Abstract
Background
Making an accurate diagnosis in patients with disorders of consciousness remains challenging. 18F-fluorodeoxyglucose (FDG)–PET has been validated as a diagnostic tool in this population, and allows identifying unresponsive patients with a capacity for consciousness. In parallel, the perturbational complexity index (PCI), a new measure based on the analysis of the electroencephalographic response to transcranial magnetic stimulation, has also been suggested as a tool to distinguish between unconscious and conscious states. The aim of the study was to cross-validate FDG–PET and PCI, and to identify signs of consciousness in otherwise unresponsive patients.
Methods
We jointly applied the Coma Recovery Scale-Revised, FDG–PET and PCI to assess 24 patients with non-acute disorders of consciousness or locked-in syndrome (13 male; 19–54 years old; 12 traumatic; 9 unresponsive wakefulness syndrome, 11 minimally conscious state; 2 emergence from the minimally conscious state, and 2 locked-in syndrome).
Results
FDG–PET and PCI provided congruent results in 22 patients, regardless of their behavioural diagnosis. Notably, FDG–PET and PCI revealed preserved metabolic rates and high complexity levels in four patients who were behaviourally unresponsive.
Conclusion
We propose that jointly measuring the metabolic activity and the electrophysiological complexity of cortical circuits is a useful complement to the diagnosis and stratification of patients with disorders of consciousness.
Abbreviations: FDG, 18F-fluorodeoxyglucose; CRS-R, Coma Recovery Scale-Revised; DOC, disorders of consciousness; EMCS, emergence from the minimally conscious state; fMRI, functional MRI; LIS, locked-in syndrome; MCS, minimally conscious state; MCS*, non-behavioural minimally conscious state; PCI, perturbational complexity index; SPM, statistical parametric mapping; TMS–EEG, transcranial magnetic stimulation coupled with high-density EEG; UWS, unresponsive wakefulness syndrome
Keywords: Transcranial magnetic stimulation, Electroencephalography, Positron emission tomography, Disorders of consciousness, Unresponsive wakefulness syndrome minimally conscious state, Brain injury
Highlights
-
•
A cross validation of brain complexity measures with metabolic imaging is proposed.
-
•
TMS–EEG and 18F-FDG PET provided congruent result in post-comatose patients.
-
•
Unresponsive patients with high complexity also show preserved metabolic activity.
-
•
These patients thus probably have a specific cognitive-motor dissociation.
-
•
TMS–EEG and PET have potential clinical impact in the diagnosis of these patients.
1. Introduction
Despite major advances, making an accurate diagnosis of consciousness in patients with chronic disorders of consciousness (DOC) remains challenging (Gantner et al., 2012). The expanding nosology makes the process even more complex, as the differences between states tend to become smaller and smaller (Bodart et al., 2013, Giacino et al., 2014). The Coma Recovery Scale-Revised (CRS-R (Giacino et al., 2004)) is the gold standard behavioural scale to make the diagnosis of unresponsive wakefulness syndrome (UWS – recovery of arousal but limited reflexes-only behaviour), minimally conscious state (MCS) minus and plus (recovery of inconsistent but definite signs of consciousness such as visual pursuit, object localization or manipulation, congruent emotional responses – MCS - or command-following and language function – MCS+), and emergence from the MCS (EMCS – recovery of functional communication or use of objects) (Seel et al., 2010). However, due to sensorimotor impairment, fluctuation of vigilance, pain, unnoticeable motor activity, apraxia, and aphasia issues, some patients might have covert consciousness not accessible through behavioural evaluation (Gosseries et al., 2014, Schiff and Fins, 2016). Several patients, otherwise considered unconscious, showed signs of command-following with functional magnetic resonance imaging (fMRI, e.g. Bardin et al., 2011, Bardin et al., 2012, Monti et al., 2010, Owen et al., 2006) and electroencephalography (EEG) active paradigms (e.g. Cruse et al., 2011, Goldfine et al., 2011). Considering these patients unconscious seems inadequate, and the diagnosis of non-behavioural MCS (MCS*) was proposed as a better way to describe their state (Gosseries et al., 2014), while other authors preferred the term “cognitive motor dissociation” (Schiff and Fins, 2016).
Cerebral 18F-fluorodeoxyglucose positron emitted tomography (FDG–PET) evaluates patterns of metabolic activity of the brain. It is a well-established technique to study consciousness (Laureys et al., 2004), and it was recently validated to distinguish between UWS and MCS patients (Stender et al., 2016, Stender et al., 2014). FDG–PET could also detect metabolic activity compatible with MCS in 29% of the UWS patients; 69% of these recovered signs of consciousness at 12 months of follow-up, suggesting that FDG–PET might be a reliable and sensitive tool to detect unresponsive patients with a covert capacity for consciousness (MCS*).
Using transcranial magnetic stimulation (TMS) coupled with EEG it is possible to study cortico-cortical interactions on a millisecond time-scale (Ilmoniemi et al., 1997, Massimini et al., 2009). Cortical EEG responses to TMS differs greatly in healthy subjects between conscious (i.e., normal wakefulness, rapid eye movements sleep (Massimini et al., 2010), ketamine anaesthesia (Sarasso et al., 2015)) and unconscious states (i.e., non-rapid eye movements sleep early in the night (Massimini et al., 2005), propofol, xenon (Sarasso et al., 2015), and midazolam anaesthesia (Ferrarelli et al., 2010)), and UWS patients (Rosanova et al., 2012). On these premises, Casali et al. (2013) implemented an algorithm, the perturbational complexity index (PCI), to assess the ability of functionally specialized modules of the thalamocortical system to interact rapidly and effectively thus producing complex patterns of activity. The distributions of this index in unconscious and conscious conditions do not overlap, ranging from 0 to 0.31 and from 0.44 to 0.7, respectively, suggesting an optimal cut-off of 0.31 (Casali et al., 2013) to distinguish between the two states. Recently, we validated this threshold with a perfect sensitivity and specificity on a larger benchmark population. We then demonstrated its ability to distinguish between UWS and MCS patients with a very high sensitivity and specificity, and detected multiple UWS patients with high complexity, whom exact state of consciousness remained to be specified (Casarotto et al., 2016).
