Abstract
Purpose
The aim of this study was to evaluate the safety and efficacy of topical besifloxacin ophthalmic suspension 0.6% compared with gatifloxacin ophthalmic solution 0.3% in the treatment of bacterial conjunctivitis in neonates.
Methods
This was a multicenter, randomized, double-masked, parallel group study. Subjects ≤31 days of age with severity grade ≥1 (scale 0–3) for both conjunctival discharge and conjunctival hyperemia were randomized to besifloxacin or gatifloxacin instilled three times daily for 7 days, and completed five study visits (three clinic visits and two phone calls). Primary endpoints included clinical resolution (absence of both conjunctival discharge and conjunctival hyperemia) at visit 5 (day 8 or 9) and ocular and non-ocular treatment-emergent adverse events (AEs). Bacterial eradication was a secondary endpoint.
Results
Thirty-three subjects were included in the intent-to-treat (ITT) population. All were aged <28 days, with a mean (standard deviation) age of 15.5 days (6.0), and 57.6% were female. Twenty-two subjects had culture-confirmed conjunctivitis in at least one eye (modified ITT [mITT] population), most often with Gram-positive bacteria. Visit 5 clinical resolution and bacterial eradication rates were comparable among besifloxacin- and gatifloxacin-treated study eyes (clinical resolution: 12/16 [75.0%] vs. 12/17 [70.6%] for the ITT population, and 11/13 [84.6%] vs. 7/9 [77.8%] for the mITT population; bacterial eradication: 12/13 [92.3%] vs. 8/9 [88.9%] for the mITT population, respectively). No AEs were reported in the besifloxacin treatment group, and AEs reported in the gatifloxacin group were considered not treatment-related.
Conclusions
In this small study in neonates, both besifloxacin and gatifloxacin appeared effective and safe in the treatment of bacterial conjunctivitis. Larger studies are warranted.
Key Points
| This study compared the use of two different topical antibiotics (besifloxacin and gatifloxacin, each administered three times daily for 7 days) in 33 neonatal subjects with bacterial conjunctivitis, a condition for which there are little published data in this age group. |
| High rates of clinical resolution were observed with both antibiotics; however, bacterial eradication occurred earlier with besifloxacin. |
| Both antibiotics were well tolerated in this small group of neonates and there were no adverse events with besifloxacin treatment. |
Background
Neonatal conjunctivitis is an acute condition characterized by conjunctival erythema, swelling, and mucopurulent discharge occurring within the first 30 days of life [1–3]. While viruses are a common cause of pediatric conjunctivitis, studies conducted in North America that included children <5 years of age with purulent conjunctival discharge identified a bacterial pathogen in 65–80% of cultures [4–6]. The American Academy of Ophthalmology recommends conjunctival cultures be taken from all cases of suspected neonatal bacterial conjunctivitis [7]. Conjunctivitis in a newborn can pose a risk for the development of secondary ocular infections, including endophthalmitis and keratitis (in cases of gonococcal etiology) [1, 8], permanent eye damage, and even blindness, although the latter is rare in industrialized countries [8]. Systemic complications such as pneumonitis, meningitis, and septicemia are also possible [1, 2, 8].
The recommended management of suspected bacterial neonatal conjunctivitis includes the use of systemic and/or topical antibiotics [1, 4]. Topical fluoroquinolones are often preferred for the treatment of bacterial conjunctivitis in the general population due to their rapid bactericidal activity, broad spectrum of activity, and low toxicity [9, 10]. Although some studies have included neonates within a broader population [11–14], to our knowledge there are no published reports of topical fluoroquinolones used specifically in neonatal conjunctivitis, with the exception of a single study published in abstract form only [15].
Besifloxacin is a topical fluoroquinolone and represents the first chloro-fluoroquinolone developed specifically for ophthalmic use. The broad spectrum activity of besifloxacin includes potent in vitro activity against drug-resistant strains such as ciprofloxacin-resistant, methicillin-resistant S. aureus (MRSA) and methicillin-resistant coagulase-negative Staphylococcus species [16–19]. Besifloxacin ophthalmic suspension 0.6% (Besivance®; Bausch & Lomb, Tampa, FL, USA) is approved by the US FDA for the treatment of bacterial conjunctivitis, with a recommended dosing regimen of three times daily for 7 days; this indication is based on clinical studies conducted in patients ≥1 year of age [20]. A prior analysis of pediatric (ages 1–17; n = 815) subgroup data from three bacterial conjunctivitis studies in patients of all ages demonstrated significant and high rates of clinical resolution (88.1%) and bacterial eradication (82.8%) with besifloxacin treatment at day 8 or 9 (visit 3) compared with vehicle (p ≤ 0.009). Rates of clinical resolution and bacterial eradication were similar between besifloxacin and moxifloxacin treatment groups for patients aged 1–17 at all visits (P=NS). Among subjects aged 1 year, there was statistically significant bacterial eradication at day 5 ± 1 (visit 2) compared with vehicle (p = 0.04) [21].
