Abstract
The invasive spotted wing drosophila Drosophila suzukii, a fruit fly of Asian origin, is a major pest of a wide variety of berry and stone fruits in Europe. One of the characteristics of this fly is its wide host range. A better knowledge of its host range outside cultivated areas is essential to develop sustainable integrated pest management strategies. Field surveys were carried out during two years in Italy, the Netherlands and Switzerland. Fruits of 165 potential host plant species were collected, including mostly wild and ornamental plants. Over 24,000 D. suzukii adults emerged from 84 plant species belonging to 19 families, 38 of which being non-native. Forty-two plants were reported for the first time as hosts of D. suzukii. The highest infestations were found in fruits of the genera Cornus, Prunus, Rubus, Sambucus and Vaccinium as well as in Ficus carica, Frangula alnus, Phytolacca americana and Taxus baccata. Based on these data, management methods are suggested. Ornamental and hedge plants in the vicinity of fruit crops and orchards can be selected according to their susceptibility to D. suzukii. However, the widespread availability and abundance of non-crop hosts and the lack of efficient native parasitoids suggest the need for an area-wide control approach.
Keywords: Spotted wing drosophila, Fruit fly, Host range, Invasive species
Key message
Drosophila suzukii a fruit fly of Asian origin has a broad host range. Field surveys in Europe identified more than 80 host plants including wild ornamental and crop plants.
Knowing the host range of D. suzukii outside cultivated habitats is essential for the development of sustainable IPM strategies.
The widespread availability of non-crop hosts and the lack of efficient parasitoids suggests the need for an area-wide control approach.
Introduction
The spotted wing drosophila, Drosophila suzukii Matsumura (Diptera: Drosophilidae), is a fruit fly of East-Asian origin that rapidly invaded other parts of the world in the late 2000s (Cini et al. 2012; Deprá et al. 2014; Asplen et al. 2015). In contrast to most other Drosophila spp. that develop only on overripe or decaying fruits, D. suzukii is able to oviposit in ripe fruits due to the female’s prominent serrated ovipositor (Lee et al. 2011; Walsh et al. 2011). Since its first notification in 2008 in Europe, it has rapidly spread to most suitable areas of the continent (Cini et al. 2014), becoming a major pest of a wide variety of berry and stone fruit crops (Asplen et al. 2015).
Before becoming a worldwide invasive species, D. suzukii was considered a relatively minor pest in its area of origin (Asplen et al. 2015; Haye et al. 2016) and, therefore, efficient management techniques were not available. However, research on management methods has been carried out recently in various parts of the world (Haye et al. 2016). Drosophila suzukii shows at least three important biological characteristics that may strongly influence the development of integrated management methods. Firstly, D. suzukii is poorly attacked by natural enemies in the invasion range (Haye et al. 2016). In particular, larval parasitism is almost never observed, while Drosophila spp. larvae are usually heavily parasitised by braconid and figitid wasps (Carton et al. 1986). Laboratory assays suggest that European and American larval parasitoids are not able to develop on D. suzukii, apparently because of the strong host immune response of the invasive fly against these parasitoids (Chabert et al. 2012; Kacsoh and Schlenke 2012; Poyet et al. 2013). Secondly, its fast development (ca. two weeks to develop from egg to adult at 22 °C (Tochen et al. 2014) allows it to produce many generations per year between spring and autumn. In the temperate climate of Oregon, Western USA, Tochen et al. (2014) estimated that D. suzukii undergoes an average of 7.1 generations per year, but up to 13 generations per year have been cited for warmer climates (Asplen et al. 2015); in temperate climates, the winter is spent as adults in reproductive diapause (Zerulla et al. 2015). Thirdly, D. suzukii has a very broad host range, including fruits of many wild and ornamental host plants (Lee et al. 2015; Poyet et al. 2015), which allow it to move regularly from cultivated to wild and urban habitats. These characteristics imply that the potential development of the fly in wild and ornamental fruits in the vicinity of orchards and fruit fields has an important impact on the level of attack in cultivated fruits.
For obvious reasons, the host range of D. suzukii among cultivated fruits has been assessed extensively (e.g. Mitsui et al. 2010; Lee et al. 2011; Bellamy et al. 2013; Burrack et al. 2013) whereas less emphasis has been placed on non-cultivated hosts. Very recently, however, wild and ornamental non-crop hosts have been studied in Michigan and Oregon (USA) by Lee et al. (2015) who found D. suzukii in 24 field-collected plant species belonging to 12 families. They also made additional assessments of host suitability in laboratory tests and provided a literature review on the host range of the fly worldwide. In Europe, the most extensive host range study is that of Poyet et al. (2015). They tested, in the laboratory, D. suzukii on 67 fruit species collected in Northern France and found out that D. suzukii laid eggs on 50 of them and successfully developed in 33, belonging to 15 families. However, there have been discrepancies between host range data gathered from field surveys and laboratory tests in North America (Lee et al. 2015) and, so far, no extensive field survey was carried out to assess the realised host range of D. suzukii in non-crop hosts in Europe.
The main objective of this study was to assess the host range realised by D. suzukii outside cultivated areas in Western and Central Europe. For this, surveys were carried out during two years in the Netherlands, Northern Italy and Switzerland to collect fruits in semi-natural and urban landscapes and rear out D. suzukii. Attempts were made to classify the host fruits according to the frequency and level of infestation.
Materials and methods
Potential host fruits, i.e. fruits that appeared sufficiently soft to allow the oviposition and development of D. suzukii, were collected through regular surveys in 2014 and 2015 at various sites in three countries: Italy, the Netherlands and Switzerland. In all three countries, sites were distant from each other’s by at least one km. All sampling regions had been infested by D. suzukii at least since 2013, and the presence of D. suzukii in the areas during the sampling periods was confirmed by trapping campaigns for monitoring adult populations. The survey focused on ripe fruits of wild and ornamental non-crop hosts in various habitats, i.e. forests, forest edges, meadows, hedges in agricultural habitats, gardens and parks, etc. In a few cases, fruit trees planted as urban or garden trees at the surveyed sites were also sampled. Plants were identified using local reference guides (Pignatti 1982; Meijden 1996; Ferrari and Medici 2008; Koning and Broek 2012; Info Flora 2015). Sampling techniques were rather similar in the three countries but with some differences. Therefore, they are described separately below.
Italy
Twenty-nine sites in semi-natural habitats located in seven different areas in North-eastern Italy (Veneto and Trentino Regions) were sampled every two weeks from March 2014 to October 2015. The fruits were collected when available from all potential host plants. Moreover, occasional collections were made in various landscapes in Liguria, Toscana and Veneto Region wherever new fruit species were found. Fruit were sampled from a total of 116 plant species. When possible, samples consisted in 2 dl of small fruits or 50 individuals of large fruits, but smaller amount of less abundant fruits were also collected. For each sample, the number of fruits was counted and their weight was measured. Fruits were then stored in containers, covered with fine mesh and kept at 23 °C. Emerging insects were collected three times a week and lasted three weeks after the last emergence of D. suzukii. Flies were stored in ethanol for later identification.
Netherlands
Three areas were selected in the centre of the Netherlands. The areas differed in respect to soil and vegetation type. The first was in the orchard dominated river clay area in Gelderland province. The second was in a semi-urban area in the Utrecht province, where river clay meets the sandy Pleistocene soils. The third was in forests and at forest edges on the sandy Pleistocene soils in Gelderland province. At each area, surveys were made at three sites of 0.5 ha each. The vegetation at each of the nine sites was sampled eight times from June to October 2014. Additionally, a large sampling effort was made on December 4, 2014, to determine whether D. suzukii could overwinter as a larva in fruits. In 2015, surveys were carried out between May and October at the same sites. At each sampling date, fruits were picked from all potential host plants. Occasional collections were also made in the region, wherever new potential host plant species were found. Fruits of 34 plant species were collected in 2014 and 68 in 2015. In total, 77 different plant species were sampled. When possible, samples consisted of ca. 50 fruits, but smaller numbers of less abundant fruits, or larger numbers of abundant but small fruits were also collected. After weighing, fruits were put in containers, covered with fine mesh and kept at 22 °C. Emerging insects were collected three times a week until three weeks after the last emergence of D. suzukii and stored in ethanol for later identification.
