Abstract
Amikacin has been one of the important antimicrobial agents against Gram-negative pathogens. However, there is discrepancy regarding the amikacin initial dosage, with some reports recently recommending ≥25 mg/kg and others the conventional dosage (15–20 mg/kg). Hence, we evaluated the optimal initial dosing regimen of amikacin. Pharmacokinetic (PK) parameters were estimated using a population PK analysis. The pharmacodynamic (PD) target was a ratio of ≥8 between the concentration achieved 1 h after beginning the infusion (C peak) and the minimal inhibitory concentration (MIC) of the liable bacteria. Based on the population PK parameters, we simulated individual C peak for several dosing regimens by Monte Carlo method and analyzed the C peak/MIC ratio for MICs from 0.5 to 32 μg/mL. This study included 35 infected patients (25 males), with a median (range) age and body weight of 70 (15–95) years and 49.5 (32.5–78) kg, respectively. A two-compartment model was used, and total body clearance (CL) significantly correlated with creatinine clearance, and volume of distribution (V d) with body weight. Regarding the probability to achieve a C peak/MIC of ≥8, the 15 mg/kg regimen was sufficient to achieve the PK/PD target in ≥90% of patients for a MIC of 4 μg/mL or less. The cumulative fraction of response in Pseudomonas aeruginosa was that 76% of patients achieved a C peak/MIC of 8 with the amikacin dosage of 15 mg/kg/day. We suggest that the 15-mg/kg once-daily dosage of amikacin be recommended as the initial dosage. As its maintenance dosage, the 15 mg/kg/day amikacin dosage is needed for a MIC of ≤4 μg/mL, and amikacin monotherapy for a MIC of ≥8 μg/mL should be avoided.
Key Points
| A 15-mg/kg once-daily dosage of amikacin is recommended as the initial dosage to get higher probability to achieve the pharmacodynamic/pharmacodynamic target and cumulative fraction of response with lower toxicity. |
| As its maintenance dosage, the 15 mg/kg/day amikacin dosage is needed for a minimal inhibitory concentration (MIC) of ≤4 μg/mL, and amikacin monotherapy for a MIC of ≥8 μg/mL should be avoided, especially in patients with bacteremia or pneumonia. |
Introduction
Amikacin has been one of the aminoglycosides that have been effective against Gram-negative and multidrug-resistant pathogens, such as Pseudomonas aeruginosa [1]. Pharmacodynamic (PD) considerations [including aggressive dosing with a targeted concentration achieved 1 h after beginning the infusion (C peak)/minimum inhibitory concentration (MIC) ratio] for aminoglycosides are strongly associated with clinical outcome and are essential to the appropriate management of Gram-negative bacteremia [2]. Some authors have suggested that a ratio of 8 or more between the C peak (i.e., the drug concentration of the equilibrium state between blood and tissue) and the MIC of the bacteria responsible for the Gram-negative bacilli infection is predictive of therapeutic success [3–7].
Burdet et al. showed that with a 2-mg/kg simulated infusion of amikacin, the probability to attain the target C peak/MIC was 96% for Gram-negative bacilli infections with a MIC of 4 μg/mL. They recommended that an empirical dose of 25 mg/kg or more was needed to achieve the amikacin PD predictors of clinical efficacy in Gram-negative bacilli infections [8]. Galvez et al. demonstrated that a 30-mg/kg daily dosage of amikacin presented significantly higher C peak compared with the 15- or 25-mg/kg daily dosage, with no association with higher nephrotoxicity and suggested that the standard regimen (15 mg/kg daily) might be insufficient in critically ill patients to reach the recommended C peak [9].
Of note, there is a diremption between the standard regimen (15–20 mg/kg) of amikacin and the dose (≥25 mg/kg) recently published reports recommend [8, 9]. For patients with severe infections, it is crucial to set up quickly an effective dosage regimen that will render optimum serum concentrations from the very beginning of treatment in order to maximize both microbiological and clinical response rates. Conversely, inadequate drug exposure may result in treatment failure. Therefore, the objective was to establish the optimal initial dosing regimen of amikacin using the pharmacokinetics (PK) of amikacin in patients with population PK analysis and Monte Carlo simulations.
Methods
Patients
This work was a single-center retrospective study. It included all patients admitted to Aichi Medical University Hospital (995 beds) between September 2009 and December 2014 who were older than 15 years and treated with amikacin for at least 3 days because of several types of infections. The Aichi Medical University Committee approved this study protocol. The blood concentrations of amikacin from patients were obtained as routine practice as therapeutic drug monitoring (TDM) in our hospital. Exclusion criteria were burns, pregnancy, or the use of continuous renal replacement therapy at the onset of amikacin therapy.
Antimicrobial Susceptibility Testing
All isolates were collected as part of standard patient care. Antimicrobial susceptibility of the Gram-negative isolates was tested in accordance with Clinical and Laboratory Standards Institute (CLSI) guidance by broth microdilution in triplicate for each compound [10].
Data Collection
At least 3 days before amikacin treatment started, we retrospectively collected usual clinical and demographic data, such as gender, age, ideal body weight (IBW), lean body weight (LBW) and total body weight, body mass index (BMI), serum creatinine, creatinine clearance (CCr) estimated according to the Cockroft–Gault equation [11], albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and blood urea nitrogen (BUN). IBW was calculated as follows: IBW = 22 × (height in m)2 (kg). LBW, in kilograms, was calculated by the method of Janmahasatian and colleagues: LBW (males) = (9270 × body weight)/[6680 + (216 × BMI)]; LBW (females) = (9270 × body weight)/[8780 + (244 × BMI)] (kg) [12].