The current study was designed to evaluate the safety and efficacy of besifloxacin ophthalmic suspension 0.6% compared with gatifloxacin ophthalmic solution 0.3% (Zymar®; Allergan, Irvine, CA, USA) when administered three times daily for 7 days in neonates with bacterial conjunctivitis. Gatifloxacin was selected as an active comparator because it has an antibacterial action similar to besifloxacin, they both inhibit bacterial DNA gyrase and topoisomerase IV [22], and because it was previously reported to be safe when administered three times daily in neonates [23].
Materials and Methods
Study Design and Procedures
This multicenter, randomized, double-masked, active-controlled, parallel group study (NCT01330355) was initiated at 11 sites in the US between May 2011 and October 2012. Eligible subjects were aged ≤31 days, with a clinical diagnosis of acute bacterial conjunctivitis in one or both eyes, and a severity of grade ≥1 for both conjunctival discharge and conjunctival hyperemia in the same eye (each rated on a scale of 0 [absent/normal] to 3 [severe]). Subjects with suspected fungal, protozoal, or viral etiology in either eye, evidence of chemical or physical trauma to either eye or ocular adnexa, and subjects with corneal infiltrates or ulcer in either eye were excluded. Use of systemic or topical non-prophylactic antimicrobial therapy within 96 h of day 1 (baseline), or expected use of such during the study period, was not allowed. Subjects who had received topical antimicrobial therapy for routine prophylaxis (e.g. at the time of birth) could be enrolled 24 h or more after the last application of antibiotic prophylaxis. Systemic or topical antimicrobials were not allowed to be used by the breastfeeding mother or wet nurse. Subjects were also excluded if they required concomitant use of ophthalmic (either eye) or systemic corticosteroids, systemic non-steroidal anti-inflammatory drugs (NSAIDs), systemic antihistamines, or ocular immunosuppressants. The study was conducted in accordance with Good Clinical Practice (as described in the International Conference on Harmonisation guidelines), applicable local regulations, and the ethical principles in the Declaration of Helsinki. The protocol was approved by the Institutional Review Boards associated with individual study sites, and written informed consent was obtained by each subject’s parent or legally authorized representative prior to study participation.
Subjects who met the eligibility criteria had an initial eye examination that included an assessment of ocular signs, and had a conjunctival swab taken for culture from the affected eye(s). Subjects were then randomly assigned in a 1:1 ratio, according to a computer-generated randomization list, to receive besifloxacin or gatifloxacin instilled in the affected eye(s) three times daily for 7 days. The randomization list was produced prior to study enrollment by an unmasked statistician. The investigator, the subject’s parent/authorized representative, and all study personnel involved in study conduct and monitoring were masked to the study treatment identity. Masking was accomplished by replacing the commercial labeling on besifloxacin and gatifloxacin bottles with identical investigational labels and packaging them in identical kit boxes. A designee at each study site was given responsibility for dispensing/collecting study materials to subjects. The first dose of study medication was instilled in the clinic following the initial eye examination and conjunctival culture. Parents/guardians were instructed to continue administration of study treatment at approximately 6 h intervals. Both remaining doses on day 1 were to be administered, even if the resulting intervals were shorter than 6 h.
Following the day 1/start of treatment visit, subjects returned to the clinic at visit 3 (day 4 ± 1) and visit 5 (day 8 or 9) for clinical assessment of ocular signs and culture of the affected eye(s). Vital signs and body weight measurements were taken, an ocular examination of both eyes was performed, and ocular and non-ocular adverse events (AEs) were recorded. Ocular examinations consisted of assessment of light perception, eyelid edema, conjunctival chemosis, the pupillary reflex, and the red reflex test, as well as clinical examination of the eyelid (other than eyelid edema), the conjunctiva (other than conjunctival discharge, conjunctival hyperemia, and conjunctival chemosis), and the cornea. All assessments were performed in both eyes, with the exception of microbial cultures, which were taken from baseline-affected eyes only. A physical examination was also conducted at visit 5. In addition to the clinic visits, parents/guardians were asked to complete a telephone contact at visits 2 (day 2) and 4 (day 6 or 7) to confirm their compliance with dosing and to solicit AEs.
At each visit, samples were obtained from the conjunctival cul-de-sac of baseline-affected eye(s) using a sterile swab, and the swabs were sent to Covance Central Laboratory Services, Inc. (Indianapolis, IN, USA) for quantitative bacteriological analysis. Subjects were considered culture-positive or culture-confirmed if the bacterial colony count for a particular species (in colony forming units per mL; CFU/mL) equaled or exceeded the threshold value for that species on the Cagle list, as modified by Leibowitz [24, 25]. For isolates that met/exceeded the bacterial threshold, minimum inhibitory concentration (MIC) testing was performed for besifloxacin and comparator antibacterial agents following procedures recommended by the Clinical and Laboratory Standards Institute [26].
