Abstract
Aim
The aim of this study was to compare human pharmacokinetics and bioequivalence metrics in saliva versus plasma for azithromycin as a model class I drug of the Salivary Excretion Classification System (SECS).
Methods
A pilot, open-label, two-way crossover bioequivalence study was done, and involved a single 500-mg oral dose of azithromycin given to eight healthy subjects under fasting conditions, followed by a 3-week washout period. Blood and unstimulated saliva samples were collected over 72 h and deep frozen until analysis by a validated liquid chromatography with mass spectroscopy method. The pharmacokinetic parameters and bioequivalence metrics of azithromycin were calculated by non-compartment analysis using WinNonlin V5.2. Descriptive statistics and dimensional analysis of the pharmacokinetic parameters of azithromycin were performed using Microsoft Excel. PK-Sim V5.6 was used to estimate the effective intestinal permeability of azithromycin.
Results and Discussion
No statistical differences were shown in area under the concentration curves to 72 h (AUC0–72), maximum measured concentration (C max) and time to maximum concentration (T max) between test and reference azithromycin products (P > 0.05) in the saliva matrix and in the plasma matrix. Due to the high intra-subject variability and low sample size of this pilot study, the 90% confidence intervals of AUC0–72 and C max did not fall within the acceptance range (80–125%). However, saliva levels were higher than that of plasma, with a longer salivary T max. The mean saliva/plasma concentration of test and reference were 2.29 and 2.33, respectively. The mean ± standard deviation ratios of saliva/plasma of AUC0–72, C max and T max for test were 2.65 ± 1.59, 1.51 ± 0.49 and 1.85 ± 1.4, while for the reference product they were 3.37 ± 2.20, 1.57 ± 0.77 and 2.6 ± 1.27, respectively. A good correlation of R = 0.87 between plasma and saliva concentrations for both test and reference products was also observed. Azithromycin is considered a class I drug based on the SECS, since it has a high permeability and high fraction unbound, and saliva sampling could be used as an alternative to plasma sampling to characterize its pharmacokinetics and bioequivalence in humans when adequate sample size is used.
Key Points
| Saliva sampling offers an easy and non-invasive method as compared with plasma sampling. |
| Azithromycin pharmacokinetics and bioequivalence metrics in saliva were compared with plasma, and a good correlation was demonstrated. |
| Azithromycin is considered a class I drug based on the Salivary Excretion Classification System (SECS), and saliva sampling could be used as an alternative to plasma sampling to characterize the drug’s pharmacokinetics and bioequivalence in humans. |
Introduction
Salivary excretion of some drugs has been reported previously as a good indicator for drug bioavailability, therapeutic drug monitoring [1–6], pharmacokinetics [7–11] and also drug abuse [12]. Saliva sampling offers a simple, non-invasive and cheap method as compared [13–16] with plasma sampling, yet needs special attention so that no drug residue is left in the mouth after dosing. According to the Salivary Excretion Classification System (SECS), class I drugs of high intestinal permeability and low protein binding, such as paracetamol, are subjected to salivary excretion. Class II drugs of low permeability and low protein binding, such as metformin, are subjected to salivary excretion since low permeability is counterbalanced by low protein binding. Class III drugs of high intestinal permeability and high protein binding, such as cinacalcet, are subjected to salivary excretion since high protein binding is counterbalanced by high permeability. Class IV drugs of low intestinal permeability and high protein binding, such as montelukast, are not subjected to salivary excretion [1]. In addition, drug analysis in the clean saliva matrix is simple and can be done using the same method of analysis as plasma matrix. Azithromycin is a semi-synthetic 15-member azalide antibiotic derived from erythromycin. It is characterized by better acid stability associated with more reliable and greater oral bioavailability, more extensive tissue penetration, and significantly longer elimination half-life compared with erythromycin. Azithromycin is effective against Gram-positive and Gram-negative pathogens. Azithromycin is commonly used for the treatment and prophylaxis of respiratory tract infection, skin and soft tissue infection, and sexually transmitted diseases [17, 18].
Objectives
The aim of this study was to compare human pharmacokinetics and bioequivalence metrics in saliva versus plasma for azithromycin as a model class I drug of the SECS.