Studying the relationship between the cerebral metabolic activity (as assessed by FDG–PET) and the complexity of neuronal interactions (as assessed by TMS–EEG and PCI) could shed light on the neurophysiology of consciousness. TMS–EEG being a recently validated technique, a cross-validation with established techniques such as FDG-PET is necessary. We expect to see a low PCI in UWS patients with a severely impaired brain metabolism, and a high PCI in those who have FDG–PET results compatible with MCS. Mismatches between PCI and FDG–PET results could also provide significant information about the complementarity of both techniques. The combined metabolic and electrophysiological study of unresponsive patients with high complexity, such as those identified in Casarotto et al. (2016), would allow to better understand this subgroup of patients.
2. Material and methods
2.1. Subjects
We recruited 24 adult patients (13 male, 12 traumatic brain injuries, time since injury 52.5 weeks (5–1371), age 35 ± 12 years). All had acquired brain injuries leading to a coma then to a prolonged state of impaired consciousness, except for two of them, who suffered from a LIS. The LIS and EMCS patients were included as positive controls for this study. Patients were excluded if they had significant neurological, neurosurgical or psychiatric disorders prior to the brain insult that lead to DOC, if they had any contraindication to TMS–EEG or MRI (electronic implanted devices, active epilepsy, external ventricular drain), and if they were not medically stable. Patients were seen at least 5 weeks after the brain injury, to limit the impact of spontaneous recovery during the study. We did not set an upper limit for the time since injury, to better reflect the clinical diversity of these patients. Demographic data can be found in Table 1. The Ethics Committee of the Medical School of the University of Liege approved the study, and we received informed consent from the two LIS patients, and from the legal surrogate of all non-communicating patients. The patients' PCI were already reported in Casali et al. (2013), and Casarotto et al. (2016), and the FDG-PET of ten patients were reported in Stender et al. (2014).
Table 1.
Demographical data.
Table 1 summarizes the population demography according to the behavioural diagnosis. Gender, aetiologies, age at experiment, and time since onset are similar in all groups, with a trend for shorter interval between onset and evaluation in the UWS group.
| N | Mean age (years, SD) | Men (n) | Median time since onset (weeks, range) | TBI (n) | |
|---|---|---|---|---|---|
| UWS | 9 | 34.7 ± 9.29 | 5 | 25 (5–1116) | 4 |
| MCS | 11 | 35 ± 13.9 | 6 | 188 (23–1371) | 7 |
| EMCS/LIS | 4 | 33.8 ± 13.25 | 2 | 111.5 (21 − 200) | 1 |
2.2. Behavioural assessment
The CRS-R (Giacino et al., 2004) is a behavioural scale consisting of 23 items separated in six subscales, assessing the visual, auditory, motor, and oromotor/verbal functions, communication, and arousal. Each subscale includes items of increasing complexity, of which some are considered signs of consciousness (MCS). Functional communication and functional use of object are the diagnostic criteria for EMCS if present at least twice in a row (Giacino et al., 2002). Accredited experts administered the CRS-R a minimum of three times to each patient (except in LIS), and at least the day of TMS–EEG and the day of FDG-PET. The best result was kept as the behavioural diagnosis.
2.3. TMS–EEG
TMS–EEG data were acquired and analysed similarly to our previous TMS–EEG studies (Casali et al., 2013, Rosanova et al., 2012), using TMS stimulator, neuronavigation, and EEG amplifier from Nexstim Plc., Finland. Briefly, TMS–EEG experiments were carried out the following way. The patients' 3D T1 was loaded into the neuronavigation system, and stimulation targets (left and right medial part of the superior frontal and parietal gyri) were identified, as well as any structural lesion that needed to be avoided. A 60 channels EEG cap was installed, along with electrooculogram, reference, and ground electrodes, and connected to a TMS compatible sample-and-hold amplifier. Auditory evoked potentials were avoided by applying white noise through inserted earphones. The target areas were stimulated 400 times with an interstimulus delay of 2000 ms, with a jitter of ± 300 ms, using a figure-of-eight coil driven by a mobile stimulator unit, and with an intensity at the cortical level of around 120 V/m. This could be adjusted, between 100 and 150 V/m, to obtain a sufficient signal to noise ratio with minimal artefacts. The shape and complexity of TMS evoked potentials do not vary with limited changes of intensity (Casarotto et al., 2010). Patients were kept awake for the whole stimulation sessions, using arousal protocol if required (Giacino et al., 2004). Data were then pre-processed with in house scripts in Matlab (Matworks, Natick, MA). EEG sources were computed using weighted minimal norm constraint and PCI was calculated as in Casali et al. (2013). Patients were classified as unconscious or conscious, if their best PCI was below or over 0.31, respectively (Casali et al., 2013).