Outcomes
Primary endpoints included clinical resolution (binary outcome [yes/no], with clinical resolution defined as the absence of both conjunctival discharge and conjunctival hyperemia; severity grade = 0 at visit 5) and rates of ocular and non-ocular treatment-emergent AEs (TEAEs). Ocular discharge was rated on a scale of 0 (absent), 1 (mild), 2 (moderate), and 3 (severe), and conjunctival hyperemia was rated on a scale of 0 (normal), 1 (mild), 2 (moderate), and 3 (severe). Secondary endpoints included clinical resolution at visit 3 and bacterial eradication at visits 3 and 5 in culture-positive eyes. Bacterial eradication (binary outcome [yes/no]) was defined as the absence of all ocular bacterial species that were present at or above threshold at visit 1. Eradication of individual bacterial species was another secondary endpoint.
All TEAEs (ocular and non-ocular) observed by the investigator or reported by the subject’s parent/guardian were recorded using the Medical Dictionary for Regulatory Activities (version 15.1) body system and preferred terms, and characterized as mild, moderate, or severe. The investigator also evaluated the relationship of AEs to study treatment. Secondary safety outcomes included the results of ocular examinations, physical examination, and vital signs. Ocular examinations included light perception assessment (present or absent), eyelid edema and conjunctival chemosis (each rated on a scale from 0 = none to 3 = severe), and pupillary reflex and red reflex test (rated as normal or abnormal).
Statistical Analyses
With the exception of the endpoint of individual or species-specific bacterial eradication, one eye per subject was designated as the study eye for analysis of efficacy endpoints. In subjects with bilateral conjunctivitis, the study eye was the eye with the highest combined sum of ratings (i.e. severity) for ocular discharge and conjunctival hyperemia at baseline. For cases in which baseline severity ratings were equal for both eyes, the right eye was designated as the study eye. In the analyses by individual bacterial species, non-study eyes (i.e. fellow eyes) could contribute data provided the severity of conjunctivitis in that eye met the inclusion criteria and the bacterial species that was at or above threshold in that eye at baseline was different from the species cultured from the study eye.
The primary population for the efficacy analysis was the intent-to-treat (ITT) population, which included all randomized subjects. Additional analyses, including all analyses on bacterial endpoints, were performed in the modified ITT (mITT) population, which was defined as subjects in the ITT population with baseline bacterial culture at or above threshold for any accepted ocular bacterial species. The safety population included all subjects who received at least one dose of study drug as part of the protocol and who had at least one post-treatment safety assessment.
Efficacy findings for baseline-designated study eyes and treated fellow eyes were summarized using descriptive statistics, with missing data imputed using the last observation carried forward. Differences between treatments in clinical resolution and bacterial eradication were evaluated for baseline-designated study eyes only using the asymptotic Pearson Chi-square test. All analyses were performed using Statistical Analysis Software version 9.1 (SAS Institute, Inc., Cary, NC, USA). Safety results were reported using descriptive statistics.
The study initially sought to enroll 200 subjects (100 per treatment group) to obtain 100 culture-positive subjects, but was terminated early due to a low enrollment rate unrelated to safety or efficacy concerns.
Results
Subjects and Baseline Pathogens
A total of 33 subjects (besifloxacin, n = 16; gatifloxacin, n = 17), all neonatal (<28 days), were enrolled at seven clinical sites and comprised the ITT population. Of the 33 enrolled subjects, 32 (97%) completed the study; one subject randomized to gatifloxacin withdrew consent and discontinued. Twenty-two subjects (besifloxacin, n = 13; gatifloxacin, n = 9) had culture-positive conjunctivitis in at least one eye and were included in the mITT population. Demographic characteristics of the ITT and mITT populations are provided in Table 1. There were no apparent differences in demographic characteristics between treatment groups. Overall, the mean (± standard deviation) age of subjects was 15.5 days (6.0) and 15.7 days (5.3), and 57.6% and 54.5% of subjects in the ITT and mITT populations, respectively, were female.
Table 1.
Demographic characteristics
| ITT population | mITT population | |||
|---|---|---|---|---|
| Besifloxacin [n = 16] | Gatifloxacin [n = 17] | Besifloxacin [n = 13] | Gatifloxacin [n = 9] | |
| Age, days | ||||
| Mean (SD) | 15.8 (6.39) | 15.2 (5.75) | 15.9 (6.01) | 15.4 (4.48) |
| Min, max | 6, 26 | 5, 25 | 6, 25 | 11, 25 |
| Gender | ||||
| Male | 4 (25.0) | 10 (58.8) | 3 (23.1) | 7 (77.8) |
| Female | 12 (75.0) | 7 (41.2) | 10 (76.9) | 2 (22.2) |
| Race | ||||
| Asian | 1 (6.3) | 1 (5.9) | 1 (7.7) | 1 (11.1) |
| Black/African American | 0 | 1 (5.9) | 0 | 1 (11.1) |
| White | 12 (75.0) | 12 (70.6) | 10 (76.9) | 6 (66.7) |
| Other | 3 (18.8) | 3 (17.6) | 2 (15.4) | 1 (11.1) |
Data are expressed as n (%) unless otherwise specified
ITT intent-to-treat, mITT modified intent-to-treat, SD standard deviation, Min minimum, Max maximum
Table 2 presents bacterial pathogens above threshold isolated at baseline from all culture-positive eyes, along with the MIC of besifloxacin and gatifloxacin for these isolates. A total of 50 bacterial isolates meeting threshold criteria for pathogenicity in bacterial conjunctivitis were identified, and most were Gram-positive. The most common Gram-positive bacterial species cultured were Streptococcus mitis, Staphylococcus epidermidis, and Staphylococcus aureus, while the most common Gram-negative bacterial species cultured was Moraxella catarrhalis. Overall, the MIC or range of MICs (in cases of more than one isolate) for besifloxacin appeared lower to those noted with gatifloxacin for Gram-positive organisms; for Gram-negative organisms, besifloxacin MICs were higher than or equal to those observed with gatifloxacin.