We also collected TDM data, such as amikacin blood concentration, infusion and sampling times, the dosage, infusion time, and the period of treatment. We used these to conduct the population PK analysis with WinNonlin. Amikacin was infused over 30 min or 1 h. All blood samples were obtained at the following times: (1) immediately before amikacin administration (C trough) and (2) 1 h after amikacin administration started (C peak). Dosing and sampling times were recorded; the accuracy of the records was further assessed by a pharmacist participating in this study.
As our study population included patients with moderate to severe renal failure, the serum samples, to be assayed for amikacin C trough, were collected from all patients within 4 days after amikacin therapy started (median 44.4 h, with a range of 16.6–87.0 h). Amikacin concentrations were determined by fluorescence polarization immunoassay using the amikacin assay kit (Roche Diagnostics K.K., Tokyo, Japan). The limit of quantification of the technique is 0.8 μg/mL, and the coefficients of intra- and inter-assay variation are less than 6% over the entire calibration range (0.8–40 μg/mL).
Estimation of Individual Drug Exposure
Since the sparse sampling schedule did not enable individual PK parameters to be estimated by usual methods for most patients, a population PK method based on a nonlinear mixed-effect modeling approach was used. The Bayesian forecasting method was employed to estimate individual PK parameters. The estimated parameters allowed us to predict individual serum concentration–time curves and to estimate C peak and C trough, respectively.
Population Pharmacokinetic (PK) Analysis
Basic Model
The PK parameters of amikacin were calculated with individual serum-concentration data using the Phoenix NLME component of WinNonlin version 6.3 (Pharsight Corporation, Mountain View, CA, USA). The model has two components: (1) a structural model that characterizes the concentration-time relationship and (2) random-effect models, including inter-individual variability in the PK parameters, and residual error, including intra-individual variability and measurement errors. Assumptions about the base population PK model (one- vs. two-compartment and residual variability) were evaluated based on the objective function value (OFV), agreement between observed and predicted amikacin concentration, and visual inspection of the distribution of the weighted residual (WRES) plots.
The model enabled the computation of the amikacin concentration at any time for any given dosing regimen. An exponential inter-individual variability error term, which assumes a normal distribution with a mean of zero and a variance of ω 2, was included on all the PK parameters in the model. The residual error model was supposed to be combined, with the standard deviation of the additive component and the standard deviation of the proportional component. The addition of ω to inter-compartmental clearance (Q) and the volume of distribution in the peripheral compartment (V 2) did not improve model fitting. Therefore, ω for Q and V 2 was not included in the population model.
The model also included estimates of the residual random error for amikacin (ε). The residual random errors included assay errors, individual changes in the PK parameters, and model misspecification errors. Additive, proportional, and combined additive and proportional error models were evaluated to estimate the residual variability. The distribution of ε was assumed to obey a normal distribution and was characterized by a mean of zero and variance, σ 2, which can be estimated by WinNonlin. The residual variability was modeled by a multiple error according to the equation C p = F*(1 + ε), where C p is the observed serum amikacin concentration and F is the concentration predicted from the compartment model. Two levels of variability were considered.
Covariate Model Building
After selecting the basic model, possible relationships between individual estimates of the PK parameters and covariates were explored graphically. The influence of the following covariates at initiation of treatment on amikacin PK parameters was tested: six demographic variables (gender, age, IBW, LBW, BMI, and total body weight), three clinical variables [bacteremia, pneumonia, and urinary tract infection (UTI)], and six biochemical markers (serum albumin, total bilirubin, CCr, AST, ALT, and BUN). Covariate effects were tested by incorporating covariates into the basic model using stepwise forward addition followed by stepwise backward elimination procedures. A decrease in the OFV of ≥6.63 (p < 0.01) was required to retain the covariate in the forward addition step. Covariates were finally retained when the correlations were significant at the p < 0.001 level according to the likelihood ratio test [13].
Final Model Determination
We graphically studied the influence of covariates on their related PK parameters. Outliers were studied and excluded from the analysis when incomplete data collection was suspected. A backward selection method was used in order to obtain a final model in which all covariates had a p < 0.01 using the likelihood-ratio test.
Model Evaluation
Evaluation of the final model was conducted using graphical methods. Basic goodness-of-fit plots, individual weighted residuals (IWRES), normalized prediction distribution errors (NPDE) over time and visual predictive checks (VPC) were used to assess the model. A bootstrap resampling method was used to evaluate the stability of the final model and the precision of parameter estimates [14]. For the bootstrap method, a total of about 200 resampling replicates were obtained from the original data set. The final population PK model, including final fixed-effect parameters and random-effect parameters, was used to fit the replicates using the bootstrap option, and parameter estimates for each of the replicate data sets were obtained.
Evaluation of Amikacin Doses by Monte Carlo Simulation
Using the estimated distribution of amikacin PK parameters in the final model with covariates, we simulated the amikacin concentration obtained 1 h after the start of a 30-min infusion for 1000 patients and several dosing regimens via Monte Carlo simulation (Kozo Keikaku Engineering Inc., Tokyo, Japan). This timing for amikacin sampling is commonly used for studying aminoglycoside efficacy [15–17]. For the PK simulations, we randomly resampled 1000 vectors of covariates among those observed in the patients included in the analysis and simulated individual PK parameters from their estimated distribution in the final model with covariates. The following dose regimens were simulated: 10, 15, 20, 25, and 30 mg/kg. We compared the probability to achieve (PTA) a C peak/MIC of ≥8 for MIC regimens ranging from 0.25 to 32 μg/mL for each dosing regimen [2].
Additionally, since PTA is the probability of achieving a certain goal for a given MIC, cumulative fraction of response (CFR) takes into account the MIC distributions, and was also calculated to be more representative when assessing recommendations for initial dosing regimens. Hence, CFR was calculated with MIC distribution data of EUCAST surveillance data [18].