2.4. FDG–PET
Data were acquired and analysed similarly to our previous FDG–PET studies (e.g., see Stender et al., 2014). Before and after injection of 150–300 MBq of FDG, patients were kept awake in the dark for 30 min and were then scanned for 12 min on a Philips Gemini TF PET-CT scanner. Some patients required light sedation, during the scanning only, in order to prevent excessive movements. As this sedation was performed after the glucose uptake had happened, it did not have any influence on the metabolic results obtained. To detect brain areas exhibiting a significantly preserved or decreased metabolism in each patient, we used SPM 8 (www.fil.ion.ucl.ac.uk/spm) and a contrast consisting of 39 age-matched healthy controls' FDG-PET. The PET-based diagnosis was visually made by experts, and was classified as compatible with unconsciousness (when the statistical tool did not detect a single voxel of preserved metabolism in the whole associative fronto-parietal network bilaterally) or as compatible with consciousness (when at least some significantly preserved metabolic activity could be detected in the fronto-parietal network) (Laureys et al., 1999, Nakayama et al., 2006, Thibaut et al., 2012). We used a significance threshold of p < 0.05 uncorrected in all contrast for single subject analyses, as in our previous studies (Stender et al., 2014). TMS–EEG and FDG-PET were performed 5 days apart: FDG–PET first then TMS–EEG. All the investigators (OB, OG, SW, AT, and JA) knew the results of behavioural evaluations, but TMS–EEG investigators (OB, OG) were blinded to the results of FDG–PET (SW, AT), and vice-versa.
3. Results
Table 2 illustrates the behavioural, metabolic, and electrophysiological results, as well as the outcome of the 24 patients. FDG–PET and TMS–EEG classified patients as conscious or unconscious with no mismatch, except for two patients detailed below, regardless of their behavioural diagnosis.
Table 2.
Behavioural diagnosis, imaging results, and outcome.
Table 2 reports the results of the 24 patients. Patients are arranged from best evidence for consciousness to no evidence for consciousness. Behavioural (CRS-R), FDG–PET, PCI, and outcome are reported. Results compatible with unconsciousness are greyed. The two patients with mismatches between FDG–PET and PCI are highlighted. Note that 4 patients who are behaviourally unresponsive have FDG–PET and PCI compatible with consciousness, leading to the diagnosis of MCS*.
NA: not available. Outcomes at 12 months: + improved; − declined; = did not change.
Four patients could communicate their consciousness (two locked-in syndrome – LIS, two EMCS) according to behavioural evaluations (best CRS-R score of 22 or 23), and served as positive controls for this study. Their FDG–PET showed a hypometabolism limited to the cerebellum (four cases), brainstem (two), and motor areas (two). One showed more extensive hypometabolism of the left posterior parieto-occipital areas including V1 and Wernicke area, as well as the thalami. As expected, all four had best PCI above 0.31, hence above the distribution of unconscious subjects. Ten patients were MCS (seven MCS+ and three MCS−) according to the CRS-R (best total score ranging from 7 to 17). All had a relative preservation of cerebral metabolism of the internal or external fronto-parietal network found by the PET. Similarly, all had maximum PCI above 0.31. Four patients were behaviourally unambiguously unconscious, with a diagnosis of UWS (best CRS-R of 6 or 7). The study of their cerebral metabolism revealed preservation of only the brainstem or cerebellum. Maximal PCI values ranged from 0 to 0.27, and were in the distribution of unconsciousness. These four patients have concordant behavioural, metabolic and electrophysiological findings, and had non-traumatic brain injuries. An additional four unambiguously behaviourally UWS patients (three with traumatic and one with non-traumatic brain injuries, best CRS-R ranging from 5 to 7) had at least partial preservation of the left (two cases), right (one case) or bilateral (one case) temporo-parietal metabolism, compatible with the diagnosis of MCS. Their maximum PCI were above 0.31, hence above the distribution of unconsciousness. Notably, one of them showed preserved activation of the supplementary motor area during an active fMRI evaluation (Monti et al., 2010) (Fig. 1), while the other three did not underwent this exam because they had to be sedated to avoid movement artefacts. Three of them had their brain injury less than one year ago, while the fourth suffered from it nearly four years before the study. Typical behavioural, metabolic, and electrophysiological results of one UWS, one MCS*, one MCS, and one LIS patient are illustrated in Fig. 2.
