Abstract
Background
Acne vulgaris has been linked to the Western diet. Hyperglycemic diet increases insulin and insulin-like growth factor (IGF)-1. Deeper insights into IGF-1-mediated signal pathway are critical importance to understand the impact of Western diet.
Objective
We investigated the effect of IGF-1 on the expression of inflammatory biomarkers and sebum production in cultured sebocytes.
Methods
Polymerase chain reaction and enzyme-linked immunosorbent assay were performed to measure changes in the expression of inflammatory biomarkers including interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), IGF1R, IGFBP2, sterol response element-binding protein (SREBP), and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PI3KCA) after the treatment of cultured sebocytes with 10−7 M or 10−5 M IGF-1. Sebum production was evaluated after the treatment of cultured sebocytes with 10−7 M or 10−5 M IGF-1 using lipid analysis.
Results
The expression levels of IL-1β, IL-6, IL-8, and TNF-α in cultured sebocytes after treatment with 10−7 M or 10−5 M IGF-1 were increased. Increased gene expression levels of NF-κB in cultured sebocytes were also shown after 10−7 M or 10−5 M IGF-1 treatments. Gene expression of these inflammatory biomarkers was decreased after 10−7 M or 10−5 M IGF-1 treatment in the presence of 100 nM NF-κB inhibitor. Treatment with 10−7 M or 10−5 M IGF-1 increased the gene expression levels of IGF1R, IGFBP2, SREBP and PI3KCA in cultured sebocytes. Sebum production from cultured sebocytes treated with 10−7 M or 10−5 M IGF-1 was also increased.
Conclusion
It is suggestive that IGF-1 might be involved in the pathogenesis of acne by increasing both expression of inflammatory biomarkers and also sebum production in sebocytes.
Keywords: Biomarkers, Insulin-like growth factor-1, Sebum
INTRODUCTION
Acne is caused by multiple factors such as increased sebum secretion, inflammation, follicular keratinization, and Propionibacterium acnes1. Excessive sebum production, abnormal sebum composition, sebum peroxidation, and inflammatory lipid production contribute to the formation of primary acne lesions2. Furthermore, sebaceous glands also produce inflammatory biomarkers and antimicrobial peptides and play an important role in the formation and aggravation of acne lesions3. Production of inflammatory cytokines is induced by the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) through the Toll-like receptors (TLR)-2 or TLR-4. Upregulation of inflammatory cytokines, such as interleukin (IL)-1α, IL-6, and IL-8, is present in acne lesions4.
Diet is considered to be one of the main factors influencing the induction and aggravation of acne, though this is still debatable5. Recent research interests have focused on glycemic load, and hyperinsulinemia resulting from a high glycemic load has been linked to an increase in the concentration of insulin-like growth factor (IGF)-1, which has been reported to affect androgen metabolism and lipogenesis6,7. Additionally, IGF-1 has been shown to upregulate inflammatory cytokines in various cells including immortalized sebocytes8,10,11. A correlation between the severity of acne and the level of serum IGF-1 has also been reported12. A recent clinical study showed that a low glycemic diet can decrease both the size of the sebaceous gland and the number of inflammatory lesions6.
So far, increasing number of evidence demonstrating the relation between diet and acne is supporting the likelihood. However, the effect of IGF-1 on cultured sebocyte was not revealed enough yet and, thus, it is necessary to make clear the cellular effect of IGF-1.
This study was undertaken to evaluate the effect of IGF-1 on the expression of inflammatory biomarkers as well as on lipogenesis in cultured sebocytes.
MATERIALS AND METHODS
Sebocyte culture
Primary sebocyte cultures were prepared from occipital hair follicle sebaceous glands. The sebaceous glands were isolated from hair follicles under a binocular microscope and transferred to a tissue culture dish. The cells were maintained in Dulbecco's modified Eagle medium (DMEM; Hyclone Laboratories, Logan, UT, USA) at 37℃ in a humidified atmosphere of 5% CO2. Explants were left for a period of 4 days, and the medium was then changed to Epilife (MEPI500CA; Gibco BRL, Grand Island, NY, USA). The medium was changed every 2 or 3 days. DMEM was supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), and 20% heat-inactivated bovine serum (Hyclone Laboratories), while Epilife was supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), and fungizone (0.25 µg/ml).