Switzerland
Collections were carried out only in 2014, mainly in the Canton Ticino, in the Southern Alps. Fruits of a variety of potential host species were collected at ten sites, once per month, from early May to early October 2014. Additional collections were made along elevation gradients in the Ticino, once in July and once in August 2014. Some collections were also made in the Jura Canton, Northwestern Switzerland. A total of 39 plant species were sampled. When possible, samples consisted of ca. 50 fruits, but smaller numbers of less abundant fruits, or larger numbers of abundant but small fruits were also collected. For each sample, the number of collected fruits was recorded. Fruits were then placed in photo-eclectors made of a cardboard cylinder surrounded by a funnel ending in a translucent plastic cup, in which the flies were collected daily until three weeks after the last emergence of D. suzukii. They were then killed in ethanol to allow a careful counting of the number of D. suzukii adults. After the emergence period, the cylinders were inspected to count the few flies that had died without reaching the cup.
Data analyses
Two parameters were calculated: the rate of occurrence and the infestation level. The rate of occurrence expressed the geographical frequency at which D. suzukii was found on a particular fruit species, without taking into account the level of attack at specific sites. It was calculated as the ratio between the number of sites × years (throughout all three countries) where a fruit was found attacked by D. suzukii divided by all sites × years combinations where the fruit was collected.
To allow a comparison of the infestation level among host species, the number of flies emerging per individual fruit is not a very good parameter because fruit size strongly varies among species. Instead, the number of flies should be expressed per fruit weight, volume or skin surface. In Italy and Switzerland, all fruits were counted but the size and weight of fruits could not be measured for all samples. Thus, for each fruit species collected in Italy and Switzerland, the average diameter was gathered in the literature, mainly in Info Flora (2015) and, if not indicated, an average of the average data found in various information sources (other books on regional flora and web sites from scientific societies and organisations) was calculated. In case of oval fruits, the length and the width were averaged. The fruit surface was estimated for each species (surface = 4πr 2). Aggregate fruits composed of drupelets, e.g. Rubus spp., were treated in the same way, although we realise that, for these fruits, the surface was underestimated. Then, for each sample, a level of infestation was expressed as the number of D. suzukii adults emerged per dm2 of fruit surface. In the Netherlands, the number of fruits was not counted but, instead, samples were weighed. Thus, for these samples, the level of infestation was expressed as the number of D. suzukii adults per kg of fruit. We realise that none of these two parameters are perfect. The fruit surface is probably a better expression of the potential of the fruit to attract D. suzukii and to support the development of a certain quantity of larvae than its weight or its volume. On the other hand, for some species, the size of the sampled fruits may be rather different from the average size found in the literature. Furthermore, if the fruit is very small in size individually, the fruit surface may not matter as much, and having other measures might be useful. But the aim of this parameter was not to finely compare fruit species but rather to broadly categorise the infestation levels of host fruits in the field. For a finer comparison of infestation levels, several confounding factors such as time of collection, habitat, fruit density and population size of the flies would have to be taken into account. For the same reasons, the infestation levels were not statistically tested. Only data from the years with the most abundant collections were considered for the calculation of the infestation levels, i.e. 2014 for Italy and Switzerland, and 2015 for the Netherlands.
Results
Fruits from a total of 165 plant species were collected in the three countries, providing 24,165 D. suzukii adults, 4153 in Italy, 15,527 in the Netherlands and 4485 in Switzerland. The list of the plant species from which D. suzukii emerged is provided in Table 1, with quantitative information on the sampling and emergence. The plant species from which no D. suzukii emerged are listed in Table 2. In total, 84 plant species from 19 families gave rise to adult emergence, 39 species in Italy, 52 in the Netherlands and 24 in Switzerland. Forty-two of these are recorded for the first time as hosts of D. suzukii in the field, of which six had already been found to be suitable for larval development in laboratory studies (Baroffio et al. 2014; Lee et al. 2015; Poyet et al. 2015) (Table 1). Thirty-eight host species are not native to any of the three investigated countries. Fifty are commonly found in the wild in at least one of the three regions, 53 are commonly planted as ornamental and 16 are commonly cultivated fruits.
Table 1.
Fruit species from which D. suzukii adults emerged, in Italy (IT), the Netherlands (NL) and Switzerland (CH: Ticino TI; Jura JU), in 2014 and 2015
| Species (family) | New host recorda | Main habitat/purposeb | Native/Exoticc | IT 2014 | IT 2015 | NL 2014 | NL 2015 | CH-TI 2014 | CH-JU 2014 |
|---|---|---|---|---|---|---|---|---|---|
| Actinidia chinensis Planch. (Actinidiaceae) | F | E | 1/1 | ||||||
| Amelanchier lamarckii F.G. Schr. (Rosaceae) | ✓ | O/W | E | 0/2 | 1/2 | ||||
| Amelanchier ovalis Medik. (Rosaceae) | ✓ | W/O | N | 1/2 | |||||
| Arbutus unedo L. (Ericaceae) | W/O | N | 1/2 | 0/1 | |||||
| Arum italicum Mill. (Araceae) | ✓ | O/W | N | 0/1 | 0/2 | 1/1 | |||
| Cornus alba L. (Cornaceae) | ✓ | O | E | 1/1 | |||||
| Cornus kousa Hance (Cornaceae) | O | E | 1/1 | ||||||
| Cornus mas L (Cornaceae) | W/O | N | 1/3 | 1/1 | 1/1 | 1/1 | |||