Experimental
Design
Plasma pharmacokinetics under fasted state conditions were compared with saliva pharmacokinetics in a two-way cross-over truncated design study. The pilot bioequivalence study was conducted on in eight healthy human volunteers at Al-Hilal Hospital as per the International Council for Harmonization, Good Clinical Practice, and Helsinki declaration guidelines, after Jordan Food and Drug Administration approvals.
Oral dosing of 500 mg azithromycin test formula (Azox® tablet, batch #150912, expiry date 09/2018) or reference formula (Zithromax tablet, batch #315704, expiry date 03/2018) with 240 mL of water was given after 10 h overnight fasting without dietary restrictions.
Plasma and resting unstimulated saliva samples were collected at 0, 0.33, 0.66, 1.00, 1.50, 2.00, 2.5, 3.00, 3.50, 4.00, 4.50, 5.00, 6.00, 8.00, 10.00, 12.00, 24.00 and 48.00 and truncated at 72.00 h during each study phase. Mouth checking and thorough rinsing of the mouth was done prior to first saliva sampling to avoid contamination of saliva samples by any drug residues. All samples were deep frozen until assayed by the validated liquid chromatography with mass spectroscopy (LC-MS) assay method (detailed below).
Medical history, vital signs, physical examination and laboratory safety test results showed no evidence of clinically significant deviation from normal medical condition as evaluated by the clinical investigator.
Assay Methodology
Plasma and saliva samples were deep frozen until assayed by validated LC-MS assay method. A Hypersil BDS C18 column at 30 °C was used with roxithromycin as the internal standard. The mobile phase was 45% 0.01 M ammonium acetate plus 0.1% acetic acid and 55% methanol. The flow rate was 0.5 mL/min, with an ambient auto-sampler temperature. The extraction procedure was as follows:
Pipette 300 μL of spiked/blank plasma sample into a previously labeled test tube.
Add 50 μL of internal standard (400 ng roxithromycin/mL), and vortex for 15 s.
Add 150 μL of extraction buffer for plasma samples only (0.1 M sodium carbonate), and vortex for 30 s.
Add 5 mL of extraction solvent (MTBE), and vortex for 2 min.
Centrifuge the sample for 2 min at 4400 rpm.
Freeze the sample for about 30 min then decant the supernatant in a clean evaporating glass tube.
Evaporate the extraction solvent by compressed air in water bath at 40 °C (this step should be conducted in the fume hood), reconstitute the residue with 250 μL of reconstitution solution (50 MeOH:50 water) plus 0.1% acetic acid, and vortex for 1 min.
Transfer the sample into a flat bottom inserts vial, and inject into the instrument.
Intra-day coefficient of variation was 3%, inter-day accuracy range was 93.228–105.140%, inter-day precision range was 6.65–13.204% and linear range was 5–450 ng/mL.
Data Analysis
Pharmacokinetic Analysis
Individual pharmacokinetic parameters for drug concentration in both saliva and plasma samples were calculated by non-compartmental analysis using WinNonlin V5.2. Pharmacokinetic parameters were area under the concentration curve to 72 h (AUC0–72), maximum measured concentration (C max) and time to maximum concentration (T max). The elimination phase was not clear enough in the truncated design to calculate elimination parameters such as half-life and elimination rate constant.
Dimensional and Correlation Analysis
The following dimensionless saliva/plasma ratios were calculated in Microsoft Excel:
AUC* = saliva AUCt/plasma AUCt
T max * = saliva T max/plasma T max
C max * = saliva C max/plasma C max
C * = saliva concentration/plasma concentration = C s/C p
Correlation analysis was performed using Excel to correlate average C s versus average C p values up to median T max of the reference product.
Bioequivalence and Statistical Analysis
Analysis of variance, 90% confidence intervals, and intra-subject variability estimates for primary pharmacokinetic parameters for test and reference products after logarithmic transformation were calculated by WinNonlin V5.2. Statistical comparisons were also done using parametric t tests for AUC and C max, and the non-parametric Wicoxon test for T max.
Absorption Kinetics
The Nelder–Mead algorithm of the Parameter Estimation module, using the PK-Sim program V5.6, was used to calculate azithromycin intestinal permeability (P eff) by searching for the best parameter values that produced a plasma concentration that matched the actual plasma concentration at the same time. The objective function is the weighted sum of squared differences of the observed and model predicted values.