Once the outgrowth cells became subconfluent, the cells were harvested using 0.25% trypsin and 10 mM ethylenediamine tetraacetic acid in Hank's balanced salt solution, followed by subculturing. The cells obtained after the second passage were used in this study after identifying the cultured sebocytes using Oil Red O (Sigma, St. Louis, MO, USA) staining and immunocytofluorescence against cytokeratins 1 and 7 (Chemicon, Billerica, MA, USA).
The treatment of cultured sebocytes with IGF-1 and/or NF-κB inhibitor
IGF-1 (R&D Systems, Minneapolis, MN, USA) was added to the cultured sebocytes at concentrations ranging from 10−11 to 10−5 M for the Cell Counting Kit-8 assay. As a result, cultured sebocytes was treated with IGF-1 10−7 M or 10−5 M for 3 days in this study. IGF-1 10−7 M or 10−5 M was also added to NF-κB 100 nM (Selleckchem, Houston, TX, USA) inhibitor-pretreated sebocytes for 2 days.
Real-time polymerase chain reaction
For real-time polymerase chain reaction (PCR) of cultured sebocytes following treatment with IGF-1 and/or the NF-κB inhibitor, the Maxima SYBR Green/Fluorescein qPCR Master Mix (2×) (Thermo Scientific, Vilnius, Lithuania) was used in triplicate reactions following the manufacturer's protocol. Real-time PCR was performed using a LightCycler (Roche Diagnostics, Indianapolis, IN, USA) under the following conditions: one cycle of 2 min at 50℃, followed by 10 min at 95℃ for one cycle, 55 cycles of 10 s at 95℃, and finally 30 s at an annealing temperature. Analysis of variance (ANOVA) was used for statistical analysis of the data. A probability (p)-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Enzyme-linked immunosorbent assay
Analysis of IL-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α (R&D Systems, Shanghai, China) protein expression using enzyme-linked immunosorbent assay (ELISA) was conducted according to the manufacturer's instructions. Briefly, 50~200 µl samples were added to each well in triplicate. Next, 200 µl prepared streptavidin-horseradish peroxidase (conjugate) and 200 µl premixed 3,3′,5,5′-tetramethylbenzidine substrate solution were added to each well, in that order. The plates were developed in the dark at room temperature for approximately 20~30 min, and the reaction was halted by adding 50 µl of a stop solution to each well. Finally, the absorbance was measured on a VERSAMax microplate ELISA reader (Molecular Devices, Sunnyvale, CA, USA). ANOVA was used for statistical analysis of the data. A p-value of less than 0.05 was considered statistically significant.
Quantification of lipid production
IGF-1-treated sebocytes were homogenized in an e-tube with 50 µl 0.9% NaCl plus 1% triton ×100 followed by vortex agitation. After incubation at 4℃ for 30 min, the solution was centrifuged at 13,000 rpm for 15 min. The supernatant was collected in a clean 1.5 ml e-tube and stored at −20℃ until required. The TG-S reaction kit (Asan Pharm. Co., Seoul, Korea) was used for the detection of neutral lipids, according to the manufacturer's protocol.
RESULTS
The increased expression of IL-1β, IL-6, IL-8, and TNF-α in cultured sebocytes after treatment with IGF-1
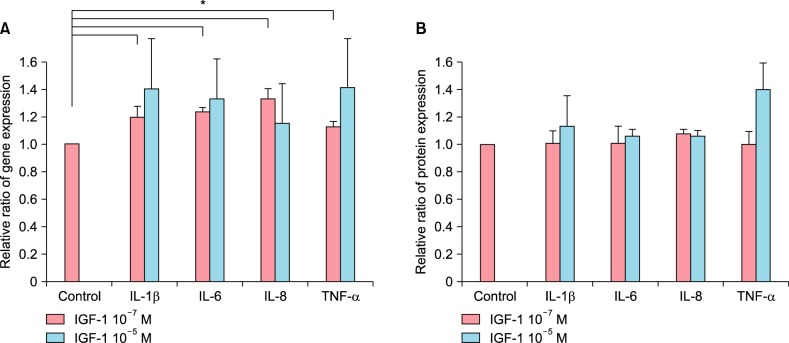
The treatment of cultured sebocytes with 10−7 M IGF-1 showed significantly increased gene expression of IL-1β, IL-6, IL-8 and TNF-α (p<0.05). The treatment of cultured sebocytes with 10−5 M IGF-1 also showed increased gene expression (p>0.05; Fig. 1A). In addition, the treatment of cultured sebocytes with 10−7 M or 10−5 M IGF-1 increased protein expression of IL-1β, IL-6, IL-8 and TNF-α (p>0.05; Fig. 1B).