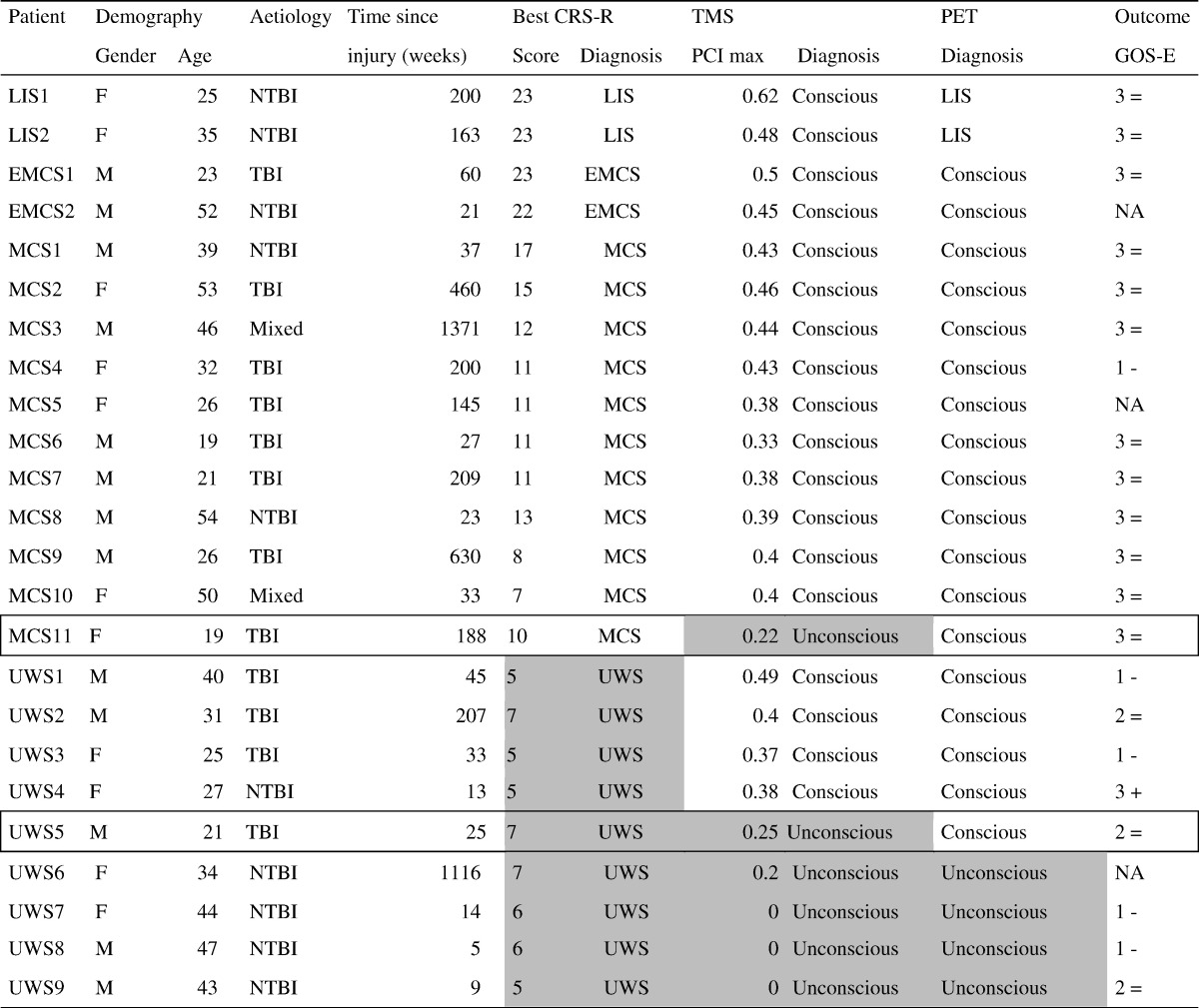
Fig. 1.
Typical results of the active fMRI paradigm in a healthy control and in an MCS* patient.
Functional MRI activation of the supplementary motor area (yellow) and of the parahippocampal gyrus (blue-green) after motor imagery and spatial imagery tasks, respectively, in healthy subjects (A). Subject UWS1 shows significant activation in the supplementary motor area (yellow) after the motor imagery task, similar to the one found in healthy subjects.
Part A adapted with permission from Monti et al., Willful modulation of brain activity in disorders of consciousness, New England Journal of Medicine, 2010, 362(7); 579–589.
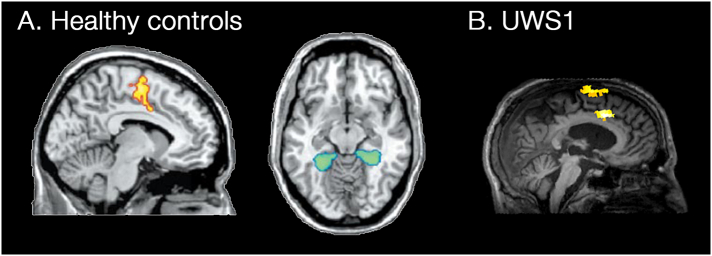
Fig. 2.
Typical behavioural, PCI, and FDG–PET results in an UWS, MCS*, MCS and LIS patients.
Top row illustrates the behavioural subscores of each assessment with the black line representing the threshold for MCS. The second row illustrates the areas on the left hemisphere in which FDG–PET finds significantly impaired (blue) or preserved (red) metabolism compared to 39 controls (p < 0.05). The third raw illustrates the TMS evoked potential traces at the cortical level, which are later used to compute PCI. Note that while behaviourally, UWS and MCS* are alike, the MCS*'s TMS evoked potentials and FDG–PET patterns are more similar to those observed in MCS and LIS patients. FDG–PET images were created by merging the impaired and preserved metabolism maps from SPM8. UWS: unresponsive wakefulness syndrome, MCS*: non-behavioural minimally conscious state, MCS: minimally conscious state, Aud. Vis. Mot. Oro. Com. Aro. are the six CRS-R subscales, A: anterior, P: posterior.
Finally, the last two patients had discordant results. One had a behavioural diagnosis of UWS after repeated CRS-R assessments. The FDG–PET revealed a relative preservation of the whole right hemisphere metabolism. The PCI were all below 0.31, hence in the distribution of unconsciousness, despite stimulations over the right hemisphere. After a follow-up of five years, this patient has not improved and is still UWS. The other patient had a behavioural diagnosis of MCS- (best CRS-R of 10), as visual pursuit was detected once during evaluations, the day of FDG–PET. The latter was compatible with this diagnosis, showing a relative preservation of cerebral metabolism including the anterior cingulate gyrus, the precuneus, and the left frontal area. The PCI was 0.22, hence in the distribution of unconsciousness. However, the day of TMS–EEG, this patient had a behavioural diagnosis of UWS (CRS-R of 4), and PCI could be computed only on the right premotor area (two areas were excluded due to the presence of a cerebrospinal fluid shunt, and one had low signal-to-noise ratio as it was close to structural lesions on the MRI). The TMS evoked potentials and FDG–PET results of these patients are illustrated in Fig. 3.