Results and Discussion
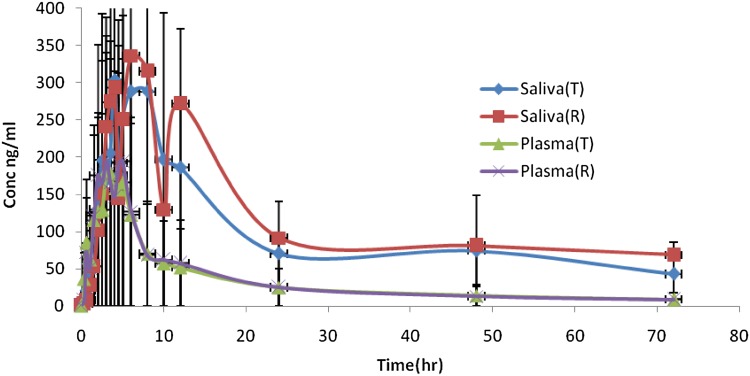
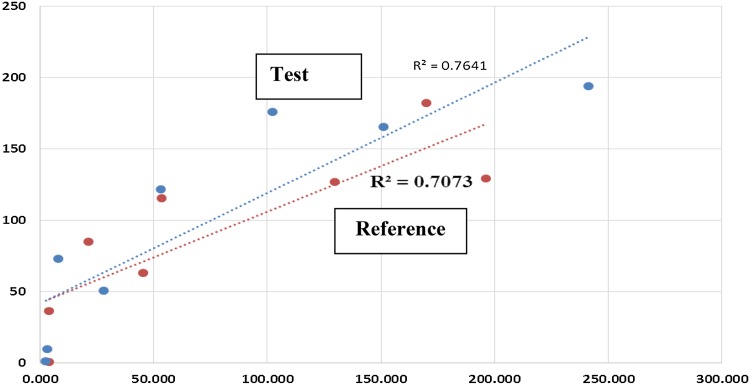
Azithromycin plasma and saliva mean concentrations are shown in Fig. 1. It is noticed that saliva concentrations are much higher than plasma concentrations. However, saliva profiles in test and reference products are closely related to those in plasma. The correlation coefficients and coefficients of determination of 0.84–0.87 and 0.71–0.76 between saliva and plasma concentrations up to median T max values of plasma profiles are shown in Fig. 2. This is in agreement with the good correlation shown previously for other drugs [1]. Pharmacokinetic parameters of AUC0–72, T max and C max in saliva and plasma are summarized in Tables 1 and 2. No statistical differences were seen for AUC0–72, C max and T max between test and reference azithromycin products (P > 0.05) in saliva matrix and in plasma matrix.
Fig. 1.
Plasma and saliva of azithromycin mean concentrations (conc). R reference, T test
Fig. 2.
Correlation of saliva and plasma azithromycin mean concentrations in test and reference
Table 1.
Pharmacokinetic parameters of azithromycin test and reference formulations in plasma
| Parameter | Test | Reference | P value; paired t test |
|---|---|---|---|
| AUC0–72 (ng/mL·h) | 2419.47 | 2517.0 | 0.44 |
| C max (ng/mL) | 291.30 | 328.3 | 0.47 |
| T max (h) | 3.38 | 3.2 | 0.67* |
* Wilcoxon test is done for T max
Table 2.
Pharmacokinetic parameters of azithromycin of test and reference formulations in saliva
| Parameter | Test | Reference | P value; paired t test |
|---|---|---|---|
| AUC0–72 (ng/mL·h) | 6992.05 | 8560.74 | 0.35 |
| C max (ng/mL) | 453.33 | 496.69 | 0.30 |
| T max (h) | 5.25 | 8.44 | 0.15* |
* Wilcoxon test is done for T max
Moreover, bioequivalence metrics and statistical comparisons for primary pharmacokinetic parameters were calculated for both saliva and plasma. The 90% confidence intervals for AUC0–72 and C max were 45.32–117.51 and 63.20–113.64, and 65.56–117.58 and 52.05–134.33% in saliva and plasma, respectively. However, geometric mean ratios of AUC0–72 and C max in saliva were 84 and 73, and 88 and 83% in plasma. Confidence intervals did not pass in both saliva and plasma because of the low sample size of this pilot study. This is reflected in a high intra-subject variability of 52% and a minimum low study power of 19% in both saliva and plasma.