Evaluation of Clinical and Microbiological Effects
Microbiological response was evaluated at the end of amikacin therapy. The microbiological cure was defined as effective when bacteria disappeared during and after amikacin therapy. Microbiological failure was defined as the persistence of pathogen(s) in laboratory samples or as the development of a new infection.
Statistical Analysis
Data were analyzed with JMP version 10.0 (SAS, Tokyo, Japan). The analysis of patient data included sex and combination therapy as categorical variables, and age, body weight, and PK parameters (including C peak and C trough) as continuous variables. Statistical significance of the difference was evaluated by Kruskal-Wallis test or paired t test for categorical data and Scheffe test for continuous data, according to whether the data distribution was normal or non-normal, respectively. A p value of 0.05 was employed.
Results
Patients
The demographics and clinical characteristics of the subjects participating in this study are summarized in Table 1. During the study period, 35 of 39 patients fulfilled the inclusion criteria (four patients were excluded because of incomplete infusion time data). The infection types of the 35 patients were UTI for eight patients, peritoneum inflammation for two, pneumonia for ten, bacteremia for 11, pneumonia and bacteremia for two, and febrile neutropenia for two. Median (minimum–maximum) age and total body weight were 70 years (15–95 years) and 49.5 kg (32.5–78 kg), respectively, and most patients were males (n = 25; 71.4%).
Table 1.
Clinical characteristics and distribution of amikacin of hospitalized patients
| Parameter | Mean ± SD | Median [range] |
|---|---|---|
| Male/female | 25/10 | |
| Age (years) | 67.5 ± 17.3 | 70.0 [15–95] |
| Weight (kg) | 50.4 ± 10.4 | 49.5 [32.5–78] |
| Lean body weight (kg) | 40.8 ± 8.8 | 42.3 [24.6–58.4] |
| BMI (kg/m2) | 19.6 ± 3.1 | 19.7 [13.4–26.4] |
| Ideal body weight (kg) | 56.8 ± 7.8 | 57.5 [40.1–68.1] |
| Albumin (g/dL) | 2.7 ± 0.6 | 2.6 [1.7–4.3] |
| Serum creatinine (mg/dL) | 1.14 ± 1.23 | 0.61 [0.2–5.6] |
| CCra (mL/min) | 60.3 ± 33.9 | 60.0 [6.1–144] |
| AST (U/L) | 41.3 ± 39.7 | 31 [6–243] |
| ALT (U/L) | 35.2 ± 21.1 | 33 [7–91] |
| BUN (mg/dL) | 24.0 ± 17.7 | 16.4 [5.9–71.6] |
| Total bilirubin (mg/dL) | 0.88 ± 0.81 | 0.55 [0.22–3.47] |
| Duration of amikacin therapy (days) | 13.6 ± 26.5 | 8 [3–162] |
| Amikacin dosage (mg/day) | 513 ± 258 | 400 [200–1000] |
| Amikacin dosage (mg/kg/day) | 10.0 ± 3.9 | 9.6 [3.1–17.7] |
| Infusion time (h) | 0.68 ± 0.38 | 0.5 [0.5–2.0] |
Numerical data are shown as mean ± SD
ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, BUN blood urea nitrogen, CCr creatinine clearance, CL total body clearance, SD standard deviation
aCCr estimates calculated according to the Cockcroft–Gault equation
A total of 65 values of amikacin concentrations were obtained from 35 patients, for a range of 1–2 points per subject. The median amikacin dose administered was 9.6 mg/kg (3.1–17.7 mg/kg). Amikacin was infused intravenously once daily for 30 min for 30 patients and for 1 h for five patients. In principle, blood was sampled within 3 days of the start of amikacin administration. Many samples were drawn 30 min after the end of infusion and/or immediately before the next administration.
Antimicrobial Susceptibility
In total, 33 causative isolates were collected from blood (42.4%), sputum (39.4%), and urine (18.2%). The others (two patients) were negative. The MIC distribution for Gram-negative isolates is presented in Table 2. The most common species isolated was P. aeruginosa (48.5%), followed by Klebsiella pneumoniae (6.1%). The MIC range for amikacin against Gram-negative pathogens isolated from patients treated with amikacin was from 0.25 to 48 µg/mL in our study. Among them were three metallo-β-lactamase-producing P. aeruginosa isolates, ten P. aeruginosa isolates with carbapenem and quinolone resistance, one extended spectrum-β-lactamase (ESBL)-producing K. pneumonia, one Escherichia coli with quinolone resistance, and one ESBL-producing E. coli.
Table 2.
MIC distribution for Gram-positive and Gram-negative bacteria for amikacin
| Isolates | MIC (μg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 1.5 | 2 | 3 | 4 | 6 | 8 | 12 | 16 | 32 | 48 | |
| Staphylococcus aureus | 1 | 1 | |||||||||||
| Staphylococcus epidermidis | 2 | 1 | |||||||||||
| Staphylococcus capitis | 1 | ||||||||||||
| Staphylococcus haemolyticus | 1 | ||||||||||||
| Staphylococcus warneri | 1 | ||||||||||||
| Pseudomonas aeruginosa | 2 | 1 | 4 | 1 | 2 | 1 | 4 | 1 | |||||
| Enterobacter cloacae | 1 | ||||||||||||
| Klebsiella pneumoniae | 1 | 1 | |||||||||||
| Serratia marcescens | 1 | ||||||||||||
| Escherichia coli | 1 | 1 | |||||||||||
| Rhizobium radiobacter | 1 | ||||||||||||
| Corynebacterium sp. | 1 | ||||||||||||
| Corynebacterium striatum | 1 | ||||||||||||
MIC minimal inhibitory concentration
Population PK Parameters of Amikacin
We first fitted the one- and two-compartment models without any covariates, and the results of this fitting suggested that the two-compartment model better described the current data set. The basic parameters were total body clearance (CL), the volume of distribution in the central compartment (V 1), V 2, and Q.