Table 2.
Bacterial species above the threshold criteria for pathogenicitya isolated at baseline, and minimum inhibitory concentrations of besifloxacin and gatifloxacin for those isolates
| Bacterial species | No. of isolatesb | Minimum inhibitory concentrations (µg/mL) | |
|---|---|---|---|
| Besifloxacin | Gatifloxacin | ||
| Gram-positive | 38 | 0.015–8 | 0.06–64 |
| CDC coryneform group G | 1 | 0.03 | 0.06 |
| Enterococcus faecalis | 1 | 0.25 | 0.5 |
| Lactococcus garvieae | 1 | 0.5 | 0.5 |
| Staphylococcus aureus | 6 | 0.015–0.06 | 0.06–0.25 |
| Staphylococcus epidermidis | 9 | 0.03–8 | 0.06–64 |
| Staphylococcus hominis | 4 | 0.06 | 0.12–0.25 |
| Staphylococcus warneri | 1 | 0.12 | 0.25 |
| Streptococcus mitis group | 11 | 0.06–2 | 0.25–32 |
| Streptococcus salivarius group | 4 | 0.06–0.12 | 0.12–0.5 |
| Gram-negative | 12 | 0.03–4 | 0.03–1 |
| Chryseobacterium indologenes | 2 | 1 | 0.5 |
| Chryseobacterium species | 1 | 4 | 1 |
| Elizabethkingia meningoseptica | 1 | 1 | 0.25 |
| Haemophilus influenzae | 1 | 0.03 | 0.03 |
| Leclercia adecarboxylata | 1 | 0.06 | 0.03 |
| Moraxella catarrhalis | 4 | 0.06–0.12 | 0.03–0.06 |
| Serratia marcescens | 1 | 0.5 | 0.25 |
| Wautersiella falsenii | 1 | 0.5 | 0.5 |
CFU colony-forming units, CDC centers for disease control
aThreshold criteria were ≥1000 CFU/mL for the CDC coryneform group G; ≥100 CFU/mL for Staphylococcus spp (except S. aureus); ≥10 CFU/mL for E. faecalis, L. garvieae, S. aureus, Streptococcus spp, and M. catarrhalis; ≥1 CFU/mL for Gram-negative species (except M. catarrhalis)
bNumber of times a specific bacterial species was isolated at or above threshold at baseline from study eyes or treated fellow eyes
Efficacy
Clinical Resolution
Table 3 presents clinical resolution rates at visits 3 and 5. In the ITT population, clinical resolution at visit 5 (day 8 or 9; primary efficacy endpoint) was observed in 75.0% of study eyes treated with besifloxacin, compared with 70.6% of study eyes treated with gatifloxacin (p = 0.78). In the mITT population, clinical resolution at visit 5 was observed in 84.6% of besifloxacin-treated study eyes compared with 77.8% of gatifloxacin-treated study eyes (p = 0.68). At visit 3 (day 4 ± 1), clinical resolution rates were 18.8 vs. 29.4% (ITT population) and 7.7 vs. 33.3% (mITT population) in besifloxacin- and gatifloxacin-treated eyes, respectively (p ≥ 0.13). In treated fellow eyes, patterns in clinical resolution rates between treatment groups appeared similar to those observed in study eyes at both visit 3 and visit 5 (statistical analyses not performed).
Table 3.