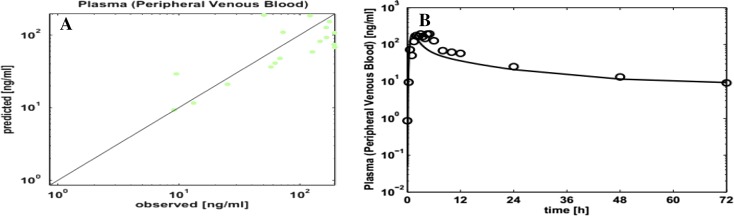
Figure 3 shows observed versus PK-Sim-predicted concentration profiles, indicating good fit. The optimized effective permeability coefficient was 0.0006 cm/s. Azithromycin falls into class I, consistent with published results [1], with high permeability (Fa = 0.99) and high fraction unbound (fu = 0.71), with good salivary excretion. Moreover, the dimensional analysis presented in Table 3 shows that saliva to plasma ratios are higher than unity. This is consistent with the higher concentrations and parameter values in saliva as compared to plasma, which makes it easier to measure and trace azithromycin concentrations in saliva.
Fig. 3.
Observed vs. PK-Sim-predicted plasma concentrations (a, b)
Table 3.
Saliva/plasma dimensional analysis of test and reference
| Parameter | Test | Reference |
|---|---|---|
| AUC* | 2.65 | 3.37 |
| C max* | 1.51 | 1.57 |
| T max* | 1.85 | 2.86 |
| C* | 2.29 | 2.33 |
AUC area under the concentration curve, AUC* saliva AUCt/plasma AUCt, C max maximum measured concentration, C max * saliva C max/plasma C max, T max time to C max, T max * saliva T max/plasma T max, C* saliva concentration/plasma concentration
It is well known that for most antibiotics, serum levels serve as a good surrogate of levels at the site of infection, but for certain classes of antibiotics (e.g., azithromycin, clarithromycin), there is a large difference between serum drug level and level at infection site (10- to 20-fold). Therefore, using the pharmacokinetic data alone is not enough to describe the antimicrobial activity of the drug, and pharmacodynamic parameters should integrate with pharmacokinetic parameters. Integrating the pharmacokinetic parameters with the minimum inhibitory concentration (MIC) gives us three pharmacokinetic/pharmacodynamic parameters that quantify the activity of an antibiotic: the peak/MIC ratio, the T > MIC, and the 24 h-AUC/MIC ratio. It was found that azithromycin killing activity is best described by 24 h-AUC/MIC calculation [19, 20]. Since the AUC of azithromycin in saliva is much higher than that of plasma, saliva AUC may be used to calculate the killing activity of azithromycin.
Conclusions
Azithromycin saliva sampling could be used as an alternative to plasma sampling to characterize the drug’s pharmacokinetics and bioequivalence in humans when adequate sample size is used. Further work is needed to fully characterize the salivary excretion of azithromycin following its oral administration using larger sample size and extended sampling time.
Indeed, it was not our goal to establish bioequivalence, but rather to confirm a concept and to show suitability and validity of saliva instead of plasma in such studies. Saliva sampling has the advantages of being easier to collect and painless compared with plasma sampling. Moreover, saliva sampling can be done at home by patients, in the case of therapeutic drug monitoring, or in a controlled study in hospitals. NASA has conducted several clinical trials in space shuttles using saliva sampling because of its validity and suitability [21]. The main disadvantage of saliva samples can be the high intra- and inter-subject variability. However, this can also be accounted for by enrolling a larger number of subjects in the clinical study.
Acknowledgements
This work was done in partial fulfillment of Master of Science requirements at the University of Petra. We thank all staff at Al-Hilal Hospital and the Jordan Center of Pharmaceutical Research.
Compliance with Ethical Standards
Conflicts of interest
Nasir Idkaidek, Tawfiq Arafat, Hazim Hamadi, Salim Hamadi and Ibrahim Al-Adham have no conflicts of interest to declare.
Funding
No funding was received for the preparation of this manuscript. This research was funded by Petra University. WinNonlin was used under an academic license from Pharsight Inc., USA. The PK-Sim program was used under an academic license from BAYER Ltd, Germany.
