%; in the base-case model, we assumed that this would translate to a 24% reduction in the number of missed dialysis treatments [15].
Model inputs are summarized in Table 2. Remuneration for dialysis (considered separately for Medicare and privately insured patients) and for injected drug administration (relevant for privately insured patients) was based on actual values, which are proprietary and cannot be shown.
Table 2.
Base-case model inputs
| Value | Source/reference | ||
|---|---|---|---|
| Model inputs derived from retrospective analysis of LDO patients | |||
| Mean (SD) starting serum phosphate, mg/dL | 5.4 (1.6) | Retrospective analysis of LDO patients | |
| Distribution of primary insurance,% | |||
| Medicare | 85 | ||
| Private | 15 | ||
| PB mix (standard of care),% | |||
| Calcium acetate | 45 | ||
| Sevelamer | 47 | ||
| Lanthanum carbonate | 7 | ||
| Starting PB strength based on starting serum phosphate,% | |||
| Serum phosphate <3.5 mg/dL, starting PB strength: | 1 1.5 2 |
19.9 27.7 52.4 |
|
| Serum phosphate = 3.5–5.5 mg/dL, starting PB strength: | 1 1.5 2 |
19.6 23.1 57.4 |
|
| Serum phosphate = 5.6–6.5 mg/dL, starting PB strength: | 1 1.5 2 |
17.8 21.2 61.0 |
|
| Serum phosphate > 6.5 mg/dL, starting PB strength: | 1 1.5 2 |
13.4 17.2 69.4 |
|
| Model inputs derived from publically available sources | |||
| Drug acquistion costs | |||
| ESA (Epogen) | US$18.24/1000 U | REDBOOK™ AWPa | |
| IV iron (iron sucrose) | US$8.64/100 mg | ||
| Model inputs derived from ferric citrate clinical trials | |||
| Effects of ferric citrate: | |||
| ESA dose | 36% reduction | [14, 15]c | |
| IV iron dose | 55% reduction | ||
| IV iron administrations | 59% reduction | ||
| Missed dialysis treatmentsb | 24% reduction | ||
AWP average wholesale price, ESA erythropoiesis-stimulating agent, Hb hemoglobin, IV intravenous, LDO large dialysis organization, PB phosphate binder, SD standard deviation
aAccessed 31 March, 2015
bInferred from hospitalizations, assuming 1:1 ratio of missed dialysis treatments to hospitalizations
cEstimates were modeled from primary clinical trial data
Base-Case Analyses
Under base-case assumptions and considered over 1 year, treatment with ferric citrate vs. standard of care was found to have a net budgetary impact of +US$213,223/year (p < 0.001) per 100 patients treated with phosphate binder (Table 3).
Table 3.
Net budgetary impact of ferric citrate vs. standard of care; base-case model
| Net budgetary impact of ferric citrate vs. SOC (US$/year per 100 patients treated) | |||
|---|---|---|---|
| Mean (SEM) | Median [p25; p75] | p valuea | |
| Overall | +213,223 (14,111) | +213,018 [203,743; 222,109] | <0.001 |
| Component: missed treatment | +12,766 (4276) | +12,474 [9622; 15,748] | <0.001 |
| Component: ESA | +194,452 (13,683) | +194,652 [185,618; 203,423] | <0.001 |
| Component: IV iron | +5775 (948) | +5806 [5171; 5807] | <0.001 |
ESA erythropoiesis-stimulating agent, IV intravenous, SEM standard error of mean, SOC standard of care
a p value for comparison of mean, ferric citrate vs. SOC
Component Costs
Under the base-case scenario, we examined the component effects of missed treatment reductions, ESA utilization reduction, and IV iron utilization reduction. Each considered in isolation, the 24% reduction in missed treatments corresponded to a net budgetary impact of +US$12,474/year per 100 patients treated; the 36% reduction in ESA utilization corresponded to a net budgetary impact of +US$194,652/year per 100 patients treated; and the 55% reduction in IV iron utilization corresponded to a net budgetary impact of +US$5806/year per 100 patients treated (Table 3).