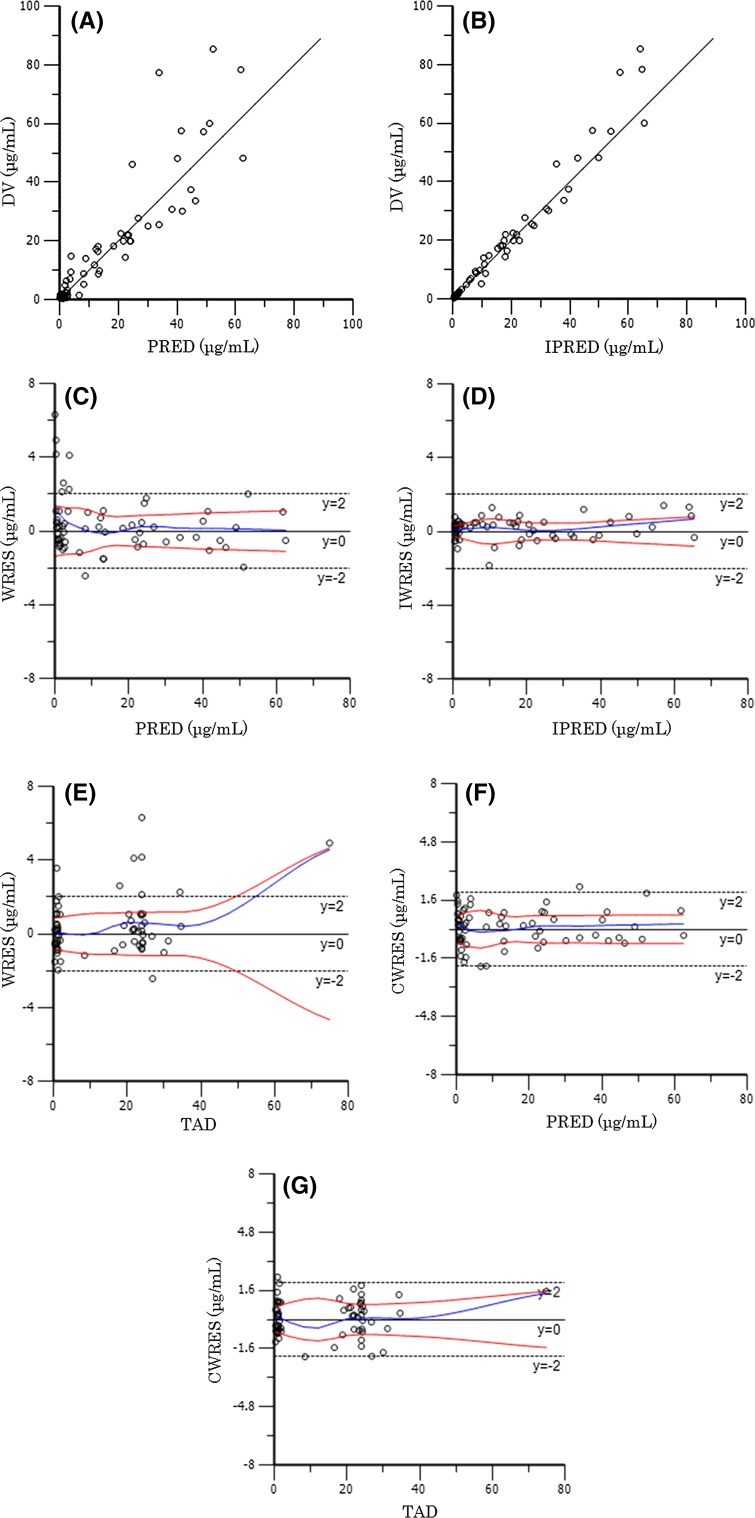
The final estimates for the population PK parameters of amikacin are summarized in Tables 3 and 4. Our data indicated that CL was related to CCr. Additionally, V 1 was related to weight. Scatter plots demonstrating the performance of the final model are presented in Fig. 1 [population predicted concentration predictions (PRED) vs. observed concentrations (DV) (Fig. 1a), and individual predicted concentrations (IPRED) vs. DV (Fig. 1b), the WRES vs. the PRED (Fig. 1c), the IWRES vs. the IPRED (Fig. 1d), the WRES vs. time after dose (TAD) (Fig. 1e), conditional weighted residuals (CWRES) vs. the PRED (Fig. 1f), and the CWRES vs. the TAD (Fig. 1g)]. The inter-individual variability in CL and V 1 were estimated as 50.8 and 29.0%, respectively. Furthermore, the intra-individual residual variability of the amikacin concentration was 0.25 μg/mL.
Table 3.
Hypothesis testing for factors affecting pharmacokinetics of amikacin
| Question | OFV | P value | |
|---|---|---|---|
| Full modela | 330.7 | ||
| Is CL proportional to CCrb? | Full model vs. θ 1 = 0 | 365.8 | <0.001 |
| Is V1 proportional to weight? | Full model vs. θ 2 = 0 | 338.6 | <0.001 |
Final estimates of population pharmacokinetic parameters for amikacin
CCr creatinine clearance, CL total body clearance, OFV objective function value, V 1 volume of distribution in the central compartment
aCL = θ 1(CCr/60.0), V 1 = θ 2(weight/49.5)
bCCr estimates calculated according to the Cockcroft–Gault equation
Table 4.