References
- 1.Idkaidek N, Arafat T. Saliva versus plasma pharmacokinetics: theory and application of a salivary excretion classification system. Mol Pharm. 2012;9:2358–2363. doi: 10.1021/mp300250r. [DOI] [PubMed] [Google Scholar]
- 2.Baumann RJ. Salivary monitoring of antiepileptic drugs. J Pharm Pract. 2007;20:147–157. doi: 10.1177/0897190007305139. [DOI] [Google Scholar]
- 3.Danhof M, Breimer D. Therapeutic drug monitoring in saliva. Clin Pharmacokinet. 1978;3:39–57. doi: 10.2165/00003088-197803010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Drobitch RK, Svensson CK. Therapeutic drug monitoring in saliva. Clin Pharmacokinet. 1992;23:365–379. doi: 10.2165/00003088-199223050-00003. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Delgado MR. Therapeutic drug concentration monitoring using saliva samples. Clin Pharmacokinet. 1999;36:453–470. doi: 10.2165/00003088-199936060-00006. [DOI] [PubMed] [Google Scholar]
- 6.Mucklow JC. The use of saliva in therapeutic drug monitoring. Ther Drug Monit. 1982;4:229–248. doi: 10.1097/00007691-198208000-00001. [DOI] [Google Scholar]
- 7.Adithan C, Thangam J. A comparative study of saliva and serum paracetamol levels using a simple spectrophotometric method. Br J Clin Pharmacol. 1982;14:107–109. doi: 10.1111/j.1365-2125.1982.tb04944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldo M, Hunzicker G, Altamirano J, Murguía M, Hein G. Saliva as a noninvasive biological sample to compare bioavailability of phenytoin formulations by LC–MS/MS. Int J Pharm Sci Res. 2015;6:3752. [Google Scholar]
- 9.Cawello W, Bökens H, Nickel B, Andreas JO, Halabi A. Tolerability, pharmacokinetics, and bioequivalence of the tablet and syrup formulations of lacosamide in plasma, saliva, and urine: saliva as a surrogate of pharmacokinetics in the central compartment. Epilepsia. 2013;54:81–88. doi: 10.1111/j.1528-1167.2012.03725.x. [DOI] [PubMed] [Google Scholar]
- 10.Koks C, Meenhorst P, Hillebrand M, Bult A, Beijnen J. Pharmacokinetics of fluconazole in saliva and plasma after administration of an oral suspension and capsules. Antimicrob Agents Chemother. 1996;40:1935–1937. doi: 10.1128/aac.40.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suryawati S, Santoso B. Pharmacokinetics of metronidazole in saliva. Int J Clin Pharmacol Ther Toxicol. 1991;29:474–478. [PubMed] [Google Scholar]
- 12.Guinan T, Ronci M, Kobus H, Voelcker NH. Rapid detection of illicit drugs in neat saliva using desorption/ionization on porous silicon. Talanta. 2012;99:791–798. doi: 10.1016/j.talanta.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Nunes LAS, Brenzikofer R, Macedo DV. Reference intervals for saliva analytes collected by a standardized method in a physically active population. Clin Biochem. 2011;44:1440–1444. doi: 10.1016/j.clinbiochem.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Nunes S, Alessandro L, Mussavira S, Sukumaran Bindhu O. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochemia Medica. 2015;25:177–192. doi: 10.11613/BM.2015.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011;57:675–687. doi: 10.1373/clinchem.2010.153767. [DOI] [PubMed] [Google Scholar]
- 16.Javaid MA, Ahmed AS, Durand R, Tran SD. Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res. 2015;6:67–76. doi: 10.1016/j.jobcr.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drew RH, Gallis HA. Azithromycin—spectrum of activity, pharmacokinetics, and clinical applications. Pharmacother: J Hum Pharmacol Drug Ther. 1992;12:161–173. [PubMed] [Google Scholar]
- 18.Ballow CH, Amsden GW. Azithromycin: the first azalide antibiotic. Ann Pharmacother. 1992;26:1253–1261. doi: 10.1177/106002809202601014. [DOI] [PubMed] [Google Scholar]
- 19.Levison ME, Levison JH. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am. 2009;23:791–815. doi: 10.1016/j.idc.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Bambeke F, Tulkens PM. Macrolides: pharmacokinetics and pharmacodynamics. Int J Antimicrob Agents. 2001;18:17–23. doi: 10.1016/S0924-8579(01)00406-X. [DOI] [PubMed] [Google Scholar]
- 21.Rhie J. The pellet gastric emptying (PGE) test: development of a non-invasive method to assess gastric emptying. Ph.D. Dissertation, University of Michigan. 1996.