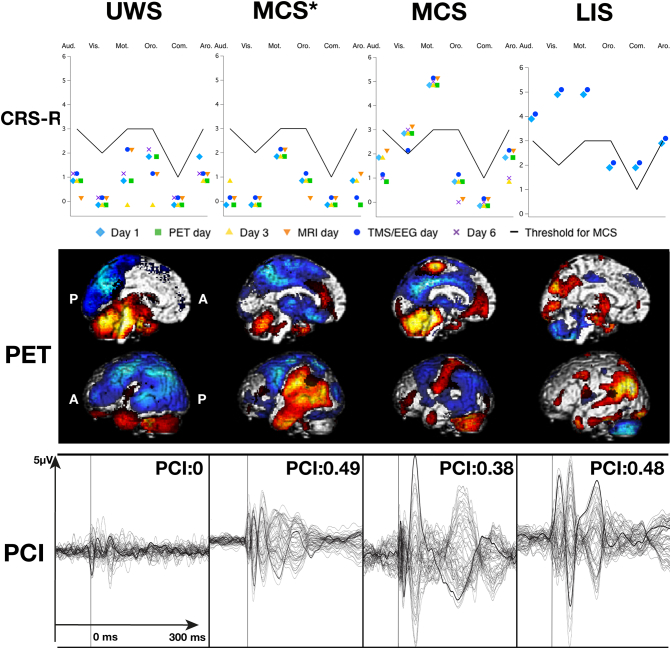
Fig. 3.
Behavioural, PCI, and FDG–PET results in the patients with incongruent results.
Top row illustrates the behavioural subscores of each assessment with the black line representing the threshold for MCS. The second row illustrates the areas in which FDG–PET finds significantly decreased (blue) or preserved (red) metabolism compared to 39 controls (p < 0.05). The third raw illustrates the TMS evoked potential traces at the cortical level, which are later used to compute the PCI. Despite being behaviourally unresponsive on all evaluations, and with a PCI < 0.31, patient UWS5's FDG–PET shows metabolism preservation of a large part of his right hemisphere. Patient MCS11 has some preserved metabolism in the fronto-parietal network, and is MCS− based on one visual pursuit, but the PCI remained < 0.31. FDG–PET images were created by merging the impaired and preserved metabolism maps from SPM8. UWS: unresponsive wakefulness syndrome, MCS*: non-behavioural minimally conscious state, MCS: minimally conscious state, Aud. Vis. Mot. Oro. Com. Aro. are the six CRS-R subscales, A: anterior, P: posterior, L: left, R: right.
One year after the evaluation, five patients had died, 15 remained stable, three were lost to follow up, and only one had improve from MCS* to MCS (Table 2).
4. Discussion
Out of our 24 patients, 22 have congruent FDG–PET and PCI results. The association of these two techniques shows the most concordance when compared to the combination of CRS-R with any or both of FDG–PET and PCI (Table 3). In four cases in which the patients could communicate (EMCS and LIS), both the FDG–PET and TMS–EEG results were compatible with the presence of consciousness: FDG-PET showed preserved metabolism in the fronto-parietal network, while TMS–EEG provided PCI above 0.31. Similarly, the FDG–PET and the PCI were compatible with the presence of consciousness in ten MCS patients. Finally, in four patients that were unambiguously UWS after repeated behavioural assessment, both the metabolic and electrophysiological results were in favour of the absence of consciousness. In these cases, PCI was always below 0.31 in patients whose FDG–PET could not identify preserved metabolism in the fronto-parietal network, suggesting that preserved metabolic rates are a minimal prerequisite for cortical circuits to engage in complex dynamics.
Table 3.
Concordance of CRS-R, PET, and PCI results.
Table 3 summarizes the number of cases in which each technique provided results compatible with consciousness or not (left part of the table). On the right part, we report the number of cases where all techniques, or any couple of two, shared results concurring with consciousness or unconsciousness. The association of FDG–PET and PCI is the one with the most concordance, with 22 out of 24 samples concurring.
| Test(s) in favour of … | CRS-R | PET | PCI | All | CRS-R PET | CRS-R PCI | PET PCI |
|---|---|---|---|---|---|---|---|
| Consciousness | 15 (62.5%) | 20 (83,3%) | 18 (75%) | 14 (58.3%) | 15 (62.5%) | 14 (58.3%) | 18 (75%) |
| Unconsciousness | 9 (37.5%) | 4 (16.7%) | 6 (25%) | 4 (16.7%) | 4 (16.7%) | 5 (20.8%) | 4 (16.7%) |
| Total | 24 (100%) | 24 (100%) | 24 (100%) | 18 (75%) | 19 (79.2%) | 19 (79.2%) | 22 (91.7%) |
Notably, four patients with otherwise unambiguous clinical diagnosis of UWS had preserved metabolic patterns and PCI levels typical of conscious conditions, suggesting that these patients were probably MCS*. Thus, preserved FDG–PET not only has prognostic value (Stender et al., 2014), but may index an actual, albeit covert, capacity for consciousness. A hypothesis further supported by the willful modulation of brain activity in our fMRI paradigm in one of these patients (Fig. 1). This also demonstrates that UWS patients with a high PCI do not reflect a lack of specificity of TMS–EEG, but really seem to be a specific population, with its own metabolic and electrophysiological characteristics. MCS* are likely to also share their own prognosis, given the results of previous studies showing they were more prone to recover behavioural signs of consciousness (Stender et al., 2014) and as the only patient in this cohort that recovered signs of consciousness was MCS* (Table 2). Adding the fact that PCI has very high sensitivity and specificity in healthy controls (Casarotto et al., 2016), we can safely support the hypothesis that both TMS–EEG and FDG–PET are able to detect non-behavioural signs of consciousness.