Final estimates of population pharmacokinetic parameters for amikacin and Bootstrap validation of the estimated population pharmacokinetic parameters in the final model
| Parameter | Final estimates | Bootstrap mean ± SD | Median [95% CI] | Difference (%) |
|---|---|---|---|---|
| θ 1 (L/h) | 2.41 | 2.54 ± 0.38 | 2.50 [1.97–3.31] | 5.39 |
| θ 2 (L) | 10.70 | 10.20 ± 2.29 | 10.26 [5.99–14.69] | −4.67 |
| Intra-individual | ||||
| ω (CL) (%) | 50.8 | 46.4 ± 36.8 | 44.7 [18.4–55.7] | −8.66 |
| ω (V 1) (%) | 29.0 | 37.0 ± 35.8 | 31.9 [27.2–65.6] | 27.6 |
| Intra-individual | ||||
| σ (μg/mL) | 0.25 | 0.20 ± 0.09 | 0.23 [0.01–0.33] | −20.0 |
Successful rate of calculation = 100% (200/200). Final model: CL (L/h) = θ 1(CCr/60.0), V1 (L) = θ 2(weight/49.5), V 2 (L) = 7.72, Q (L/h) = 7.0. Bootstrap validation of the estimated population pharmacokinetic parameters in the final model. The difference between the final model estimate and bootstrap mean is calculated as follows: [(bootstrap mean − final model estimate)/final model estimate] × 100
CCr creatinine clearance, CI confidence interval, CL total body clearance, Q inter-compartmental clearance, SD standard deviation, V 1 volume of distribution in the central compartment, V 2 volume of distribution in the peripheral compartment
Fig. 1.
Scatter plots of population predicted concentrations (PRED) vs. observed concentrations (DV) (a) and individual predicted concentrations (IPRED) vs. DV (b), weighted residuals (WRES) vs. the PRED (c), the individual weighted residuals (IWRES) vs. the IPRED (d), the WRES vs. time after dose (TAD) (e), conditional weighted residuals (CWRES) vs. the PRED (f), and the CWRES vs. the TAD (g)
Model Validation
The final model was repeatedly fitted to 200 bootstrap resampled data sets. The average parameter values obtained from the bootstrap analyses and the final estimates from the original data set are compared in Table 4. Other than the difference of 27.6% (ω V 1), the differences in all other parameters were less than 10.0%. The results of bootstrap analysis validation indicated that the reliability and robustness of the parameter estimates and thus the population PK model were acceptable. The bias, expressed as the mean prediction error, of the final model was 0.25 μg/mL, while the mean bias (95% confidence interval) obtained from the 200 bootstrap analyses was 0.20 μg/mL (0.01–0.33).
Pharmacodynamic Target Attainment
Based on the estimated population PK parameters, we simulated the serum amikacin concentration-versus-time curves for patients with parameters calculated by Monte Carlo simulation.
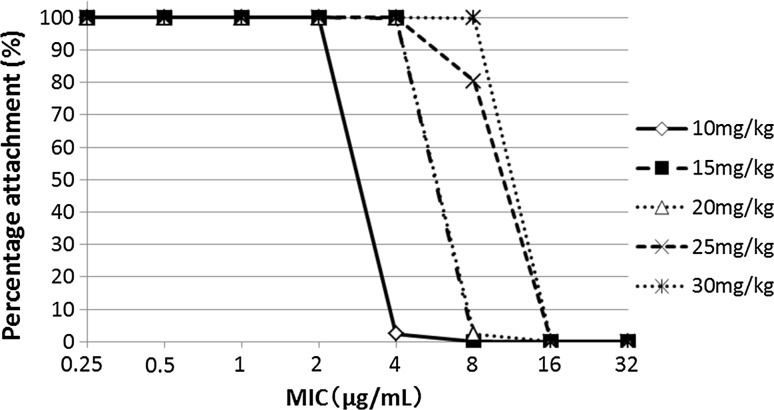
Figure 2 presents the PTA for a C peak/MIC of ≥8 according to various amikacin regimens. Amikacin 10 mg/kg/day was sufficient to achieve the PK/PD target in ≥90% of patients for a MIC of 2 μg/mL or less. A 15-mg/kg regimen was sufficient to achieve the PK/PD target in ≥90% of patients for a MIC of 4 μg/mL or less. With a 20-mg/kg regimen, 2.4 and 0% of patients achieved the target for MIC of 8 and 16 μg/mL, respectively.
Fig. 2.
Probability to achieve the pharmacokinetic target (a C peak/MIC of ≥8) according to the single-dosing regimen and the theoretical MIC of the strain in the Monte Carlo simulation. C peak concentration achieved 1 h after beginning the infusion, MIC minimal inhibitory concentration
On the other hand, for the PTA for the amikacin C trough target (<4 μg/mL), amikacin 10 mg/kg/day achieved the highest probabilities (86.6%), followed by 15 mg/kg/day (77.1%), 20 mg/kg/day (67.9%), 25 mg/kg/day (61.5%), and 30 mg/kg/day (54.5%).
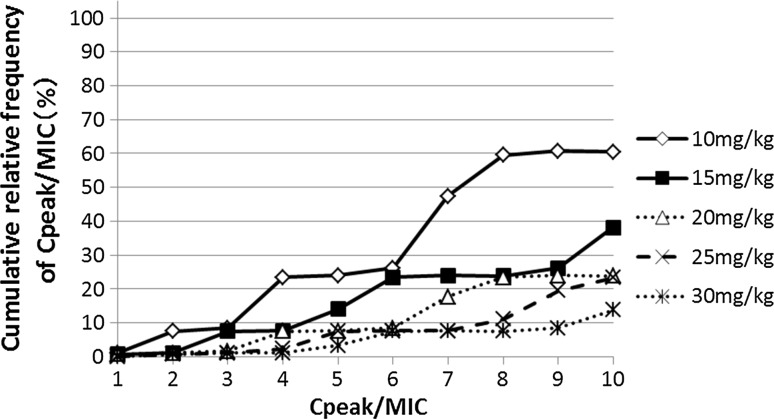
Additionally, CFR considering the amikacin MIC distribution (refer to EUCAST data) in P. aeruginosa at various C peak/MIC levels is presented in Fig. 3. Seventy-six percent of the patients achieved a C peak/MIC of 8 with amikacin 15 mg/kg/day (40% at 10 mg/kg/day and 77% at 20 mg/kg/day).