| Cornus sanguinea L. (Cornaceae) | W/O | N | 2/4 | 0/1 | 2/4 | 2/2 | 0/6 | 0/3 | |
| Cotoneaster franchetii Boiss. (Rosaceae) | ✓ | O | E | 1/1 | |||||
| Cotoneaster lacteus W.W. Smith (Rosaceae) | O | E | 1/2 | 0/1 | |||||
| Cotoneaster rehderi Pojark. (Rosaceae) | ✓ | O | E | 1/1 | |||||
| Crataegus chrysocarpa Ashe (Rosaceae) | ✓ | O | E | 1/1 | |||||
| Crataegus monogyna Jacq. (Rosaceae) | ✓ | W/O | N | 0/4 | 0/1 | 2/6 | 0/1 | 0/2 | |
| Daphne mezereum L. (Thymelaeaceae) | ✓ | W | N | 1/2 | 0/1 | ||||
| Duchesnea indica (Andr.) Focke (Rosaceae) | Lab | O/W | E | 1/2 | 0/1 | 3/3 | 2/5 | ||
| Eriobotrya japonica (Thunb.) Lindl. (Rosac.) | O | E | 1/2 | ||||||
| Ficus carica (L.) (Moraceae) | F | N | 1/3 | 3/3 | |||||
| Fragaria vesca L. (Rosaceae) | W | N | 0/1 | 1/1 | 1/1 | 9/22 | |||
| Frangula alnus Mill. (Rhamnaceae) | W | N | 3/3 | 2/2 | 1/1 | 3/3 | |||
| Gaultheria x wisleyensis M.&M. (Ericaceae) | ✓ | O | E | 1/1 | |||||
| Hippophae rhamnoides L. (Elaeagnaceae) | W/F | N | 0/1 | 1/1 | |||||
| Lonicera alpigena L. (Caprifoliaceae) | W | N | 2/3 | 2/3 | |||||
| Lonicera caerulea L. (Caprifoliaceae) | W | N | 1/1 | 1/1 | |||||
| Lonicera caprifolium L. (Caprifoliaceae) | ✓ | W/O | N | 0/1 | 2/2 | ||||
| Lonicera ferdinandii Franch. (Caprifoliaceae) | ✓ | O | E | 1/1 | |||||
| Lonicera nigra L. (Caprifoliaceae) | W | N | 1/2 | 2/2 | |||||
| Lonicera nitida E. H. Wilson (Caprifoliaceae) | Lab | O | E | 2/2 | |||||
| Lonicera sp (Caprifoliaceae) | 6/8 | ||||||||
| Lonicera xylosteum L. (Caprifoliaceae) | W | N | 1/3 | 1/4 | |||||
| Lycium barbarum L. (Solanaceae) | ✓ | O/F/W | N | 1/2 | |||||
| Mahonia aquifolium (Pursh) Nutt. (Berberid.) | Lab | O | E | 0/1 | 1/5 | ||||
| Mahonia sp. (Berberidaceae) | O | E | 0/1 | 1/1 | |||||
| Malus baccata Borkh. (Rosaceae) | ✓ | O | E | 1/1 | |||||
| Paris quadrifolia L. (Melanthiaceae) | ✓ | O | N | 0/3 | 0/1 | 1/1 | |||
| Parthenocissus quinquefolia (L.) (Vitaceae) | O | E | 0/2 | 2/2 | |||||
| Photinia beauverdiana C. K. Schn. (Rosaceae) | ✓ | O | E | 1/1 | |||||
| Photinia villosa (Thunb.) DC. (Rosaceae) | ✓ | O | E | 1/1 | |||||
| Photinia prunifolia Lindl. (Rosaceae) | ✓ | O | E | 1/1 | |||||
| Phytolacca americana L. (Phytolaccaceae) | O/W | E | 4/4 | 0/1 | 0/1 | 5/5 | |||
| Phytolacca esculenta Van Houtte (Phytolacc.) | ✓ | O/W | E | 1/1 | |||||
| Polygonatum multiflorum (L.) All. (Liliaceae) | ✓ | W | N | 1/1 | 0/1 | ||||
| Prunus armeniaca L. (Rosaceae) | F | E | 1/1 | ||||||
| Prunus avium (L.) (Rosaceae) | W/F/O | N | 0/2 | 1/2 | 5/10 | ||||
| Prunus cerasifera Ehrh. (Rosaceae) | O/W | E | 1/1 | ||||||
| Prunus cerasus L. (Rosaceae) | F/W | N | 1/1 | 0/1 | |||||
| Prunus domestica L. (Rosaceae) | F | E | 2/2 | 0/1 | |||||
| Prunus laurocerasus L. (Rosaceae) | O/W | E | 1/1 | 0/1 | 1/1 | 1/1 | 2/2 | ||
| Prunus lusitanica L. (Rosaceae) | O | E | 1/1 | ||||||
| Prunus mahaleb L. (Rosaceae) | W/O | N | 2/3 | 1/3 | |||||
| Prunus padus L. (Rosaceae) | Lab | W/O | N | 0/1 | 1/1 | 1/1 | |||
| Prunus serotina Ehrhart (Rosaceae) | W | E | 1/2 | 1/1 | |||||
| Prunus spinosa L. (Rosaceae) | Lab | W/O | N | 1/5 | 0/2 | 2/5 | 2/3 | 0/1 | |
| Pyracantha sp. (Rosaceae) | O | E | 1/1 | ||||||
| Rhamnus cathartica L. (Rhamnaceae) | W | N | 2/2 | ||||||
| Ribes rubrum L. (Rosaceae) | Lab | F | N | 0/1 | 2/3 | 0/1 | |||
| Rosa acicularis Lindl. (Rosaceae) | ✓ | O | E | 1/1 | |||||
| Rosa canina L. (Rosaceae) | ✓ | W/O | N | 0/7 | 0/5 | 3/5 | 0/2 | ||
| Rosa glauca Pourr. (Rosaceae) | ✓ | O/W | N | 1/1 | |||||
| Rosa pimpinellifolia L. (Rosaceae) | ✓ | O/W | N | 1/1 | |||||
| Rosa rugosa Thunb. (Rosaceae) | ✓ | W/O | E | 0/1 | 3/3 | ||||
| Rubus caesius L. (Rosaceae) | ✓ | W | N | 1/2 | 3/3 | ||||
| Rubus fruticosus aggr. (Rosaceae) | W/F | N | 4/5 | 8/9 | 6/6 | 29/32d | 5/7 | ||
| Rubus idaeus L. (Rosaceae) | W/F | N | 2/2 | 0/3 | 1/2 | 12/16 | |||
| Rubus phoenicolasius Maxim. (Rosaceae) | ✓ | F | E | 2/2 | |||||
| Rubus saxatilis L. (Rosaceae) | ✓ | W | N | 2/2 | 0/1 | ||||
| Sambucus ebulus L. (Adoxaceae) | W | N | 1/2 | ||||||
| Sambucus nigra L. (Adoxaceae) | W | N | 2/3 | 0/2 | 33/34 | 4/4 | 5/8 | 2/3 | |
| Sambucus racemosa L. (Adoxaceae) | W/O | N | 1/3 | 5/6 | 0/3 | 1/1 | 4/5 | ||
| Solanum dulcamara L. (Solanaceae) | W | N | 0/3 | 0/1 | 0/2 | 3/4 | 1/6 | ||
| Solanum nigrum L. (Solanaceae) | W | N | 0/4 | 1/4 | 1/1 | ||||
| Sorbus aria (L.) (Rosaceae) | ✓ | W/O | N | 1/3 | 0/1 | ||||
| Sorbus aucuparia L. (Rosaceae) | ✓ | W/O | N | 0/4 | 0/1 | 1/4 | 0/1 | ||
| Symphoricarpos albus (L.) (Caprifoliaceae) | O/W | E | 0/1 | 2/3 | 0/1 | 0/1 | |||
| Tamus communis L. (Dioscoreaceae) | ✓ | W | N | 2/4 | 2/3 | ||||
| Taxus baccata L. (Taxaceae) | O/W | N | 2/3 | 0/1 | 1/1 | 1/1 | |||
| Vaccinium myrtilloides Michx. (Ericaceae) | ✓ | F/O | E | 1/1 | |||||
| Vaccinium myrtillus L. (Ericaceae) | W/F | N | 1/1 | 1/1 | |||||
| Vaccinium oldhamii Miquel. (Ericaceae) | ✓ | O/F | E | 1/1 | |||||
| Vaccinium praestans Lamb. (Ericaceae) | ✓ | O | E | 1/1 | |||||
| Vaccinium vitis-idea L. (Ericaceae) | O | E | 0/1 | 1/1 | |||||
| Viburnum lantana L. (Adoxaceae) | W/O | N | 0/1 | 1/3 | 0/1 | ||||
| Viburnum rhytidophyllum Hemsl. (Adoxaceae) | ✓ | O | E | 1/1 | |||||
| Vitis vinifera L. (Vitaceae) | F | N | 1/1 | 0/3 |
a/b: a number of sites where D. suzukii was obtained; b number of sites were the fruit was collected
aNew host record: ✓ = species not yet reported in the literature as host in the field, based on Cini et al. (2012), Baroffio et al. (2014), Asplen et al. (2015) and Lee et al. 2015; Lab species not yet found as host in the field but suitable host in laboratory tests in Baroffio et al. (2014), Lee et al. (2015) or Poyet et al. (2015)
bMain habitat/purpose: W commonly found in the wild in at least one of the three regions, O commonly planted as ornamental, F commonly planted as fruit crop; minor habitats/purposes are not indicated
cNative (N) = native in at least one of the investigated regions; Exotic (E) exotic in the three regions
dIn Ticino, Rubus fruticosus aggr. may have also included Rubus caesius
Table 2.