Fig. 1. The increased expression of inflammatory cytokines in cultured sebocytes after treatment with insulin-like growth factor (IGF)-1. (A) The treatment of cultured sebocytes with 10−7 M or 10−5 M IGF-1 showed increased gene expression of interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α. *p<0.05. (B) The treatment of cultured sebocytes with 10−7 M or 10−5 M IGF-1 increased protein expression of IL-1β, IL-6, IL-8 and TNF-α.
The decreased expression of IL-1β, IL-6, IL-8, and TNF-α after treatment of NF-κB-inhibitor pretreated sebocytes with IGF-1
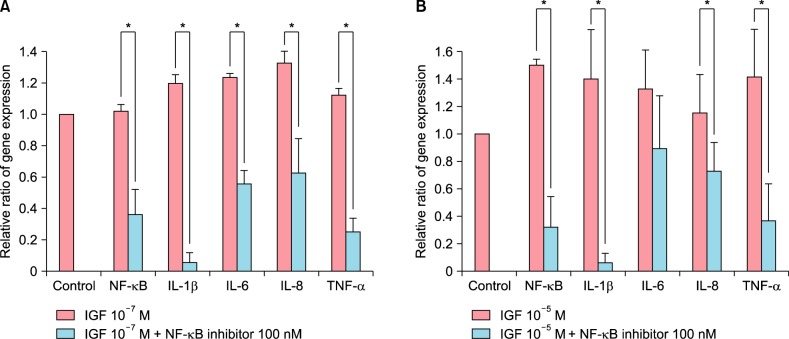
The treatment of cultured sebocytes with 10−7 M IGF-1 showed an increase in the gene expression of NF-κB compared with the untreated control. The treatment of 100 nM NF-κB inhibitor-pretreated sebocytes with 10−7 M IGF-1 showed a significant decrease in the gene expression of NF-κB, IL-1β, IL-6, IL-8, and TNF-α compared with the treatment of cultured sebocytes with 10−7 M IGF-1 (p<0.05; Fig. 2A). The treatment of cultured sebocytes with 10−5 M IGF-1 also showed an increase in the gene expression of NF-κB compared with the untreated control. In addition, the treatment of 100 nM NF-κB inhibitor-pretreated sebocytes with 10−5 M IGF-1 showed a significant decrease in the gene expression of NF-κB, IL-1β, IL-6, IL-8, and TNF-α compared with the treatment of cultured sebocytes with 10−5 M IGF-1 (p<0.05).
Fig. 2. The decreased expression of inflammatory cytokines after treatment of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) inhibitor-pretreated sebocytes with insulin-like growth factor (IGF)-1. (A) The treatment of cultured sebocytes with 10−7 M IGF-1 showed an increase in the gene expression of NF-κB. The treatment of 100 nM NF-κB inhibitor-pretreated sebocytes with 10−7 M IGF-1 showed a significant decrease in the gene expression of NF-κB, interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α. *p<0.05. (B) The treatment of cultured sebocytes with 10−5 M IGF-1 showed an increase in the gene expression of NF-κB. The treatment of 100 nM NF-κB inhibitor-pretreated sebocytes with 10−5 M IGF-1 showed a significant decrease in the gene expression of NF-κB, IL-1β, IL-6, IL-8, and TNF-α. *p<0.05.
The increased expression of IGF1R and IGFBP-2 in cultured sebocytes after treatment with IGF-1
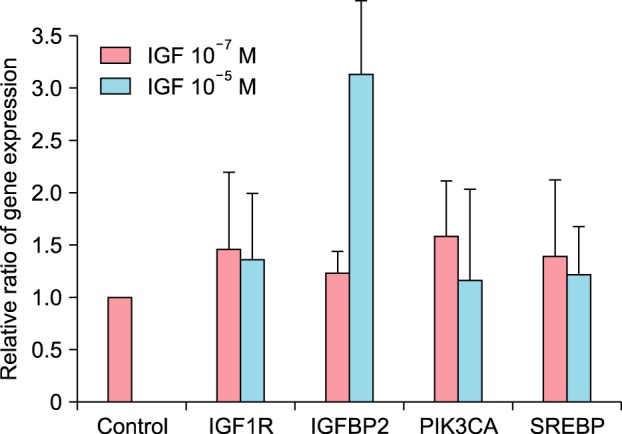
The treatment of cultured sebocytes with 10−7 M or 10−5 M IGF-1 showed increased gene expression of IGF1R and IGRBP-2 (p>0.05; Fig. 3).