Clinical resolutiona at visits 3 and 5 (LOCF)
| Baseline-designated study eye [n/N (%)] | Fellow treated eye [n/N (%)] | ||||
|---|---|---|---|---|---|
| Besifloxacin | Gatifloxacin | p valueb | Besifloxacin | Gatifloxacin | |
| Visit 3 (day 4 ± 1) | |||||
| ITT population | 3/16 (18.8) | 5/17 (29.4) | 0.48 | 1/8 (12.5) | 2/5 (40.0) |
| mITT population | 1/13 (7.7) | 3/9 (33.3) | 0.13 | 0/5 (0.0) | 2/3 (66.7) |
| Visit 5c (day 8 or 9) | |||||
| ITT population | 12/16 (75.0) | 12/17 (70.6) | 0.78 | 5/8 (62.5) | 2/5 (40.0) |
| mITT population | 11/13 (84.6) | 7/9 (77.8) | 0.68 | 4/5 (80.0) | 2/3 (66.7) |
ITT intent-to-treat, mITT modified intent-to-treat, LOCF last observation carried forward
aClinical resolution defined as the absence of both conjunctival discharge and conjunctival hyperemia
bPearson Chi-square test; LOCF
cPrimary outcome visit (study eye only)
Bacterial Eradication
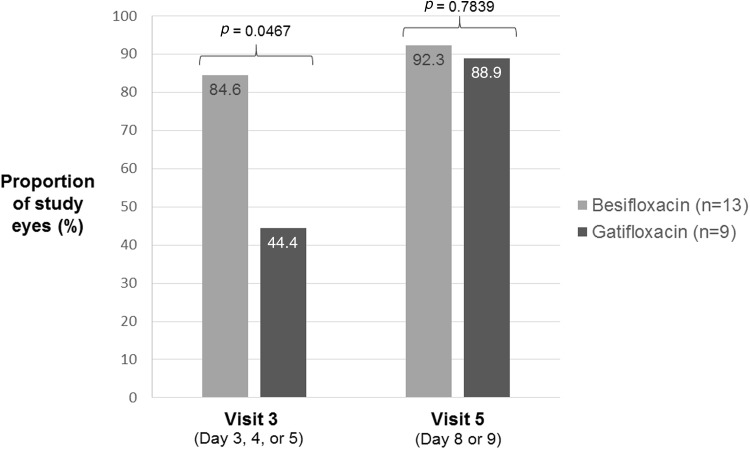
Figure 1 presents bacterial eradication rates at visits 3 and 5 for culture-positive, baseline-designated study eyes (mITT population). There was a significant difference between treatments in favor of besifloxacin at visit 3 (84.6 vs. 44.4%; p = 0.0467) but not at visit 5 (92.3 vs. 88.9%: p = 0.7839). Among treated fellow eyes, bacterial eradication at visit 3 was achieved in three of five eyes treated with besifloxacin and two of three eyes treated with gatifloxacin; at visit 5, bacterial eradication occurred in four of five eyes treated with besifloxacin and all eyes (3/3) treated with gatifloxacin.
Fig. 1.
Bacterial eradication rates at visits 3 and 5 (culture-positive, baseline-designated study eyes, modified intent-to-treat population, last observation carried forward). p values from the Pearson Chi-square test
Table 4 presents bacterial eradication data for individual bacterial species at visits 3 and 5. As indicated earlier, treated fellow eyes could contribute data if the bacterial species isolated from that eye was different from that cultured from the baseline-designated study eye. At visit 3, 88.9% (16/18) of Gram-positive organisms were eradicated in besifloxacin-treated eyes, compared with 46.2% (6/16) in gatifloxacin-treated eyes. The percentage of all culture-positive treated eyes showing eradication of Gram-negative organisms by visit 3 was 100% (6/6) in the besifloxacin group and 75.0% (3/4) in the gatifloxacin group. At visit 5, all species were eradicated, with the only exceptions being Staphylococcus epidermidis in a gatifloxacin-treated eye and Staphylococcus hominis in a besifloxacin-treated eye. However, the bacterial count for these isolates was observed to be reduced to below threshold levels, with no new species present in both cases.
Table 4.
Bacterial eradication by species at visits 3 and 5, mITT population (LOCF)
| Bacterial species | Species-specific study eye [n/N] | |||
|---|---|---|---|---|
| Besifloxacin | Gatifloxacin | |||
| Visit 3 | Visit 5 | Visit 3 | Visit 5 | |
| Gram-positive | 16/18 | 17/18 | 6/13 | 12/13 |
| CDC coryneform group G | 1/1 | 1/1 | – | – |
| Enterococcus faecalis | 1/1 | 1/1 | – | – |
| Lactococcus garvieae | 1/1 | 1/1 | – | – |
| Staphylococcus aureus | 3/3 | 3/3 | 1/2 | 2/2 |
| Staphylococcus epidermidis | 2/2 | 2/2 | 2/5 | 4/5 |
| Staphylococcus hominis | 1/2 | 1/2 | 0/1 | 1/1 |
| Staphylococcus warneri | – | – | 1/1 | 1/1 |
| Streptococcus mitis group | 5/6 | 6/6 | 2/3 | 3/3 |
| Streptococcus salivarius group | 2/2 | 2/2 | 0/1 | 1/1 |
| Gram-negative | 6/6 | 6/6 | 3/4 | 4/4 |
| Chryseobacterium indologenes | 1/1 | 1/1 | – | – |
| Chryseobacterium species | 1/1 | 1/1 | – | – |
| Elizabethkingia meningoseptica | – | – | 1/1 | 1/1 |
| Haemophilus influenzae | – | – | 1/1 | 1/1 |
| Leclercia adecarboxylata | 1/1 | 1/1 | – | – |
| Moraxella catarrhalis | 2/2 | 2/2 | 1/1 | 1/1 |
| Serratia marcescens | – | – | 0/1 | 1/1 |
| Wautersiella falsenii | 1/1 | 1/1 | – | – |
mITT modified intent-to-treat, LOCF last observation carried forward, CDC centers for disease control
Safety
Study eyes had a mean exposure of 6.94 (±0.25) and 6.65 (±1.46) days in the besifloxacin and gatifloxacin treatment groups, respectively. The mean exposure for all treated eyes (i.e. sum of study eye and treated fellow eye exposure) was 10.38 (±3.52) and 8.71 (±3.60) eye-days for the besifloxacin and gatifloxacin treatment groups, respectively.