In two patients, FDG–PET and PCI provided different results. This is not surprising since these two techniques clearly index different aspects of neuronal functioning at distinct spatial and temporal scales. FDG–PET evaluates the metabolic activity in terms of patterns of preserved and impaired glucose uptake over many minutes, allows inferring on the level of neuronal activity across distributed cortical areas. On the other hand, PCI evaluates the electrophysiological complexity of cortico-cortical neuronal interactions over milliseconds, allowing computing effective connectivity between cortical areas, and fluctuates on a minute-by-minute base according to the subject's current state (Massimini et al., 2005). The two patients with incongruent results had preserved metabolic activity, while their PCI values were in the range of unconsciousness. The first patient was behaviourally unambiguously UWS, showed a conservation of the whole right hemisphere metabolism, whereas PCI computed across several sites at optimal intensity were always in the distribution range of unconsciousness. Over a five year long follow-up this patient remained behaviourally UWS. A parsimonious explanation of this result is that preserved metabolic rates may be necessary but not sufficient to recover complex interactions among cortical areas and consciousness. Hence, neurons that are still active may not be able to engage in complex network dynamics due to an insufficient level of connectivity or functional imbalances, such as sleep-like neuronal bistability induced by increased potassium conductance or by an altered excitation/inhibition balance (Massimini et al., 2012). The second patient only showed visual pursuit on one occasion, the day of the FDG–PET. The day of TMS–EEG, and the rest of the week, this patient was behaviourally UWS and showed no signs of consciousness. In this case PCI could be computed from only one session and was low. Assuming that this patient was conscious, the discrepancy between FDG–PET may be explained by the inability of TMS to effectively stimulate the cortex, or by a drop in the level of vigilance during the PCI measurement. Apart from fluctuation of consciousness levels, aetiology and comorbidities may be the source of divergent results. Patients with anoxic brain injuries may exhibit low cortical excitability, hindering the emergence of complex EEG potentials (Gosseries et al., 2015). Stimulating over brain lesions should be avoided, but in traumatic brain injuries, they may be so widespread that gathering artefact-free signal in TMS–EEG is impossible. Antiepileptic drugs also lower cortical excitability, and this should be taken into account while performing TMS–EEG. Diabetes, if treated with insulin, and any serious infection may artificially decrease the measure of brain metabolism in FDG–PET, and these conditions should be looked for before the acquisition.
Overall, these results, by showing a substantial concordance between FDG–PET and TMS-EEG results, provides a first cross-validation of two different techniques in assessing DOC patients and suggest that their complementarities could be exploited in the clinical setting in a two-step procedure (Fig. 4). In this scenario, patients diagnosed as UWS with repeated CRS-R should first be screened with FDG–PET, as it is easier to perform, well-standardized, and might be more sensitive than TMS–EEG. In patients showing preserved fronto-parietal metabolic activity, TMS–EEG could be performed, to assess the ability of cortical neurons to interact effectively and engage in complex pattern of distributed activity, which provides a more specific index of a capacity for consciousness. The use of these costly techniques should be limited to the patients for whom an accurate diagnostic might influence important clinical decisions, such as end-of-life issues. Moreover, we think that patients with traumatic, or at least non-anoxic, brain injuries might benefit more from these additional assessments, as they are more likely to exhibit non-behavioural signs of consciousness (e.g. Cruse et al., 2011, Monti et al., 2010, Stender et al., 2014). Although both FDG–PET and TMS–EEG are not readily available in the clinical setting and require specific expertise, their joint application may increase the sensitivity and specificity of our diagnostic process of DOC patients and provide relevant physiopathological insight. Indeed, early identification of MCS* could lead to the planning of new assessments and increased attention toward the identification of the emergence of subtle signs of consciousness, and could change the rehabilitation program these patients undergo. Nevertheless, one of our MCS* patient could be identified several years after the brain injury, and could also benefit from this more accurate diagnosis. Moreover, the joint assessment of metabolic activity and electrophysiological complexity of cortical circuits may help guiding pharmaceutical or electrophysiological trials of consciousness promoting agents, such as amantadine (Giacino et al., 2012), zolpidem (Whyte and Myers, 2009), transcranial direct current stimulation (Thibaut et al., 2014) or deep brain stimulation (Schiff et al., 2007).
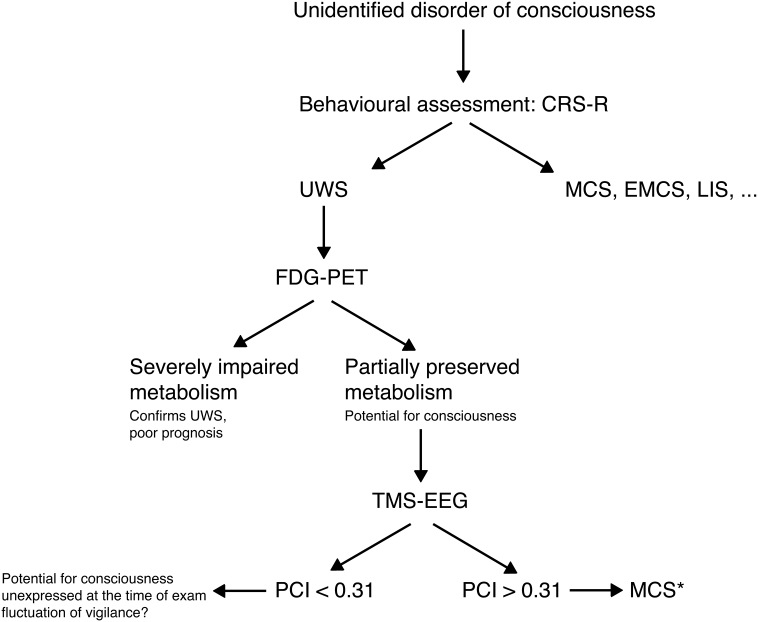
Fig. 4.