One-Way Sensitivity Analysis: Effect of Ferric Citrate on Missed Treatment Rate
The effects of ferric citrate on missed dialysis treatments per se have not been studied; however, clinical trial data indicate that ferric citrate use results in a 24% lower hospitalization rate [15]. In base-case models, we assumed that the number of missed treatments corresponded 1:1 with hospitalizations; therefore, a 24% reduction in missed treatments was assumed. However, because ferric citrate specifically reduced types of hospitalizations that typically incur longer lengths of stay (cardiovascular, sepsis, gastrointestinal), the ratio of missed treatments to hospitalizations may be greater than 1:1. In a sensitivity analysis, we assessed the net budgetary impact of ferric citrate varying the assumed ratio of missed treatments to hospitalizations. Varying the ratio of missed sessions to hospitalizations to 1.25:1 (30% reduction in missed sessions) or 1.5:1 (36% reduction in missed sessions) resulted in a net budgetary impact of ferric citrate of +US$220,366/year per 100 patients treated and +US$223,281/year per 100 patients treated, respectively (p < 0.001 for both, Table 4). Under a maximally conservative assumption whereby ferric citrate use did not translate into any reduction in missed hemodialysis sessions, the net budgetary impact of ferric citrate was +US$199,220/year per 100 patients treated (standard error of mean, 14,281; p < 0.001).
Table 4.
Net budgetary impact of ferric citrate vs. standard of care; one-way sensitivity analyses
| Net budgetary impact of ferric citrate vs. SOC (US$/year per 100 patients treated) | ||
|---|---|---|
| Mean (SEM) | p valuea | |
| Ratio of missed treatments to hospitalizations | ||
| 1:1 (base case; 24% reduction in missed treatments) | +213,223 (14,111) | <0.001 |
| 1.25:1 (30% reduction in missed treatments) | +220,366 (14,340) | <0.001 |
| 1.5:1 (36% reduction in missed treatments) | +223,281 (14,839) | <0.001 |
| Implications of Hb differential on ESA utilization, +0.35 g/dL greater Hb results in: | ||
| 0% further reduction in ESA (base case) | +213,223 (14,111) | <0.001 |
| 10% further reduction in ESA | +246,690 (15,871) | <0.001 |
| 20% further reduction in ESA | +281,758 (19,262) | <0.001 |
| 30% further reduction in ESA | +316,296 (21,974) | <0.001 |
ESA erythropoiesis-stimulating agent, Hb hemoglobin, SEM standard error of mean, SOC standard of care
a p value for comparison of mean, ferric citrate vs. SOC
One-Way Sensitivity Analysis: Effect of Hemoglobin Differential
In the phase III clinical trial, mean Hb was 0.33 g/dL higher in ferric citrate-treated patients compared with control patients. In clinical practice, this magnitude of the Hb differential may be sufficient to prompt ESA down-titration in ferric citrate-treated patients, which would result in incremental reductions in ESA utilization. We conducted a sensitivity analysis to evaluate the economic impact of ferric citrate over varied assumptions regarding the impact of the Hb differential on further ESA reductions. Specifically, scenarios were considered where the +0.35 g/dL increase in Hb translated into additional 10, 20, or 30% reductions in ESA. Net budgetary impact was +US$246,690, +US$281,758, and +US$316,296/year per 100 patients treated, respectively (Table 4).
Two-Way Sensitivity Analysis Varying ESA and Iron Costs
Typically, dialysis providers do not pay full wholesale price for ESAs and IV iron because of rebating. The precise acquisition cost for these agents varies from provider to provider and over time within a provider based on negotiated contracts and data are not publically available. To explore the impact of ESA and iron costs on the net budgetary impact of ferric citrate, we performed a two-way sensitivity analysis in which acquisition costs for ESAs and for IV iron were permuted: each from 100 to 50% of listed AWP so as to encompass all foreseeable circumstances. Across these scenarios, the net budgetary impact varied from +US$104,840 to +US$213,223/year per 100 patients treated (Table 5). The net budgetary impact was more sensitive to differences in ESA acquisition costs than IV iron acquisition costs.
Table 5.