There were no serious AEs during the conduct of the study. No TEAEs, either ocular or non-ocular, were reported in either study or fellow eyes of subjects in the besifloxacin treatment group. In the gatifloxacin treatment group, a total of six AEs (five non-ocular, one ocular) were reported. All AEs were mild or moderate in severity and judged as ‘unrelated’ or ‘unlikely related’ to the study drug. The one ocular AE consisted of mild bacterial conjunctivitis in an initially untreated fellow eye, which occurred after visit 1. The eye was treated with study treatment (gatifloxacin) and then resolved. The five non-ocular AEs occurring in four subjects included abdominal pain, irritability, rhinorrhea, acne infantile, and dermatitis. No AEs resulted in treatment discontinuation.
There were no meaningful findings noted for vital sign measurements or results of ocular or physical examinations performed during the study. In addition, there were no cases of severe eyelid edema or severe chemosis at any visit, and all cases of eyelid edema and chemosis resolved at visit 5. Pupillary reflex, red reflex test, and light perception were normal throughout the study. No major findings were identified on eyelid and conjunctival examinations in either treatment group, and all corneal examinations were normal.
Discussion
In this report, we describe the results of a double-masked, multicenter study comparing besifloxacin ophthalmic suspension 0.6% with gatifloxacin ophthalmic solution 0.3% in 33 neonatal subjects with bacterial conjunctivitis. Other than a study published in abstract form only [15], this is the first published study evaluating the safety and efficacy of two topical fluoroquinolones in neonatal patients with bacterial conjunctivitis. Both besifloxacin and gatifloxacin appeared to be well-tolerated in this group of neonates. No TEAEs were reported in the besifloxacin group, and the six AEs in the gatifloxacin group were not considered related to treatment.
Both besifloxacin and gatifloxacin appeared effective for clinical resolution in neonates with bacterial conjunctivitis. The percentages of eyes showing clinical resolution were not statistically different between the besifloxacin and gatifloxacin groups at either visit 3 (day 4 ± 1) or visit 5 (day 8 or 9). However, the proportion of eyes showing bacterial eradication was significantly higher in the besifloxacin group at visit 3, almost double that of the gatifloxacin group. Besifloxacin has previously been shown to have more rapid in vitro bactericidal activity compared with gatifloxacin [27], possibly evidenced in the current study by the higher rate of bacterial eradication at visit 3 in the besifloxacin group. However, by visit 5 the two treatment groups showed similarly high percentages of bacterial eradication, likely due to the action of the antibacterial in conjunction with the host immune response in this self-limited condition. Bacterial eradication findings with besifloxacin were consistent with rates observed at treatment completion in several previous clinical studies [28–30].
The range of MICs for cultured pathogens at baseline differed somewhat between besifloxacin and gatifloxacin. Besifloxacin MIC ranges appeared better (i.e. lower) than gatifloxacin MIC ranges for Gram-positive pathogens, while gatifloxacin MICs were similar or slightly better to besifloxacin MICs for Gram-negative organisms. The greatest differences in in vitro susceptibility between the two antibacterials was noted among the Streptococcus mitis group, for which besifloxacin MICs were 4- to 16-fold lower than those for gatifloxacin. Yet, by visit 5, all S. mitis organisms were found to have been eradicated in both treatment groups, indicating that in vitro data may not always predict in vivo efficacy when antibacterial drugs are used topically in ocular infections. At visit 5, each treatment group had one species-specific eye in which a bacterial species failed to be eradicated (S. epidermidis in a gatifloxacin-treated eye and S. hominis in a besifloxacin-treated eye); however, the bacterial count for these species was reduced compared with visit 3.
Very little published data on the use of topical fluoroquinolones in neonates with bacterial conjunctivitis are available. In a study published in abstract form only, 142 culture-positive patients <31 days of age received either moxifloxacin or ciprofloxacin three times daily for 4 days; at the test-of-cure visit (day 9), clinical cure was 80% for both treatments, and microbiological eradication was 92% versus 87% for moxifloxacin and ciprofloxacin, respectively [15]. Both medications were well tolerated, with no treatment-related serious AEs or treatment-related changes in ocular and cardiovascular examination parameters. In an analysis of safety data from five separate studies of moxifloxacin for the treatment of bacterial conjunctivitis, the incidences of common AEs among subjects <28 days of age (n = 100) were generally similar to or lower than the incidences observed in other age groups, and there were no serious AEs in newborns [11]. The findings from a study comparing gatifloxacin (n = 84) and moxifloxacin (n = 86) in neonates with conjunctivitis have been reported on the ClinicalTrials.gov website [23]. Rates of non-serious AEs (all ‘conjunctivitis’ or ‘conjunctivitis bacterial’) were 13.1% with gatifloxacin and 9.3% with moxifloxacin; one serious AE (pyrexia) was reported in the moxifloxacin group.