Fig. 3.
Cumulative relative frequency of C peak/MIC considering MIC distribution data from EUCAST surveillance data. C peak concentration achieved 1 h after beginning the infusion, MIC minimal inhibitory concentration
Comparison Between the Cured and Failed to Cure Groups
Satisfactory microbiological efficacies were obtained in 23 of the 35 patients. The others were failed to clinical cure (n = 9) and unknown (n = 3). Table 5 shows the differences between the microbiologically cured and the failed to cure groups. In the data, excluding a patient whose duration of amikacin therapy was 162 days, there was no significant difference regarding the duration of amikacin therapy between the cured and failed groups (data not shown). Most of the parameter values did not differ significantly between the groups. However, BMI for the cured group [19.9 kg/m2 (15.4–26.4 kg/m2)] was significantly higher than that of the failed to cure group [17.1 kg/m2 (14.9–20.2 kg/m2)]. Additionally, while no significant differences in C trough and C peak values were observed between the cured and failed to cure groups, C peak/MIC for the cured group [10.0 (1.5–102.4)] was higher than that of the failed group [5.1 (0.28–19.3)]. The ratio of co-administrated antibiotics [anti-methicillin resistant Staphylococcus aureus (MRSA) drugs, carbapenems, quinolones, β-lactams] with amikacin did not show significant difference between the two groups (anti-MRSA drugs, p = 0.61; carbapenems, p = 0.43; quinolones, p = 0.81; β-lactams, p = 0.21).
Table 5.
Comparison of cured and failed groups
| Parameter | Cured group | Failed group | P value |
|---|---|---|---|
| Male/female | 17/6 | 8/1 | 0.36 |
| Age (year) | 66.8 [15–89] | 72 [22–83] | 0.96 |
| Weight (kg) | 54 [32.5–78] | 46 [39–57.5] | 0.23 |
| Lean body weight (kg) | 43.9 [24.6–58.4] | 43.1 [30.3–49.7] | 0.89 |
| BMI (kg/m2) | 19.9 [15.4–26.4] | 17.1 [14.9–20.2] | 0.01 |
| Ideal body weight (kg) | 56.3 [44.4–66.8] | 61.4 [48.2–68.1] | 0.16 |
| Albumin (g/dL) | 2.6 [1.7–4.3] | 2.5 [2.4–3.8] | 0.51 |
| Serum creatinine (mg/dL) | 0.69 [0.27–5.58] | 0.51 [0.81–3.47] | 0.48 |
| CCra (mL/min) | 61.0 [6.1–143.5] | 65.2 [14.4–115.3] | 0.76 |
| AST (U/L) | 40 [6–243] | 26 [11–48] | 0.12 |
| ALT (U/L) | 38 [11–91] | 24 [7–44] | 0.04 |
| BUN (mg/dL) | 18.1 [5.9–71.3] | 21.7 [6.8–60.5] | 0.99 |
| Total bilirubin (mg/dL) | 0.55 [0.32–3.47] | 0.65 [0.39–1.37] | 0.81 |
| Duration of amikacin therapy (days) | 8 [3–162] | 7 [4–15] | 0.47 |
| C peak (μg/mL) | 33.7 [14.5–85.3] | 29 [8.8–77.3] | 0.38 |
| C peak/MIC | 10 [1.5–102.4] | 5.1 [0.28–19.3] | 0.30 |
Numerical data are shown as the median [minimum–maximum]. Cured group: the eradication of Gram-positive and Gram-negative organisms. Failed group: the persistence of pathogen in laboratory samples or the development of a new infection
ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, BUN blood urea nitrogen, CCr creatinine clearance, C peak concentration achieved 1 h after beginning the infusion, MIC minimal inhibitory concentration
aCCr estimates calculated according to the Cockcroft–Gault equation
In a subpopulation analysis, patients with UTI showed an 83.3% microbiological cure rate (five of six patients). One patient was not able to detect the microbiological effect. Furthermore, C peak/MIC for the cured group [10.0 (1.5–102.4)] was over 8, as per the PK/PD target. On the other hand, bacteremia and pneumonia patients showed 100% and 62.5% microbiological cure rates, respectively. C peak/MIC for the cured group with bacteremia [21.4 (3.6–39.2)] was over 8, as per the PK/PD target.
Discussion
Despite the introduction of new antimicrobial agents, amikacin has maintained an important role in the treatment of severe bacterial infections [1, 19]. This is especially true in the current era of multiple drug-resistant pathogens, such as P. aeruginosa, where the aminoglycosides have appeared to still have maintained high levels of in vitro susceptibility [2]. Optimal dosage of aminoglycoside antimicrobials is complicated by the narrow therapeutic window for aminoglycosides and mandates accuracy in predicting drug exposure when attempting to reduce the risk of nephrotoxicity. The rationale for once-daily dosing of aminoglycosides is well established [20], and several recent studies have documented the clinical and microbiological efficacies of once-daily dosing of amikacin in combination with β-lactams during febrile neutropenia [21, 22]. Recently, some authors recommended the higher dose regimen of amikacin on the basis of the results of population PK analysis, compared with the conventional dosage (15–20 mg/day) [8, 23, 24]. Therefore, the objective of our study was to evaluate the optimal initial dosing regimen of amikacin with population PK analysis and Monte Carlo simulations.