Net budgetary impact of ferric citrate vs. standard of care in two-way sensitivity analysis in which acquisition costs for ESA and IV iron are varied
| Net budgetary impact of ferric citrate vs. SOC (US$/year per 100 patients treated) | Acquisition cost for ESAa | ||
|---|---|---|---|
| 50% AWP (US$9.12/1000 U) | 75% AWP (US$13.68/1000 U) | 100% AWP (US$18.24/1000 U) | |
| Acquisition cost for IV irona | |||
| 50% AWP (US$4.32/100 mg) | +104,840 | +157,138 | +209,802 |
| 75% AWP (US$6.46/100 mg) | +106,946 | +158,680 | +210,447 |
| 100% AWP (US$8.64/100 mg) | +108,407 | +160,399 | +213,223 |
AWP average wholesale price, ESA erythropoiesis-stimulating agent, IV intravenous, SOC standard of care
aBased on REDBOOK™ AWP; accessed 31 March, 2015
Discussion
The net budgetary impact model presented demonstrates that if ferric citrate was adopted in wide clinical practice as a first-line phosphate binder rather than the current standard of care, cost savings to the dialysis provider would be approximately US$213,000/year per 100 patients receiving phosphate binders under current reimbursement policies. Extrapolation of this value gives estimated cost savings of US$14.29 per patient per treatment among patients receiving phosphate binders. Estimated cost savings arise principally from reductions in ESA utilization but also from reductions in missed dialysis treatments and IV iron utilization. The potential range of cost savings depends in part on a provider’s acquisition cost for ESA, and whether and how physicians further adjust ESA dose in response to the +0.35 g/dL differential in Hb level seen in ferric citrate-treated patients compared with control patients. To a lesser extent, potential cost savings depend on a provider’s acquisition cost for IV iron and to the extent to which reductions in missed dialysis treatments mirror the observed reductions in hospitalizations.
Changes to Centers for Medicare and Medicaid Services reimbursement for hemodialysis in recent years have affected dialysis facilities’ financial status, with the costs of providing care exceeding payments received for Medicare patients. With the implementation of the ESRD Prospective Payment System in 2011, the dialysis composite rate payment was expanded to cover the cost of injectable drugs and laboratory tests that were previously separately billable. [12] In November 2013, Centers for Medicare and Medicaid Services issued its final rule for calendar year 2014, updating payment rates for the ESRD Prospective Payment System based on an observed >30% drop in drug utilization (primarily in ESAs) from 2007 to 2012 [17], which will result in an additional 12% reduction in payments, implemented over a 3- to 4-year transition period.
A previously published cost-offset model developed from the perspective of a managed care payer indicated potential cost savings with ferric citrate between US$626,000 and US$1,106,000 per year for a very large individual dialysis facility serving 500 patients [18]. The current model considered savings from the perspective of a dialysis provider based on the observed payer mix of patients who initiated phosphate binders, thus comparison of the two models is difficult. In addition to the difference in perspective, several important methodological differences between the studies should be noted. The previously published model considered savings as a result of reductions in ESA and IV iron utilization alone. Moreover, that model was developed prior to completion of the phase III US trial of ferric citrate; effects of ferric citrate on ESA and IV iron use were therefore inferred from changes in serum ferritin levels and transferrin saturation observed in phase II trials [19]. Since then, phase III trials have been completed and the effects of ferric citrate on drug utilization have been assessed directly, thereby providing real inputs for the model we describe here.
Several important notes are offered with respect to interpretation of these data. First, all results are expressed as differences in the net budget between ferric citrate and standard of care. These should not be misinterpreted as absolute profit/loss estimates. Second, cost savings pertain strictly to patients who are treated with ferric citrate and not patients who do not receive phosphate binders. Payer mix among phosphate binder initiators is skewed toward a higher prevalence of commercial payers because such patients are, on average, younger than the overall dialysis population. Therefore, extrapolation of findings to a facility or provider level should be undertaken cautiously. Third, the data used to estimate costs were derived based on contracted rates for a single provider in the US; actual costs will therefore vary across provider organizations. Fourth, the effects of ferric citrate on drug utilization and hospitalization rates were derived from the findings of a clinical trial and the effects of the drug in practice are likely to differ from those observed in randomized controlled trials. Fifth, model inputs were derived from a retrospective analysis of incident phosphate binder users who were enrolled in the LDO’s pharmacy management program. It cannot be empirically determined whether such patients are representative of phosphate binder initiators who are not enrolled in the pharmacy management program because oral prescription drug data are not available for the latter. Sixth, the model was developed using data from patients undergoing hemodialysis. The net budgetary impact of ferric citrate in patients undergoing hemodiafiltration or peritoneal dialysis, and in patients with advanced (stage 4 or 5) non-dialysis-requiring chronic kidney disease, who may also require treatment with phosphate binders, IV iron, and ESAs, was not estimated. Finally, the model assessed the net budgetary impact of ferric citrate under the current reimbursement paradigm where oral medications are reimbursed separately from dialysis. Therefore, these data do not pertain to the net budgetary impact of ferric citrate in the context of an even more comprehensive bundle, under which dialysis providers assume financial responsibility for the costs of oral drugs used in patients undergoing dialysis, including phosphate binders.