Fig. 3. The increased expression of insulin-like growth factor (IGF)1R and IGFBP2 in cultured sebocytes after treatment with IGF-1. In addition, the increased gene expression of sterol response element-binding protein (SREBP) and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PI3KCA) after treatment of cultured sebocytes with IGF-1.
The increased lipid production after treatment of cultured sebocytes with IGF-1
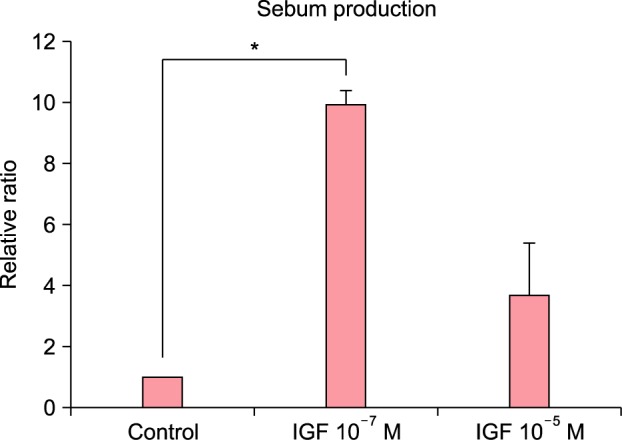
The treatment of cultured sebocytes with 10−7 M or 10−5 M IGF-1 increased the gene expression of sterol response element-binding protein (SREBP) (p>0.05; Fig. 3). The treatment of cultured sebocytes with 10−7 M or 10−5 M IGF-1 revealed an increase in the gene expression of phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PI3KCA) compared with the untreated control (p>0.05; Fig. 3). Lipid production was significantly increased following the treatment of the cultured sebocytes with 10−7 M IGF-1 (p<0.05) and increased following the treatment of the cultured sebocytes with 10−5 M IGF-1 (p>0.05; Fig. 4).
Fig. 4. The increased lipid production after treatment of cultured sebocytes with insulin-like growth factor (IGF)-1. Lipid production was increased following the treatment of the cultured sebocytes with 10−7 M or 10−5 M IGF-1. *p<0.05.
DISCUSSION
Acne is a complex, chronic and common skin disorder of pilosebaceous units. Current research has shown the induction of inflammatory signaling in the pilosebaceous unit to be a major process in the initiation of acne lesions2,13,14.
Inflammation is a key component of acne pathogenesis15. The pilosebaceous unit is an immunocompetent organ, and sebocytes and follicular keratinocytes, the major components of the pilosebaceous unit, may act as immune-active cells. Sebocytes can recognize microbia and abnormal lipid presentation, followed by the production of inflammatory cytokines4. Genes involved in the pathways that regulate inflammation are upregulated in acne lesions compared with normal skin, and NF-κB, a transcription factor critical for the upregulation of several inflammatory cytokine genes, has been shown to be activated in acne lesions16.
Recent research suggests that diet may influence the development of acne17,18,19. A diet with a high glycemic load may have a potentiating effect on the levels of serum insulin and IGF-1, thereby promoting the development of acne5. Clinical research has demonstrated a correlation between the severity of acne and IGF-1 levels in female acne patients12. In contrast, patients with a low glycemic load showed significant increases in both IGFBP-1 and IGFBP-3, suggesting reduced IGF-1 activity and bioavailability20.
IGF-1 has a direct influence on androgen regulation in the skin and potentiates androgen signaling by the activation of 5α-reductase and the androgen receptor5. In vitro, IGF-1 upregulated the expression of SREBP-1 through the PI3K/Akt signaling pathway, resulting in increased lipogenesis in SEB-1 sebocytes7.
Cytokines are present in normal sebaceous glands and are affected by many factors21. Cytokine regulation of sebocytes is an important aspect in acne pathophysiology and can be modulated by various factors. While IL-1, TNF-α, IL-6, and IL-8 are released into the supernatant in unstressed sebocyte cultures, cytokine released is increased significantly in a stressed environment22. In a previous report, we showed that well-known factors such as substance P and dihydrotestosterone play key roles in the regulation of inflammatory cytokines in sebocytes23,24,25.