Proposed screening algorithm.
Patients with unidentified DOC should be first assessed using validated standardized behavioural scale, such as the CRS-R. If no signs of consciousness can be detected, potential for consciousness can be identified using 18FDG-PET. In case this exam shows at least partial preservation of the fronto-parietal network metabolism, TMS-EEG could be used to detect the presence of covert consciousness. This would be the case if the PCI was above the distribution found in unconsciousness (> 0.31).
5. Conclusion
For the first time, metabolic and complexity measures of the brain are studied in the same challenging population of patients with disorders of consciousness. Our results offer a cross validation of these two techniques, despite their very different characteristics and own specificities. This allows us to suggest the use of both FDG-PET and TMS-EEG to improve our diagnostic accuracy in the clinical setting. Moreover, we demonstrated that they could both detect non-behavioural minimally conscious patients, and that UWS patients showing high level of complexity in TMS–EEG did not reflect a lack of specificity of this technique. This also has an important potential clinical impact, as these patients seem to have a better prognosis than patients with unresponsive wakefulness syndrome and thus require increased attention toward potential appearance of subtle signs of consciousness, and more efforts in rehabilitation centre to allow these signs to emerge.
Funding
OB is a research fellow, OG a post-doctoral fellow and SL a research director at the national fund for scientific research (FNRS).
This research was supported by the Belgian National Fund for Scientific Research (FNRS), Human Brain Project (EU-H2020-FETFLAGSHIP-HBP-SGA1-GA720270), Luminous project (EU-H2020-FETOPEN-GA686764), the Belgian American Education Foundation, the Wallonie-Bruxelles International, the European Commission, the Fonds Léon Fredericq, the James S. McDonnell Foundation, the Mind Science Foundation, the French Speaking Community Concerted Research Action (ARC-06/11-340), and the University and University Hospital of Liège.
Competing financial interests
The authors do not report any conflict of interest.
Acknowledgements
The authors would like to thank their colleagues C. Aussems, M-A. Bruno, J-F. Tshibanda, R. Hustinx, and C. Bernard for their help in data acquisition, and the patients and their families for their precious participation to this study.
References
- Bardin J.C., Fins J.J., Katz D.I., Hersh J., Heier L.A., Tabelow K., Dyke J.P., Ballon D., Schiff N.D., Voss H.U. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain. 2011;134:769–782. doi: 10.1093/brain/awr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin J.C., Schiff N.D., Voss H.U. Pattern classification of volitional functional magnetic resonance imaging responses in patients with severe brain injury. Arch. Neurol. 2012;69:176–181. doi: 10.1001/archneurol.2011.892. [DOI] [PubMed] [Google Scholar]
- Bodart O., Laureys S., Gosseries O. Coma and disorders of consciousness: scientific advances and practical considerations for clinicians. Semin. Neurol. 2013;33:83–90. doi: 10.1055/s-0033-1348965. [DOI] [PubMed] [Google Scholar]
- Casali A.G., Gosseries O., Rosanova M., Boly M., Sarasso S., Casali K.R., Casarotto S., Bruno M.-A., Laureys S., Tononi G., Massimini M. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013;5:1–10. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
- Casarotto S., Romero Lauro L.J., Bellina V., Casali A.G., Rosanova M., Pigorini A., Defendi S., Mariotti M., Massimini M. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S., Comanducci A., Rosanova M., Sarasso S., Fecchio M., Napolitani M., Pigorini A., Casali A.G., Trimarchi P., Boly M., Gosseries O., Bodart O., Curto F., Landi C., Mariotti M., Devalle G., Laureys S., Tononi G., Massimini M. Stratification of unresponsive patients by an independantly validated index of brain complexity. Ann. Neurol. 2016 doi: 10.1002/ana.24779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse D., Chennu S., Chatelle C., Bekinschtein T.A., Fernandez-Espejo D., Pickard J.D., Laureys S., Owen A.M. Bedside detection of awareness in the vegetative state: a cohort study. Lancet. 2011;378:2088–2094. doi: 10.1016/S0140-6736(11)61224-5. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F., Massimini M., Sarasso S., Casali A.G., Riedner B.A., Angelini G., Tononi G., Pearce R.A. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner I.S., Bodart O., Laureys S., Demertzi A. Our rapidly changing understanding of acute and chronic disorders of consciousness: challenges for neurologists. Future Neurol. 2012;8:43–54. [Google Scholar]
- Giacino J.T., Ashwal S., Childs N., Cranford R., Jennett B., Katz D.I., Kelly J.P., Rosenberg J.H., Whyte J., Zafonte R.D., Zasler N.D. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Kalmar K., Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Whyte J., Bagiella E., Kalmar K., Childs N., Khademi A., Eifert B., Long D., Katz D.I., Cho S., Yablon S.A., Luther M., Hammond F.M., Nordenbo A., Novak P., Mercer W., Maurer-Karattup P., Sherer M. Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 2012;366:819–826. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Fins J.J., Laureys S., Schiff N.D. Disorders of consciousness after acquired brain injury: the state of the science. Nat. Rev. Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- Goldfine A.M., Victor J.D., Conte M.M., Bardin J.C., Schiff N.D. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin. Neurophysiol. 2011;122:2157–2168. doi: 10.1016/j.clinph.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosseries O., Zasler N.D., Laureys S. Recent advances in disorders of consciousness: focus on the diagnosis. Brain Inj. 2014;28:1141–1150. doi: 10.3109/02699052.2014.920522. [DOI] [PubMed] [Google Scholar]