Strengths of the current model include the robust source data on which it was based (including phase III trial results as well as analysis of detailed data from >25,000 incident patients), the wide array of health states and inputs considered, and robust sensitivity analyses to examine the sensitivity of findings to assumptions made regarding inputs that could not be observed directly in source data.
Conclusion
The use of ferric citrate as first-line phosphate binder therapy in patients undergoing hemodialysis was associated with a favorable US$213,000 budgetary impact per 100 patients receiving phosphate binders per year under the current reimbursement paradigm.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Abigail Hunt, PhD, of DaVita Clinical Research for medical writing and editorial support.
Compliance with Ethical Standards
Funding
Funding for this study and for medical writing support was provided by Keryx Biopharmaceuticals, Inc.
Conflict of interest
Steven Brunelli is employed by DaVita Clinical Research, his spouse is employed by AstraZeneca. Scott Sibbel is employed by DaVita Clinical Research. David Van Wyck is employed by and owns stock in DaVita Inc. Amit Sharma was an employee of Keryx Biopharmaceuticals, Inc. at the time the study was conducted. Andrew Hsieh was an employee of Keryx Biopharmaceuticals, Inc. at the time the study was conducted. Glenn Chertow has served as a consultant to Keryx Biopharmaceuticals, Inc. for work related to the design and oversight of clinical trials, and for service on an advisory board. This study and subsequent manuscript development were funded by Keryx Biopharmaceuticals, Inc. Medical writing support for manuscript development was provided by Abigail E. Hunt, PhD, an employee of DaVita Clinical Research.
References
- 1.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 4.Menon V, Greene T, Pereira AA, et al. Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am J Kidney Dis. 2005;46(3):455–463. doi: 10.1053/j.ajkd.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Saran R, Bragg-Gresham JL, Rayner HC, et al. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64(1):254–262. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 6.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52(3):519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 7.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl. 3):S1–201. S0272638603009053 [pii]. [PubMed]
- 8.Astor BC, Muntner P, Levin A, et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162(12):1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 9.Obrador GT, Roberts T, St Peter WL, et al. Trends in anemia at initiation of dialysis in the United States. Kidney Int. 2001;60(5):1875–1884. doi: 10.1046/j.1523-1755.2001.00002.x. [DOI] [PubMed] [Google Scholar]
- 10.K/DOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(5 Suppl. 3):S11–145. doi:10.1053/j.ajkd.2006.03.010. [DOI] [PubMed]
- 11.Horl WH. Clinical aspects of iron use in the anemia of kidney disease. J Am Soc Nephrol. 2007;18(2):382–393. doi: 10.1681/ASN.2006080856. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services End-stage renal disease prospective payment system and quality incentive program: final rule. Fed Regist. 2011;76(218):70228–70318. [PubMed] [Google Scholar]
- 13.Fuller DS, Pisoni RL, Bieber BA, et al. The DOPPS practice monitor for U.S. dialysis care: update on trends in anemia management 2 years into the bundle. Am J Kidney Dis. 2013;62(6):1213–1216. doi: 10.1053/j.ajkd.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JB, Sika M, Koury MJ, et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26(2):493–503. doi: 10.1681/ASN.2014020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodby R, Umanath K, Niecestro R, et al. Phosphorus binding with ferric citrate is associated with fewer hospitalizations and reduced hospitalization costs. Expert Rev Pharmacoecon Outcomes Res. 2015;15(3):545–550. doi: 10.1586/14737167.2015.995169. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama K, Hirakata H, Akiba T, et al. Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol. 2012;36(5):478–487. doi: 10.1159/000344008. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Medicare and Medicaid Services End-stage renal disease prospective payment system and quality incentive program: final rule. Fed Regist. 2013;78(231):72156–72251. [Google Scholar]
- 18.Mutell R, Rubin JL, Bond TC, Mayne T. Reduced use of erythropoiesis-stimulating agents and intravenous iron with ferric citrate: a managed care cost-offset model. Int J Nephrol Renovasc Dis. 2013;6:79–87. doi: 10.2147/IJNRD.S40729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinsakul M, Sika M, Koury M, et al. The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract. 2012;121(1–2):c25–c29. doi: 10.1159/000341922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.