Fruit species from which no D. suzukii adults emerged, in Italy (IT), the Netherlands (NL) and Switzerland (CH: Ticino TI; Jura JU), in 2014 and 2015, with the number of sites sampled
| Species (family) | Known host of D. suzukii 1 | Number of sites where fruits were sampled | |||||
|---|---|---|---|---|---|---|---|
| IT 2014 | IT 2015 | NL 2014 | NL 2015 | CH-TI 2014 | CH-JU 2014 | ||
| Actaea spicata L. (Ranunculaceae) | 2 | 1 | 3 | ||||
| Ampelopsis brevipedunculata (Max.) Tr. (Vitaceae) | 1 | ||||||
| Aronia x prunifolia (Rosaceae) | 1 | ||||||
| Asparagus acutifolius L. (Asparagaceae) | 1 | ||||||
| Asparagus officinalis L. (Asparagaceae) | 1 | ||||||
| Atropa bella-donna L. (Solanaceae) | Lab | 1 | |||||
| Aucuba japonica Thunberg (Garryaceae) | Field | 1 | 2 | ||||
| Berberis x media (Berberidaceae) | 1 | ||||||
| Berberis vulgaris L. (Berberidaceae) | 3 | 3 | |||||
| Berberis sp. (Berberidaceae) | 2 | ||||||
| Bryonia dioica Jacq. (Cucurbitaceae) | 1 | 1 | |||||
| Callicarpa bodinieri H. Lév. (Lamiaceae) | 1 | 1 | |||||
| Cephalotaxus harringtonia (K. ex F.) Koch (Cephalotaxaceae) | 1 | ||||||
| Chamaerops sp. (Arecaceae) | 1 | ||||||
| Convallaria majalis L. (Nolinoideae) | 2 | ||||||
| Cotoneaster acutifolius Turcz. (Rosaceae) | 1 | ||||||
| Cotoneaster dammeri C. K. Schneid. (Rosaceae) | 1 | ||||||
| Cotoneaster horizontalis Decne. (Rosaceae) | Field | 1 | 1 | 1 | |||
| Cotoneaster microphyllus Wall. ex Lindl. (Rosaceae) | 2 | ||||||
| Cotoneaster salicifolius Franch. (Rosaceae) | 1 | 2 | |||||
| Cotoneaster suecicus G.Klotz (Rosaceae) | 1 | ||||||
| Cotoneaster × watereri Exell (Rosaceae) | 1 | ||||||
| Crataegus azarolus L. (Rosaceae) | 1 | ||||||
| Crataegus coccinea L. (Rosaceae) | 1 | ||||||
| Crataegus crus-galli L. (Rosaceae) | 1 | ||||||
| Crataegus kansuensis E. H. Wilson (Rosaceae) | 1 | ||||||
| Crataegus laevigata (Poir.) DC. (Rosaceae) | 1 | 1 | |||||
| Crataegus sp. (Rosaceae) | 2 | ||||||
| Diospyros kaki Thunberg (Ebenaceae) | Field | 1 | |||||
| Euonymus europaeus L. (Celastraceae) | 1 | 3 | |||||
| Gaultheria shallon Pursh (Ericaceae) | 1 | ||||||
| Gaultheria sp. 1 (Ericaceae) | 1 | ||||||
| Gaultheria sp. 2 (Ericaceae) | 1 | ||||||
| Hedera helix L. (Araliaceae) | 1 | 3 | 1 | 3 | |||
| Hypericum sp. (Hypericaceae) | 1 | ||||||
| Hypericum androsaemum L. (Hypericaceae) | 1 | 1 | |||||
| Ilex aquifolium L. (Aquifoliaceae) | 1 | 1 | 1 | ||||
| Ilex sp. (Aquifoliaceae) | 1 | ||||||
| Juniperus sp. (Cupressaceae) | 1 | ||||||
| Laurus nobilis L. (Lauraceae) | 1 | 1 | |||||
| Ligustrum lucidum W. T. Aiton (Oleaceae) | 1 | ||||||
| Ligustrum vulgare L. (Oleaceae) | 4 | 1 | 2 | 1 | 2 | ||
| Lonicera etrusca Santi (Caprifoliaceae) | 1 | ||||||
| Lonicera henryi Hemsl. (Caprifoliaceae) | 1 | 1 | |||||
| Lonicera periclymenum L. (Caprifoliaceae) | Field | 1 | |||||
| Lonicera pileata Oliv. (Caprifoliaceae) | 1 | ||||||
| Malus floribunda Siebold ex Van Houtte (Rosaceae) | 1 | 1 | |||||
| Malus x Red Sentinel (Rosaceae) | 1 | ||||||
| Mespilus germanica L. (Rosaceae) | 1 | 1 | |||||
| Morus alba L. (Moraceae) | Field | 1 | |||||
| Myrteola sp.(Myrtaceae) | 1 | ||||||
| Myrtus communis L. (Myrtaceae) | 2 | ||||||
| Nandina domestica Thunb. (Berberidaceae) | 2 | 2 | |||||
| Olea europaea L. cv. Leccino (Oleaceae) | 1 | ||||||
| Parthenocissus tricuspidata (Sieb. & Zucc.) Planch. (Vitaceae) | 1 | ||||||
| Phillyrea angustifollia L. (Oleaceae) | 2 | ||||||
| Phillyrea latifolia L. (Oleaceae) | 3 | ||||||
| Prunus persica (L.) var. florepleno (Rosaceae) | Field | 1 | |||||
| Punica granatum L. (Lythraceae) | 2 | ||||||
| Pyracantha coccinea M. Roem. (Rosaceae) | 1 | 1 | |||||
| Pyracantha ‘navaho’ (Rosaceae) | 1 | 1 | |||||
| Rhamnus pumila Turra (Rhamnaceae) | 1 | 1 | |||||
| Rhodotypos scandens (Thunb.) Makino (Rosaceae) | 1 | ||||||
| Ribes alpinum L. (Rosaceae) | Field | 1 | |||||
| Rosa pendulina L. (Rosaceae) | 2 | ||||||
| Rubus ulmifolius Schott. (Rosaceae) | 4 | ||||||
| Ruscus aculeatus L. (Asparagaceae) | 3 | 5 | |||||
| Skimmia japonica Thunberg (Rutaceae) | 1 | ||||||
| Smilax aspera L. (Smilacaceae) | 1 | ||||||
| Solanum pseudocapsicum L. (Solanaceae) | 1 | ||||||
| Solanum sisymbrifolium Lam. (Solanaceae) | 1 | ||||||
| Sorbus chamaemespilus (L.) Crantz (Rosaceae) | 2 | 1 | |||||