The major limitation of the current study was the small sample size and resulting lack of statistical power, a consequence of early study termination for low enrollment, unrelated to safety concerns or efficacy findings. Despite the smaller-than-planned population, the findings presented are of interest simply because of the paucity of published data on topical fluoroquinolone use in neonates. Further studies of adequate sample size are required to confirm the favorable efficacy and safety data observed in this study and to add to the generalizability of the findings. No cases of MRSA were identified in this small study. Antibiotic efficacy against MRSA is increasingly being recognized as a critical issue in the management of ocular bacterial infections overall and among newborns [31, 32]. Notably, in vitro susceptibility studies conducted over recent years have demonstrated potent activity of besifloxacin against ocular MRSA isolates compared with other antibiotics, including other fluoroquinolones [19, 33]. If MRSA isolates had been recovered in the current neonatal study, potential differences between besifloxacin and gatifloxacin may have been more apparent. Finally, the lack of a vehicle control group did not allow for an efficacy comparison for either fluoroquinolone versus no active treatment.
Conclusions
In this study of neonatal subjects with bacterial conjunctivitis, the rates of clinical resolution and bacterial eradication were high and were similar in eyes treated with besifloxacin compared with gatifloxacin after 7 days, while bacterial eradiation appeared to be more rapid with besifloxacin use. While larger studies are warranted to confirm these findings, both treatments were well-tolerated and no safety concerns were noted in this small study.
Acknowledgements
This study was funded by Bausch & Lomb, Inc. Writing assistance was provided by Churchill Communications (Maplewood, NJ, USA), funded by Bausch & Lomb, Inc.
Compliance with Ethical Standards
Conflict of interest
Christine M. Sanfilippo, Catherine M. Allaire, Heleen H. DeCory are employees of Bausch & Lomb, Inc.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.American Optometric Association. Care of the patient with conjunctivitis. 2002. Available at: https://www.aoa.org/documents/CPG-11.pdf. Accessed 22 June 2016.
- 2.Palafox SK, Jasper S, Tauber AD, Foster SC. Ophthalmia neonatorum. J Clin Exp Ophthalmol. 2011;2:1000119. [Google Scholar]
- 3.Matejcek A, Goldman RD. Treatment and prevention of ophthalmia neonatorum. Can Fam Physician. 2013;59(11):1187–1190. [PMC free article] [PubMed] [Google Scholar]
- 4.Block SL. Etiologic and therapeutic pitfalls of newborn conjunctivitis. Pediatr Ann. 2012;41(8):310–313. doi: 10.3928/00904481-20120727-05. [DOI] [PubMed] [Google Scholar]
- 5.Jackson WB, Low DE, Dattani D, Whitsitt PF, Leeder RG, MacDougall R. Treatment of acute bacterial conjunctivitis: 1% fusidic acid viscous drops vs. 0.3% tobramycin drops. Can J Ophthalmol. 2002;37(4):228–237. doi: 10.1016/S0008-4182(02)80114-4. [DOI] [PubMed] [Google Scholar]
- 6.Patel PB, Diaz MC, Bennett JE, Attia MW. Clinical features of bacterial conjunctivitis in children. Acad Emerg Med. 2007;14(1):1–5. doi: 10.1111/j.1553-2712.2007.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Ophthalmology. Conjunctivitis summary benchmarks for preferred practice pattern® guidelines. 2015. Available at: https://prodc.aao.org/summary-benchmark-detail/conjunctivitis-summary-benchmark--october-2012. Accessed 19 July 2016.
- 8.Andalibi S, Haidara M, Bor N, Levin M. An update on neonatal and pediatric conjunctivitis. Curr Ophthalmol Rep. 2015;3:158–169. doi: 10.1007/s40135-015-0080-x. [DOI] [Google Scholar]
- 9.Smith A, Pennefather PM, Kaye SB, Hart CA. Fluoroquinolones: place in ocular therapy. Drugs. 2001;61(6):747–761. doi: 10.2165/00003495-200161060-00004. [DOI] [PubMed] [Google Scholar]
- 10.Mah FS. Fourth-generation fluoroquinolones: new topical agents in the war on ocular bacterial infections. Curr Opin Ophthalmol. 2004;15:316–320. doi: 10.1097/00055735-200408000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Silver LH, Woodside AM, Montgomery DB. Clinical safety of moxifloxacin ophthalmic solution 0.5% (VIGAMOX) in pediatric and nonpediatric patients with bacterial conjunctivitis. Surv Ophthalmol. 2005;50(Suppl 1):S55–S63. doi: 10.1016/j.survophthal.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Cochereau I, Meddeb-Ouertani A, Khairallah M, et al. 3-day treatment with azithromycin 1.5% eye drops versus 7-day treatment with tobramycin 0.3% for purulent bacterial conjunctivitis: multicentre, randomised and controlled trial in adults and children. Br J Ophthalmol. 2007;91(4):465–469. doi: 10.1136/bjo.2006.103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bremond-Gignac D, Nezzar H, Bianchi PE, et al. Efficacy and safety of azithromycin 1.5% eye drops in paediatric population with purulent bacterial conjunctivitis. Br J Ophthalmol. 2014;98(6):739–745. doi: 10.1136/bjophthalmol-2013-303888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bremond-Gignac D, Messaoud R, Lazreg S, Speeg-Schatz C, Renault D, Chiambaretta F. A 3-day regimen with azithromycin 1.5% eyedrops for the treatment of purulent bacterial conjunctivitis in children: efficacy on clinical signs and impact on the burden of illness. Clin Ophthalmol. 2015;9:725–32. [DOI] [PMC free article] [PubMed]
- 15.Gross RD, Silas P, Oshman S, et al. A comparison of the safety and efficacy of moxifloxacin and ciprofloxacin in the treatment of presumed bacterial conjunctivitis in neonatal patients. Invest Ophthalmol Vis Sci. 2003;44(13):1465. [Google Scholar]
- 16.Haas W, Pillar CM, Zurenko GE, Lee JC, Brunner LS, Morris TW. Besifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 2009;53(8):3552–3560. doi: 10.1128/AAC.00418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas W, Gearinger LS, Usner DW, DeCory HH, Morris TW. Integrated analysis of three bacterial conjunctivitis trials of besifloxacin ophthalmic suspension, 0.6%: etiology of bacterial conjunctivitis and antibacterial susceptibility profile. Clin Ophthalmol. 2011;5:1369–79. [DOI] [PMC free article] [PubMed]
- 18.Haas W, Pillar CM, Torres M, Morris TW, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011;152(4):567–574. doi: 10.1016/j.ajo.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the United States: five-year results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015;133:1445–1454. doi: 10.1001/jamaophthalmol.2015.3888. [DOI] [PubMed] [Google Scholar]
- 20.Besivance [package insert]. Tampa: Bausch & Lomb; 2012.