In our study, we collected sparse data during routine clinical care and analyzed the PK behavior of amikacin in infected patients using the nonlinear mixed-effect model (WinNonlin). As a result, the patient population comprised 35 infected patients (65 values regarding blood concentration data). A two-compartment model was used, and the following covariates were significantly correlated: CL and CCr, and volume of distribution (V d) and weight (Table 3). In previous reports, a one-compartment model has been usually employed, while several studies reported that the PK of amikacin is better characterized by a two-compartment model [8]. In the present study, the two-compartment model better described the PK of amikacin, and its clearance was associated with CCr in our study. Similar findings were made in other population studies involving the other aminoglycosides [8, 25].
Our results revealed that amikacin 15 mg/kg/day was sufficient to achieve the PK/PD target for efficacy (a C peak/MIC of ≥8) in 99.9 and 0% of patients for MIC of 4 and 8 μg/mL. On the other hand, amikacin dosage at 25 mg/kg/day was sufficient to achieve the PK/PD target in 80.4% of patients for a MIC of 8 μg/mL. Considering that the PK/PD breakpoint of the CLSI against Staphylococci, Enterobacteriaceae and P. aeruginosa is ≤16 μg/mL [10], current recommended doses (15–20 mg/kg/day) are unlikely to attain optimal PK/PD endpoints, particularly for bacteremia and pneumonia patients when bacterial susceptibility of amikacin is ≥8 μg/mL. On the other hand, the PTA for the C trough target (<4 μg/mL) with amikacin dosage at 10–15 mg/kg/day was more than 70%.
Additionally, we assessed the PD characteristics of the tested regimens (10–30 mg/kg/day) by CFR considering the distribution of MIC data of P. aeruginosa derived from EUCAST surveillance data. We did not include a list of pathogens with MIC distributions of P. aeruginosa isolated from our patients. This data set cannot be used for the CFR calculations because of the abovementioned presumed selection bias and partly because of the small number of isolates. Hence, this could provide data useful for clinicians from different countries, and it could make the comparison with other literature easier. Consequently, 77% of the patients achieved a C peak/MIC of ≥8 with amikacin 15 mg/kg/day. Hence, the 15-mg/kg once-daily dosage of amikacin is recommended as the initial dosage to get higher CRF with lower toxicity.
On the other hand, the clinical efficacy rate was 71.9% even though lower dosage of amikacin was used in this study (average 10.0 mg/kg) (Table 1). However, most patients received combination therapy (97.1%). Therefore, our clinical evaluation of amikacin does not refer to the exact microbiological efficacy of amikacin. But, in general, amikacin is used as combination therapy, and higher C peak as well as higher dose level produced higher efficacy, and an initial dose of amikacin at 15–30 mg/kg or higher has been recommended [26]. On the basis of the estimated population PK parameters, we simulated the serum amikacin concentration-time curves for infectious patients with Monte Carlo simulation, and simulated probability of target attainment of various amikacin regimens with the estimated population PK parameters. This simulation demonstrated that a conventional amikacin regimen (15 mg/kg/day) may be too low to treat infection for an amikacin MIC of 8 μg/mL. Moreover, for pathogens with amikacin MIC of 16 μg/mL, PK/PD target attainment was extremely poor, even at the highest recommended dosage of 20 mg/kg/day, especially for bacteremia patients.
This study is subject to several limitations that should be considered. First, this study was retrospective. Second, our study population included not only patients infected with P. aeruginosa, but also patients infected with other bacteria who received amikacin. Third, the number of patients was small. Hence, we could not assess the infection type as PK parameter covariates. We think, and as a previous study has revealed [27], the variety of infectious diseases is one of the influential factors in antimicrobial PK. However, this study was retrospective and small. The number of each infection type was too small to evaluate the effect on amikacin clearance and distribution volume; 11 sepsis patients were included in our study. For just in case, while the number was limited, we evaluated whether specific infection type would be a covariate on amikacin clearance and distribution volume. It was not. As a further limitation of our study, all patients were administered concomitant drugs. We also think our results did not show the exact clinical effects of amikacin treatment, as mentioned above, while we analyzed the ratio of concomitant therapies to compare cured and failed patients. In addition, from the PK standpoint, we made sure that concomitant treatments did not significantly influence amikacin clearance and distribution volume in population PK analysis. Amikacin is used as combination therapy since some clinical studies have shown the combined effect of amikacin and other antimicrobials [26]. Despite these limitations, our analyses provide a strong rationale for higher amikacin doses, especially for bacteremia and pneumonia patients.
In conclusion, we suggest that the 15-mg/kg once-daily dosage of amikacin is recommended as the initial dosage to get higher PTA and CRF with lower toxicity. But, as its maintenance dosage, the 15 mg/kg/day amikacin dosage is needed for a MIC of ≤4 μg/mL, and we should avoid amikacin monotherapy for a MIC of ≥8 μg/mL, especially in patients with bacteremia or pneumonia.
Acknowledgements
All authors thank all clinicians and pharmacists that assisted with the provision of data for this project.
Compliance with Ethical Standards
Funding
None.
Conflict of interest
Hideo Kato, Mao Hagihara, Jun Hirai, Daisuke Sakanashi, Hiroyuki Suematsu, Naoya Nishiyama, Yusuke Koizumi, Yuka Yamagishi, Katsuhiko Matsuura, and Hiroshige Mikamo have no conflicts of interest to declare pertaining to this manuscript.