| Sorbus intermedia (Ehrh.) Pers. (Rosaceae) | 1 | ||||||
| Symphoricarpos x chenaultii Hanc. (Caprifoliaceae) | 1 | ||||||
| Symphoricarpos orbiculatus Moen. (Caprifoliaceae) | 1 | ||||||
| Vaccinium uliginosum L. (Ericaceae) | 1 | ||||||
| Viburnum opulus L. (Adoxaceae) | Field | 2 | 4 | 2 | 2 | ||
| Viburnum tinus L. (Adoxaceae) | 1 | ||||||
| Viscum album L. (Santalaceae) | Lab | 1 | |||||
| Vitis labrusca L. (Vitaceae) | 2 | ||||||
| Withania somnifera (L.) Dunal, (Solanaceae) | 1 | ||||||
In bold: six species collected in at least five combinations of sites × years
1Known host of D. suzukii: Field = plant already recorded as host in the field, based on the reviews of Cini et al. (2012), Baroffio et al. (2014) and Lee et al. 2015; Lab species not yet found as host in the field but suitable host in laboratory tests in Baroffio et al. (2014), Lee et al. (2015) or Poyet et al. (2015)
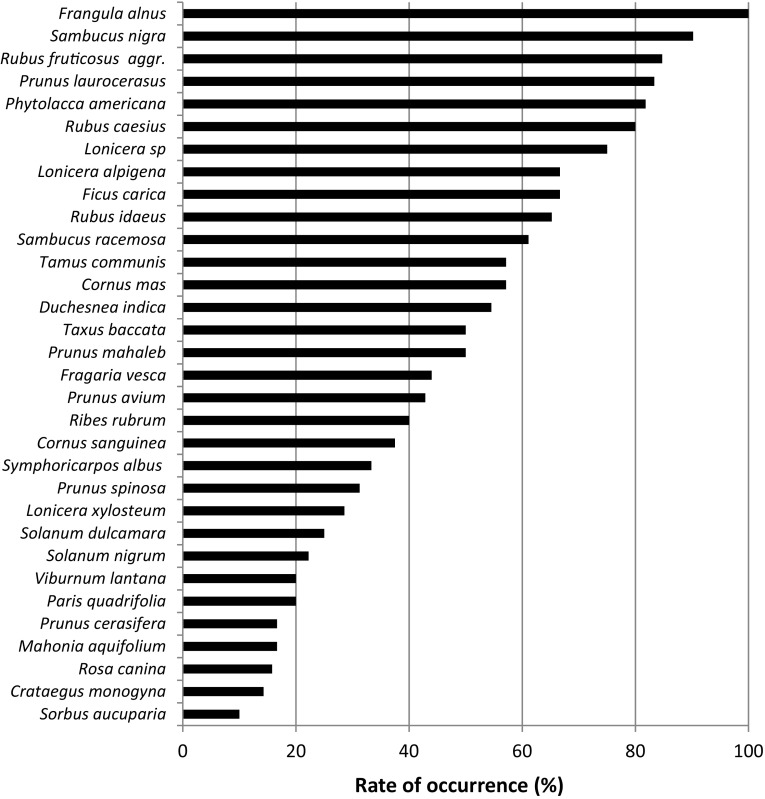
The rate of occurrence is presented for all fruit species collected in at least five different sites × year (Fig. 1). Drosophila suzukii emerged from fruit of Frangula alnus, Sambucus nigra, Rubus fruticosus aggr., Rubus caesius, Prunus laurocerasus and Phytolacca americana in at least 80 % of the sites × years. In contrast, Sorbus aucuparia, Crataegus monogyna, Rosa canina, Mahonia aquifolium, Prunus cerasifera, Paris quadrifolia and Viburnum lantana were only occasional hosts, with D. suzukii emerging at maximum 20 % of the sites × years. Among the 81 plant species that did not provide D. suzukii, only six were frequently collected (at least five sites × year) and 10 of them had been found as field or laboratory hosts in previous studies (Table 2).
Fig. 1.
Rate of occurrence of D. suzukii in the host plants in which it emerged, expressed as the % of sites × years in which D. suzukii was found. Only the fruits found in at least 5 sites × years are presented
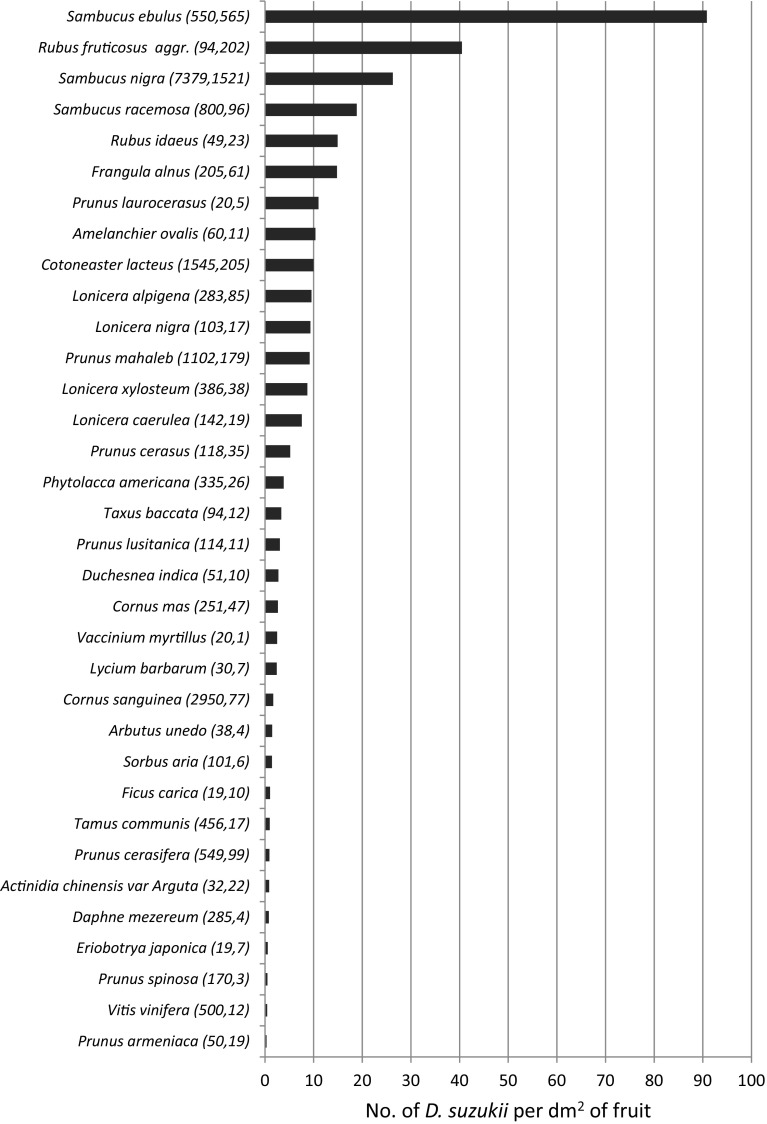
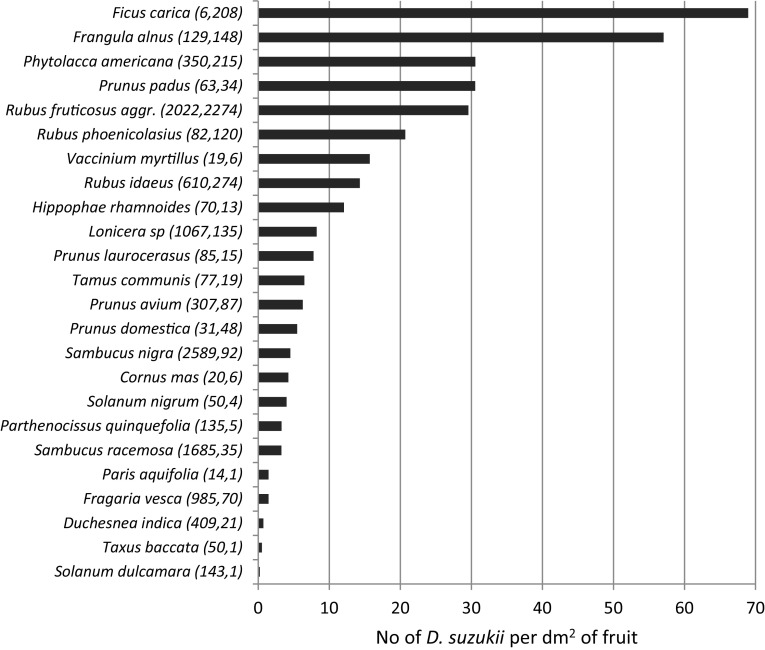
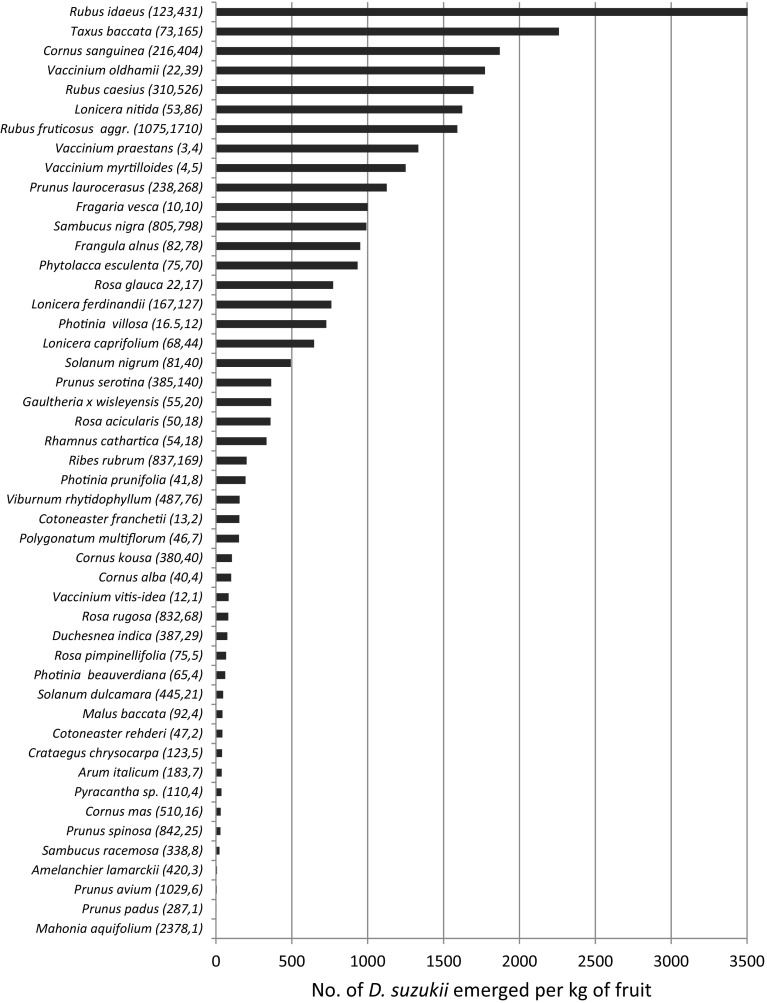
The infestation levels are presented for Italy 2014, Switzerland (Ticino only) 2014 and the Netherlands 2015 in Figs. 2, 3 and 4. In Italy, the highest levels, measured as the number of flies emerging per dm2 of fruit, were found in plants of the genera Sambucus and Rubus followed by Frangula alnus (Fig. 2). The high score in Sambucus ebulus, a species sampled only in Italy, was obtained on the basis of a single site providing an enormous amount of flies emerging from its small fruits. In Switzerland, the same parameter provided slightly different results, with the highest scores obtained from a few figs (Ficus carica), followed by Frangula alnus, Phytolacca americana and Prunus padus. Rubus spp. showed a similar infestation level in Italy and Switzerland, while fewer flies emerged from Sambucus spp. in Switzerland. In the Netherlands, where the infestation level was measured as the number of D. suzukii adults per kg of fruit, the highest scores were also obtained by Rubus spp. but, more surprisingly, also by Cornus sanguinea, a species that was not or only poorly attacked in Switzerland and Italy. Similarly, Taxus baccata was heavily infested in the Netherlands and much less so in the two other countries. Other heavily attacked species in the Netherlands included three Vaccinium species and several species that also scored high in the other countries such as Prunus laurocerasus, Sambucus nigra and Frangula alnus (Fig. 4).
Fig. 2.
No. of D. suzukii emerged per dm2 of fruit collected in 2014 in Italy. Numbers in parentheses after the fruits’ names indicate the total number of fruits collected and the total number of D. suzukii adults emerged from these fruits, respectively. Only fruits from which D. suzukii emerged are shown
Fig. 3.
No. of D. suzukii emerged per dm2 of fruit collected in 2014 in Ticino, Switzerland. Numbers in parentheses after the fruits’ names indicate the total number of fruits collected and the total number of D. suzukii adults emerged from these fruits, respectively. Only fruits from which D. suzukii emerged are shown
Fig. 4.