- 21.Comstock TL, Paterno MR, Usner DW, Pichichero ME. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% in children and adolescents with bacterial conjunctivitis: a post hoc, subgroup analysis of three randomized, double-masked, parallel-group, multicenter clinical trials. Paediatr Drugs. 2010;12(2):105–112. doi: 10.2165/11534380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Zymar [package insert]. Irvine: Allergan, Inc.; 2015.
- 23.Allergan. A study to evaluate the safety and efficacy of gatifloxacin for the treatment of bacterial conjunctivitis. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2007 [cited 1 Sep 2016]. ClinicalTrials.gov identifier: NCT00464438. Available at: https://clinicaltrials.gov/ct2/show/NCT00464438.
- 24.Cagle GD, Abshire RL. Quantitative ocular bacteriology: a method for the enumeration and identification of bacteria from the skin-lash margin and conjunctiva. Invest Ophthalmol Vis Sci. 1981;20:751–757. [PubMed] [Google Scholar]
- 25.Leibowitz HM. Antibacterial effectiveness of ciprofloxacin 0.3% ophthalmic solution in the treatment of bacterial conjunctivitis. Am J Ophthalmol. 1991;112(4 Suppl):29S–33S. [PubMed]
- 26.Clinical and Laboratory Standards Institute. M07-A10. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard–tenth edition. 2015. Available at: http://shop.clsi.org/site/Sample_pdf/M07A10_sample.pdf. Accessed 19 July 2016.
- 27.Haas W, Pillar CM, Hesje CK, Sanfilippo CM, Morris TW. In vitro time-kill experiments with besifloxacin, moxifloxacin and gatifloxacin in the absence and presence of benzalkonium chloride. J Antimicrob Chemother. 2011;66:840–844. doi: 10.1093/jac/dkq531. [DOI] [PubMed] [Google Scholar]
- 28.Tepedino ME, Heller WH, Usner DW, et al. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin. 2009;25(5):1159–1639. doi: 10.1185/03007990902837919. [DOI] [PubMed] [Google Scholar]
- 29.Karpecki P, DePaolis M, Hunter JA. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: a multicenter, prospective, randomized, double-masked, vehicle-controlled, 5-day efficacy and safety study. Clin Ther. 2009;31(3):514–526. doi: 10.1016/j.clinthera.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 30.McDonald MB, Protzko EE, Brunner LS, et al. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology. 2009;116(9):1615–1623. doi: 10.1016/j.ophtha.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Amato M, Pershing S, Walvick M, Tanaka S. Trends in ophthalmic manifestations of methicillin-resistant Staphylococcus aureus (MRSA) in a northern California pediatric population. J AAPOS. 2013;17(3):243–247. doi: 10.1016/j.jaapos.2012.12.151. [DOI] [PubMed] [Google Scholar]
- 32.Lessa FC, Edwards JR, Fridkin SK, Tenover FC, Horan TC, Gorwitz RJ. Trends in incidence of late-onset methicillin-resistant Staphylococcus aureus infection in neonatal intensive care units: data from the National Nosocomial Infections Surveillance System, 1995–2004. Pediatr Infect Dis J. 2009;28(7):577–581. doi: 10.1097/INF.0b013e31819988bf. [DOI] [PubMed] [Google Scholar]
- 33.Miller D, Chang JS, Flynn HW, Alfonso EC. Comparative in vitro susceptibility of besifloxacin and seven comparators against ciprofloxacin- and methicillin-susceptible/nonsusceptible staphylococci. J Ocul Pharmacol Ther. 2013;29(3):339–344. doi: 10.1089/jop.2012.0081. [DOI] [PubMed] [Google Scholar]