- Gosseries O., Sarasso S., Casarotto S., Boly M., Schnakers C., Napolitani M., Bruno M.-A., Ledoux D., Tshibanda J.F., Massimini M., Laureys S., Rosanova M. On the cerebral origin of EEG responses to TMS: insights from severe cortical lesions. Brain Stimul. 2015;8:142–149. doi: 10.1016/j.brs.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi R.J., Virtanen J., Ruohonen J., Karhu J., Aronen H.J., Naatanen R., Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Laureys S., Goldman S., Phillips C., Van Bogaert P., Aerts J., Luxen A., Franck G., Maquet P. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. NeuroImage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- Laureys S., Owen A.M., Schiff N.D. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Massimini M., Ferrarelli F., Huber R., Esser S.K., Singh H., Tononi G. Breakdown of cortical effective connectivity during sleep. Science (80-) 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Massimini M., Boly M., Casali A.G., Rosanova M., Tononi G. A perturbational approach for evaluating the brain's capacity for consciousness. Prog. Brain Res. 2009;177:201–214. doi: 10.1016/S0079-6123(09)17714-2. [DOI] [PubMed] [Google Scholar]
- Massimini M., Ferrarelli F., Murphy M., Huber R., Riedner B., Casarotto S., Tononi G. Cortical reactivity and effective connectivity during REM sleep in humans. Cogn. Neurosci. 2010;1:176–183. doi: 10.1080/17588921003731578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M., Ferrarelli F., Sarasso S., Tononi G. Cortical mechanisms of loss of consciousness: insight from TMS/EEG studies. Arch. Ital. Biol. 2012;150:44–55. doi: 10.4449/aib.v150i2.1361. [DOI] [PubMed] [Google Scholar]
- Monti M.M., Vanhaudenhuyse A., Coleman M.R., Boly M., Pickard J.D., Tshibanda L., Owen A.M., Laureys S. Willful modulation of brain activity in disorders of consciousness. N. Engl. J. Med. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Okumura A., Shinoda J., Nakashima T., Iwama T. Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: an FDG-PET study with statistical parametric mapping analysis. J. Neurol. Neurosurg. Psychiatry. 2006;77:856–862. doi: 10.1136/jnnp.2005.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M., Coleman M.R., Boly M., Davis M.H., Laureys S., Pickard J.D. Detecting awareness in the vegetative state. Science (80–) 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Rosanova M., Gosseries O., Casarotto S., Boly M., Casali A.G., Bruno M.-A., Mariotti M., Boveroux P., Tononi G., Laureys S., Massimini M. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain. 2012;135:1308–1320. doi: 10.1093/brain/awr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasso S., Boly M., Napolitani M., Gosseries O., Charland-verville V., Casarotto S., Rosanova M., Casali A.G., Brichant J.-F., Boveroux P., Rex S., Tononi G., Laureys S., Massimini M. Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr. Biol. 2015;25:1–7. doi: 10.1016/j.cub.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Schiff N.D., Fins J.J. Brain death and disorders of consciousness. Curr. Biol. 2016;26:R572–R576. doi: 10.1016/j.cub.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Schiff N.D., Giacino J.T., Kalmar K., Victor J.D., Baker K., Gerber M., Fritz B., Eisenberg B., Biondi T., O'Connor J., Kobylarz E.J., Farris S., Machado A., McCagg C., Plum F., Fins J.J., Rezai A.R. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Seel R.T., Sherer M., Whyte J., Katz D.I., Giacino J.T., Rosenbaum A.M., Hammond F.M., Kalmar K., Pape T.L., Zafonte R.D., Biester R.C., Kaelin D., Kean J., Zasler N.D. Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research. Arch. Phys. Med. Rehabil. 2010;91:1795–1813. doi: 10.1016/j.apmr.2010.07.218. [DOI] [PubMed] [Google Scholar]
- Stender J., Gosseries O., Bruno M.-A., Charland-Verville V., Vanhaudenhuyse A., Demertzi A., Chatelle C., Thonnard M., Thibaut A., Heine L., Soddu A., Boly M., Schnakers C., Gjedde A., Laureys S. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet. 2014;6736:8–16. doi: 10.1016/S0140-6736(14)60042-8. [DOI] [PubMed] [Google Scholar]
- Stender J., Mortensen K.N.N., Thibaut A., Darkner S., Laureys S., Gjedde A., Kupers R. The minimal energetic requirement of sustained awareness after brain injury. Curr. Biol. 2016;26:1494–1499. doi: 10.1016/j.cub.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Thibaut A., Bruno M.-A., Chatelle C., Gosseries O., Vanhaudenhuyse A., Demertzi A., Schnakers C., Thonnard M., Charland-Verville V., Bernard C., Bahri M., Phillips C., Boly M., Hustinx R., Laureys S. Metabolic activity in external and internal awareness networks in severely brain-damaged patients. J. Rehabil. Med. 2012;44:487–494. doi: 10.2340/16501977-0940. [DOI] [PubMed] [Google Scholar]
- Thibaut A., Bruno M.-A., Ledoux D., Demertzi A., Laureys S. tDCS in patients with disorders of consciousness. Neurology. 2014;82:1–7. doi: 10.1212/WNL.0000000000000260. [DOI] [PubMed] [Google Scholar]
- Whyte J., Myers R. Incidence of clinically significant responses to zolpidem among patients with disorders of consciousness: a preliminary placebo controlled trial. Am. J. Phys. Med. Rehabil. 2009;88:410–418. doi: 10.1097/PHM.0b013e3181a0e3a0. [DOI] [PubMed] [Google Scholar]