No. of D. suzukii emerged per kg of fruit collected in 2015 in the Netherlands. Numbers in parentheses after the fruits’ names indicate the total weight of fruits collected and the total number of D. suzukii adults emerged from these fruits, respectively. Only fruits from which D. suzukii emerged are shown
The fruits that were found early in the season, i.e. before June 1 in Italy, before July 1 in the Netherlands and on June 6 in Ticino (no fruits were found in Ticino at the first survey on May 9) are listed in Table 3. In general, fruits found in spring fall into two categories: those that are produced in spring and those that are produced in late summer and autumn but that last until spring of the following year. Fruit species of the first category were all infested by D. suzukii. In contrast, most fruit species that are able to last over winter were not infested and, from the six species that were, five of them contained D. suzukii only in autumn (Table 3). Only Cotoneaster lacteus fruits were found infested in November and again throughout April. A large collection of fruits was carried out in the Netherlands on 4 December 2014 to assess whether some could potentially host overwintering larvae. However, no fly emerged, even from fruits that had provided numerous flies until October. Plant species that were sampled that date included Crataegus monogyna, Prunus spinosa, Rosa canina, Rosa rugosa, Rubus fruticosus aggr., Symphoricarpos albus and Taxus baccata.
Table 3.
Host suitability of fruits found early in the year: before June 1st in Italy, before July 1st in the Netherlands and on June 6 in Switzerland
| Fruits formed in spring | Fruits formed in summer or autumn and lasting until the following year | |||
|---|---|---|---|---|
| Suitable hosts | Host suitability not proved in this study | Suitable hosts in autumn and spring | Suitable host only in autumn | Host suitability not proved in this study |
| Amelanchier lamarckii | Cotoneaster lacteus | Crataegus monogyna a | Aucuba japonica b | |
| Eriobotrya japonica | Duchesnea indica a | Cotoneaster microphyllus | ||
| Fragaria vesca | Prunus laurocerasus a | Cotoneaster salicifolia | ||
| Lonicera xylosteum | Pyracantha sp. | Hedera helix | ||
| Mahonia aquifolium | Rosa canina | Ilex aquifolium | ||
| Prunus avium | Laurus nobilis | |||
| Prunus mahaleb | Ligustrum vulgare | |||
| Prunus padus | Malus floribunda | |||
| Ribes rubrum | Mespilus germanica | |||
| Rubus sp. | Morus alba b | |||
| Sambucus racemosa | Nandina domestica | |||
| Pyracantha ‘navaho’ | ||||
| Ruscus aculeatus | ||||
| Viburnum opulus | ||||
aFor these species, only few fruits pass the winter
bPlants already recorded as suitable hosts in the field in Japan (Lee et al. 2015)
Discussion
This survey confirmed that D. suzukii is highly polyphagous and can attack and develop in a wide range of fruits of wild and ornamental plants as well as cultivated fruits. Forty-one plant species, both indigenous and exotic, have been added to the list of suitable hosts. Several hosts such as Rubus spp., Sambucus spp., Prunus spp., Lonicera spp. and Frangula alnus were consistently found throughout the sites and years as being heavily infested, confirming similar observations made in other studies (Mitsui et al. 2010; Baroffio et al. 2014; Lee et al. 2015; Poyet et al. 2015). Other results were more surprising. In particular, we did not expect so many adults emerging from species such as Rosa spp. and Malus baccata, which tend to have a rather tough skin. Similarly, flies were obtained from many other plant species considered to be unsuitable in laboratory tests carried out by Poyet et al. (2015), such as Sorbus aria, Sorbus aucuparia, Polygonatum multiflorum, Paris quadrifolia and Crataegus monogyna. These unexpected infestations can be partly explained by the very high population levels of D. suzukii in the second half of 2014 (Italy and Switzerland) and 2015 (the Netherlands). Moreover, although surveys focused on ripe, undamaged fruits, it is likely that some adults emerged from “hard” fruits, such as Malus baccata, resulted from eggs laid in unnoticed damaged fruits. Lee et al. (2015) also obtained D. suzukii from field-collected fruits that appeared unsuitable in laboratory tests. They attributed these discrepancies to differences in fruit suitability among picked (laboratory) versus hanging (field) fruit and the timing of sampling.