References
- 1.Tally FP, Louie TJ, Weinstein WM, Bartlett JG, Gorbach SL. Amikacin therapy for severe Gram-negative sepsis. Emphasis on infections with gentamicin-resistant organisms. Ann Intern Med. 1975;83:484–488. doi: 10.7326/0003-4819-83-4-484. [DOI] [PubMed] [Google Scholar]
- 2.Zelenitsky SA, Harding GK, Sun S, Ubhi K, Ariano RE. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J Antimicrob Chemother. 2003;52:668–674. doi: 10.1093/jac/dkg403. [DOI] [PubMed] [Google Scholar]
- 3.Begg EJ, Barclay ML, Kirkpatrick CM. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 2001;52(Suppl 1):35S–43S. doi: 10.1046/j.1365-2125.2001.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawchuk RJ, Zaske DE, Cipolle RJ, Wargin WA, Strate RG. Kinetic model for gentamicin dosing with the use of individual patient parameters. Clin Pharmacol Ther. 1997;21:362–369. doi: 10.1002/cpt1977213362. [DOI] [PubMed] [Google Scholar]
- 5.Demczar DJ, Nafziger AN, Bertino JS., Jr Pharmacokinetics of gentamicin at traditional versus high doses: implications for once-daily aminoglycoside dosing. Anitimicrob Agents Chemother. 1977;41:1115–1119. doi: 10.1128/aac.41.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155(1):93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 7.Kashuba AD, Nafziger AN, Drusano GL, Bertio JS., Jr Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob Agents Chemother. 1999;43(3):623–629. doi: 10.1128/aac.43.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdet C, Pajot O, Couffignal C, Armand-Lefevre L, Foucrier A, Laouenan C, Wolff M, Massias L, Mentre F. Population pharmacokinetics of single-dose amikacin in critically ill patients with suspected ventilator-associated pneumonia. Eur J Clin Pharmacol. 2015;71:75–83. doi: 10.1007/s00228-014-1766-y. [DOI] [PubMed] [Google Scholar]
- 9.Galvez R, Luengo C, Crnejo R, Kosche J, Romero C, Tobar E, Illanes V, Llanos O, Castro J. Higher than recommended amikacin loading doses achieve pharmacokinetic targets without associated toxicity. Int J Antimicrob Agents. 2011;38:146–151. doi: 10.1016/j.ijantimicag.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; Twenty-Third informational supplement. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 12.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean body weight. Clin Pharmacokinet. 2005;44:1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sherwin CM, Kiang TK, Spigarelli MG, Ensom MH. Fundamentals of population pharmacokinetic modelling: validation methods. Clin Pharmacokinet. 2012;51:573–590. doi: 10.1007/BF03261932. [DOI] [PubMed] [Google Scholar]
- 14.Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997;37(6):486–495. doi: 10.1002/j.1552-4604.1997.tb04326.x. [DOI] [PubMed] [Google Scholar]
- 15.Begg EJ, Barclay ML, Kirkpatrick CM. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 2001;52(Suppl 1):35S–43S. doi: 10.1046/j.1365-2125.2001.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawchuk RJ, Zaske DE, Cipolle RJ, Wargin WA, Strate RG. Kinetic model for gentamicin dosing with the use of individual patient parameters. Clin Pharmacol Ther. 1977;21(3):362–369. doi: 10.1002/cpt1977213362. [DOI] [PubMed] [Google Scholar]
- 17.Demczar DJ, Nafziger AN, Bertino JS., Jr Pharmacokinetics of gentamicin at traditional versus high doses: implications for once-daily aminoglycoside dosing. Antimicrob Agents Chemother. 1997;41(5):1115–1119. doi: 10.1128/aac.41.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European committee on antimicrobial susceptibility testing. MIC distribution for a wide range of organisms and antimicrobial agents, including antifungals, version 5.16b. 2007.
- 19.Khan MA, Faiz A. Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann Saudi Med. 2016;36:23–28. doi: 10.5144/0256-4947.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig WA. Once-daily versus multiple-daily dosing of aminoglycosides. J Chemother. 1995;7(Suppl. 2):47–52. [PubMed] [Google Scholar]
- 21.Cometta A, Zinner S, de Bock R, Calandra T, Gaya H, Klastersky J, Langenaeken J, Paesmans M, Viscoli C, Glauser MP. Piperacillin-tazobactam plus amikacin versus ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. The international antimicrobial therapy cooperative group og the European organization for research and treatment of cancer. Antimicrob Agents Chemother. 1995;39:445–452. doi: 10.1128/AAC.39.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer Efficacy and toxicity of single daily doses of amikacin and ceftriaxone versus multiple daily doses of amikacin and ceftazidime for infection in patients with cancer and granulocytopenia. Ann Intern Med. 1993;119:584–593. doi: 10.7326/0003-4819-119-7_Part_1-199310010-00006. [DOI] [PubMed] [Google Scholar]
- 23.Yu T, Stockmann C, Healy DP, Olson J, Wead S, Neely AN, Kagan RJ, Spigarelli MG, Sherwin CM. Determination of optimal amikacin dosing regimens for pediatric patients with burn wound sepsis. J Burn Care Res. 2015;36:244–252. doi: 10.1097/BCR.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoudi L, Mohammadpour AH, Ahmadi A, Niknam R, Mojtahedzadeh M. Influence of sepsis on higher daily dose of amikacin pharmacokinetics in critically ill patients. Eur Rev Med Pharmacol Sci. 2013;17:285–291. [PubMed] [Google Scholar]
- 25.Tod M, Lortholary O, Seytre D, Semaoun R, Uzzan B, Guillevin L, Casassus P, Petitjean O. Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults. 1998;42:849–856. doi: 10.1128/aac.42.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahar JR, Timsit JF, Garrouste-Orgeas M, Francais A, Vesin A, Descorps-Declere A, Dubois Y, Souweine B, Haouache H, Goldgran-Toledano D, Allaluchiche B, Azoulay E, Adrie C. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 27.Hagihara M, Kato H, Hamada Y, Hirai J, Sakanashi D, Sematsu H, Nishiyama N, Koizumi Y, Yamagishi Y, Matsuura K, Mikamo H. Population pharmacokinetics of arbekacin in different infectious disease settings and evaluation of dosing regimens. 2016;22:436–443. doi: 10.1016/j.jiac.2016.03.008. [DOI] [PubMed] [Google Scholar]