Eighty-one plant species did not give rise to D. suzukii adults, among which ten have been reported as hosts in other field surveys or laboratory tests (Baroffio et al. 2014; Lee et al. 2015; Poyet et al. 2015). These negative results must be considered with great caution. Most of the fruit species from which nothing emerged were collected in low numbers or at few sites. Laboratory tests could be carried out to confirm the unsuitability of these fruits, taking into account that discrepancies between laboratory tests and field surveys may occur (Lee et al. 2015). Only the six fruit species collected in high numbers in at least five combinations of sites × years can be regarded as “unsuitable”: Actaea spicata, Berberis vulgaris, Hedera helix, Ligustrum vulgare, Ruscus aculeatus and Viburnum opulus, even though, for the latter species, some larval development but no adult emergence had been observed in laboratory tests (Poyet et al. 2015).
This survey also illustrated the close association between D. suzukii and invasive plants. Forty plants identified as hosts are exotic to the survey areas and many of them are considered as invasive species. The interaction between D. suzukii and the invasive American black cherry Prunus serotina has been studied in France by Poyet et al. (2014), who suggest that the heavy infestation of Prunus serotina fruits by D. suzukii could reduce the life span of fruits and their attractiveness to seed consumers and dispersers. In contrast, Prunus serotina could represent a suitable plant reservoir enhancing D. suzukii invasion. A similar scenario was proposed by Asplen et al. (2015) regarding the invasion of the European buckthorn, Rhamnus cathartica, in North America, which was found to be a suitable host of D. suzukii both in North America and in our study. The invasive ‘Himalayan’ blackberry, Rubus armeniacus, is also known to favour the spread and abundance of D. suzukii in berry production systems in Western North America (Klick et al. 2016). Besides Prunus serotina, other important invasive plants infested by D. suzukii in our samples include, e.g. Duchesnea indica, Phytolacca americana and Phytolacca esculenta, Prunus laurocerasus, Rosa rugosa and Symphoricarpos albus. The interactions between the invasion processes of D. suzukii and these invasive plants should be further investigated.
Implications for sustainable Drosophila suzukii management
Knowing the realised host range and the preferred host plants outside cultivated habitats is essential for the development of sustainable IPM strategies against D. suzukii (Klick et al. 2016; Lee et al. 2015). Pelton et al. (2016) showed that the amount of woodland in the landscape positively affects early season crop risk and the high numbers of D. suzukii in the woods have implications for understanding overwintering. Non-crop hosts in the vicinity of susceptible fruit crops may also enhance D. suzukii populations before or during the crop season, as shown by Klick et al. (2016) in a raspberry crop system in Western North America. These alternative hosts may also be used as refuges for D. suzukii when crops are sprayed with insecticides. Therefore the management of these non-crop hosts should be integrated in control strategies. For example, our results and other host range studies (Lee et al. 2015; Poyet et al. 2015) now allow us to advise on suitable and unsuitable ornamental plants to be used in the vicinity of susceptible crops. Species such as Cornus spp., Lonicera spp., Prunus spp., Sambucus spp. and Taxus baccata, which are abundantly used as hedge plants in Europe, should be avoided. In contrast, there is now sufficient evidence showing that, e.g. Ligustrum spp., Viburnum spp., Crataegus spp. or Pyracantha spp. do not increase populations of D. suzukii on site. Similarly, field margins could be cleared of susceptible wild plants (Klick et al. 2016). However, the management of wild hosts in the surroundings of crops is often more problematic than ornamental hosts because of the high number of highly susceptible species and the difficulty in managing them in areas that do not always belong to the fruit producer. Furthermore, more should be known on the natural dispersal capacities of D. suzukii to determine the areas requiring management. If dispersal studies show that D. suzukii can be attracted over long distances, removing native wild host plants from a large area may become unpractical and have a negative effect on the functioning of local ecosystems.
The fruiting period of host plants is also an essential consideration when developing management strategies. In Europe, populations of D. suzukii often dramatically increase from spring to autumn, due to the high number of generations (Asplen et al. 2015). Only a few plants produce fruits in spring, suggesting that the availability of suitable fruits in spring is a key element in the population dynamics of D. suzukii. Therefore, efforts should be made to control the presence of these early fruits in the surroundings of fruit crops and orchards (Asplen et al. 2015; Poyet et al. 2015). Not only non-crop hosts should be controlled. In the surveyed area in Northern Italy and the Netherlands, the first heavy infestations occur on abandoned or untreated cherry trees, which probably play an important role in the local increase of D. suzukii populations in summer (Ioriatti et al. 2015; Helsen and van der Sluis unpublished data). This survey showed that all plants fruiting in spring were attacked by D. suzukii. In contrast, most fruits that are formed in autumn and overwinter until spring were used neither as overwintering hosts nor as early hosts in spring, with the possible exception of fruits of C. lacteus that were found attacked in April. We did not find any evidence that D. suzukii larvae may overwinter in fruits. In Northern Italy, the monitoring and dissection of female flies throughout the winter showed that D. suzukii overwinters as adults in reproductive diapause from November to April (Zerulla et al. 2015).
More generally, the ability of D. suzukii to attack such a large number of widely distributed ornamental and wild host plants strongly suggests the need for an area-wide control approach. Since insecticides are often not effective (e.g. Rogers et al. 2016) and cannot be used in many of the non-crop habitats, in particular forests, and since sanitation is impossible on a large scale, classical biological control through the introduction of specific parasitoids from the region of origin of the fly could be a long term solution (Haye et al. 2016; Daane et al. 2016). Preliminary studies in Japan have suggested that some larval parasitoids are specific to D. suzukii (Kasuya et al. 2013; Nomano et al. 2015) and recent surveys in East Asia have shown that larval parasitism rates are not negligible, e.g. less than 10 % in the Tokyo region (Kasuya et al. 2013), up to 16 % in South Korea (Daane et al. 2016) and over 50 % in Yunnan, China (Kenis unpublished data). It would be worth assessing the suitability of these parasitoids for introduction into invaded areas, including the evaluation of potential non-target effects of such introductions on the community of native Drosophilidae.
Author contribution statement
MK conceived the experiment and coordinated the writing up of the manuscript, which was carried out jointly by all authors. All authors participated in the field sampling and laboratory rearing.
Acknowledgments
We thank Andrea Battisti and three anonymous reviewers for their useful comments on the manuscript. The research leading to these results has received funding from the European Union’s Seventh Framework programme for research, technological development and demonstration under grant agreement number 613678 (DROPSA).
Footnotes
Special Issue: Spotted Wing Drosophila
An erratum to this article is available at http://dx.doi.org/10.1007/s10340-017-0843-2.
References
- Asplen MK, Anfora G, Biondi A, Choi DS, Chu D, Daane KM, Gibert P, Gutierrez AP, Hoelmer KA, Hutchison WD, Isaacs R, Jiang Z-L, Kárpáti Z, Kimura M, Pascual M, Philips CR, Plantamp C, Ponti L, Vétek G, Vogt H, Walton VM, Yu Y, Zappalá L, Desneux N. Invasion biology of spotted-wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci. 2015;88:469–494. doi: 10.1007/s10340-015-0681-z. [DOI] [Google Scholar]
- Baroffio C, Fischer S, Kehrli P, Kuske S, Linder C, Mazzi D, Richoz P (2014) Attractivité des plantes face à la Drosophile suzukii. Plantes, Agroscope Fiche technique No. 3. Ed. Agroscope, Conthey
- Bellamy DE, Sisterson MS, Walse SS. Quantifying host potentials: indexing postharvest fresh fruits for spotted wing drosophila, Drosophila suzukii. PLoS One. 2013;8(4):e61227. doi: 10.1371/journal.pone.0061227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrack HJ, Fernandez GE, Spivey T, Kraus DA. Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumura (Diptera: Drosophilidae), an invasive frugivore. Pest Manag Sci. 2013;69:1173–1180. doi: 10.1002/ps.3489. [DOI] [PubMed] [Google Scholar]
- Carton Y, Bouléreau M, van Alphen JJ, van Lenteren JC. The Drosophila parasitic wasps. In: Ashburner CT, editor. The genetics and biology of Drosophila. London: Academic Press; 1986. pp. 347–393. [Google Scholar]
- Chabert S, Allemand R, Poyet M, Eslin P, Gibert P. Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol Control. 2012;63:40–47. doi: 10.1016/j.biocontrol.2012.05.005. [DOI] [Google Scholar]
- Cini A, Ioratti C, Anfora G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectol. 2012;65:149–160. [Google Scholar]
- Cini A, Anfora G, Escudero-Colomar LA, Grassi A, Santosuosso U, Seljak G, Papini A. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J Pest Sci. 2014;87:559–566. doi: 10.1007/s10340-014-0617-z. [DOI] [Google Scholar]
- Daane KM, Wang XG, Bondi A, Miller JC, Riedl Miller BE, Shearer PW, Guerrieri E, Giorgini M, Buffington M, van Achterberg C, Song Y, Kang T, Yi H, Jung C, Lee DW, Chung B-K, Hoelmer KA, Walton VM. First foreign exploration for Asian parasitoids of Drosophila suzukii. J Pest Sci. 2016 [Google Scholar]
- de Koning J, van den Broek JW. Dendrologie van de lage landen. KNNV Uitgeverij: Zeist; 2012. [Google Scholar]
- Deprá M, Poppe J, Schmitz H, De Toni D, Valente V. The first records of the invasive pest Drosophila suzukii in the South American continent. J Pest Sci. 2014;87:379–383. doi: 10.1007/s10340-014-0591-5. [DOI] [Google Scholar]
- Ferrari M, Medici D. Alberi e arbusti: manuale di riconoscimento delle principali specie ornamentali. Bologna: Edagricole; 2008. [Google Scholar]
- Haye T, Girod P, Cuthbertson AGS, Wang XG, Daane KM, Hoelmer KA, Baroffio C, Zhang J, Desneux N. Current SWD IPM tactics and their practical implementation in fruit crops across different regions around the world. J Pest Sci. 2016 [Google Scholar]
- Info Flora (2015) Centre national de données et d’informations sur la flore de Suisse. https://www.infoflora.ch/fr/. Accessed 6 Nov 2015
- Ioriatti C, Boselli M, Caruso S, Galassi T, Gottardello A, Grassi A, Tonina L, Vaccari G, Mori N. Approccio integrato per la difesa dalla Drosophila suzukii. Frutticoltura. 2015;4:32–36. [Google Scholar]
- Kacsoh BZ, Schlenke TA. High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS One. 2012;7:e34721. doi: 10.1371/journal.pone.0034721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya N, Mitsui H, Ideo S, Watada M, Kimura MT. Ecological, morphological and molecular studies on Ganaspis individuals (Hymenoptera: Figitidae) attacking Drosophila suzukii (Diptera: Drosophilidae) Appl Entomol Zool. 2013;48:87–92. doi: 10.1007/s13355-012-0156-0. [DOI] [Google Scholar]
- Klick J, Yang WQ, Walton VM, Dalton DT, Hagler JR, Dreves AJ, Lee JC, Bruck DJ. Distribution and activity of Drosophila suzukii in cultivated raspberry and surrounding vegetation. J Appl Entomol. 2016;140:37–46. doi: 10.1111/jen.12234. [DOI] [Google Scholar]
- Lee JC, Bruck DJ, Curry H, Edwards DL, Haviland DR, Van Steenwyk R, Yorgey B. The susceptibility of small fruits and cherries to the spotted wing drosophila, Drosophila suzukii. Pest Manag Sci. 2011;67:1358–1367. doi: 10.1002/ps.2225. [DOI] [PubMed] [Google Scholar]
- Lee JC, Dreves AJ, Cave AM, Kawai S, Isaacs R, Miller JC, van Timmeren S, Bruck DJ. Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae) Ann Entomol Soc Am. 2015;108:117–129. doi: 10.1093/aesa/sau014. [DOI] [Google Scholar]
- Mitsui H, Beppu K, Kimura MT. Seasonal life cycles and resource uses of flower- and fruit-feeding drosophilid flies (Diptera: Drosophilidae) in central Japan. Entomol Sci. 2010;13:60–67. doi: 10.1111/j.1479-8298.2010.00372.x. [DOI] [Google Scholar]
- Nomano FY, Mitsui H, Kimura MT. Capacity of Japanese Asobara species (Hymenoptera; Braconidae) to parasitize a fruit pest Drosophila suzukii (Diptera; Drosophilidae) J Appl Entomol. 2015;139:105–113. doi: 10.1111/jen.12141. [DOI] [Google Scholar]
- Pelton E, Gratton C, Isaacs R, Van Timmeren S, Blanton A, Guedot C. Earlier activity of Drosophila suzukii in high woodland landscapes but relative abundance is unaffected. J Pest Sci. 2016 [Google Scholar]
- Pignatti S. Flora d’Italia. Bologna: Edagricole; 1982. [Google Scholar]
- Poyet M, Havard S, Prévost G, Chabrerie O, Doury G, Gibert P, Eslin P. Resistance of Drosophila suzukii to the larval parasitoids Leptopilina heterotoma and Asobara japonica is related to haemocyte load. Physiol Entomol. 2013;38:45–53. doi: 10.1111/phen.12002. [DOI] [Google Scholar]
- Poyet M, Eslin P, Héraude M, Le Roux V, Prévost G, Gibert P, Chabrerie O. Invasive host for invasive pest: when the Asiatic cherry fly (Drosophila suzukii) meets the American black cherry (Prunus serotina) in Europe. Agric For Entomol. 2014;16:251–259. doi: 10.1111/afe.12052. [DOI] [Google Scholar]
- Poyet M, Le Roux V, Gibert P, Meirland A, Prévost G, Eslin P, Chabrerie O. The wide potential trophic niche of the Asiatic fruit fly Drosophila suzukii: the key of its invasion success in temperate Europe? PlosOne. 2015;10(11):e0142785. doi: 10.1371/journal.pone.0142785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Burkness EC, Hutchison WD. Evaluation of high tunnels for management of Drosophila suzukii in fall-bearing red raspberries: potential for reducing insecticide use. J Pest Sci. 2016 [Google Scholar]
- Tochen S, Dalton DT, Wiman NG, Hamm C, Shearer PW, Walton VM. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ Entomol. 2014;43:501–510. doi: 10.1603/EN13200. [DOI] [PubMed] [Google Scholar]
- van der Meijden R. Heukels’ Flora van Nederland. 22. Groningen: Wolters-Noordhoff; 1996. [Google Scholar]
- Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ, Walton VM, O’Neal SD, Zalom FG. Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Integ Pest Manag. 2011;2:G1–G7. doi: 10.1603/IPM10010. [DOI] [Google Scholar]
- Zerulla FN, Schmidt S, Streitberger M, Zebitz CPW, Zelger R. On the overwintering ability of Drosophila suzukii in South Tyrol. J Berry Res. 2015;5:41–48. [Google Scholar]