In this study, we investigated the effect of IGF-1 on inflammatory gene and protein profiles in cultured sebocytes. IGF-1 induced upregulation of inflammatory cytokines in several cell lines. Im et al.11 demonstrated increased release of inflammatory cytokines in SZ-95 cells treated with IGF-1. This study showed that IGF-1 induced the dose-related upregulation of inflammatory biomarker gene and protein expression in cultured sebocytes.
This study suggested that IGF-1 might be involved in acne induction or aggravation through upregulation of inflammatory biomarkers in sebaceous glands. Increased gene expression of NF-κB was observed in cultured sebocytes treated with 10−7 M or 10−5 M IGF-1. Moreover, the gene expression levels of IL-1β, IL-6, IL-8 and TNF-α in cultured sebocytes were significantly decreased after treatment with 10−7 M or 10−5 M IGF-1 in the presence of the NF-κB inhibitor, indicating that the upregulation of inflammatory biomarkers by IGF-1 might be mediated via the NF-κB signaling pathway.
IFG-1 binds to IGF1R to activate its effects. IGF-1 revealed an increase in IGF1R gene expression. IGFBPs are also involved in the regulation to IGF bioactivity, acting to enhance or attenuate IGF signaling. Although IGFBP-3 is the most abundant protein among IGFBPs, IGFBP-2 was evaluated in this study. IGF-1 showed an increase in IGFBP-2 gene expression.
In addition, IGF-1 resulted in increased sebum production in cultured sebocytes. SREBP, a transcription factor regulating fatty acid synthesis, was upregulated by 10−7 M or 10−5 M IGF-1 in cultured sebocytes. The inverse dose-related effect in sebum production could be a result from the earlier suppression of sebocytes proliferation26. Increased gene expression of PI3KCA was also demonstrated. Taken together, the effect of IGF-1 on lipogenesis in cultured sebocytes was in accordance with that reported in SEB-1 sebocytes in a previous study7.
Here, we showed that the NF-κB signaling pathway transduces the IGF-1 signal, resulting in increased inflammatory biomarkers in cultured sebocytes and IGF-1 increases lipogenesis in sebocytes, mediated by increased SREBP through PI3K. As IGF-1 is related to a high glycemic index, it is plausible that a strict diet may be helpful for controlling acne, possibly through the inhibition of sebum production and inflammation in sebocytes.
ACKNOWLEDGMENT
This research was supported by grant of Amore-Pacific Corporation 2014.
References
- 1.Winston MH, Shalita AR. Acne vulgaris. Pathogenesis and treatment. Pediatr Clin North Am. 1991;38:889–903. doi: 10.1016/s0031-3955(16)38158-5. [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Zouboulis CC, Adjaye J, Akamatsu H, Moe-Behrens G, Niemann C. Human skin stem cells and the ageing process. Exp Gerontol. 2008;43:986–997. doi: 10.1016/j.exger.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 5.Melnik BC. Diet in acne: further evidence for the role of nutrient signalling in acne pathogenesis. Acta Derm Venereol. 2012;92:228–231. doi: 10.2340/00015555-1358. [DOI] [PubMed] [Google Scholar]
- 6.Kwon HH, Yoon JY, Hong JS, Jung JY, Park MS, Suh DH. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241–246. doi: 10.2340/00015555-1346. [DOI] [PubMed] [Google Scholar]
- 7.Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renier G, Clément I, Desfaits AC, Lambert A. Direct stimulatory effect of insulin-like growth factor-I on monocyte and macrophage tumor necrosis factor-alpha production. Endocrinology. 1996;137:4611–4618. doi: 10.1210/endo.137.11.8895324. [DOI] [PubMed] [Google Scholar]
- 9.Kooijman R, Coppens A, Hooghe-Peters E. IGF-I stimulates IL-8 production in the promyelocytic cell line HL-60 through activation of extracellular signal-regulated protein kinase. Cell Signal. 2003;15:1091–1098. doi: 10.1016/s0898-6568(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 10.Tu W, Cheung PT, Lau YL. IGF-I increases interferon-gamma and IL-6 mRNA expression and protein production in neonatal mononuclear cells. Pediatr Res. 1999;46:748–754. doi: 10.1203/00006450-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Im M, Kim SY, Sohn KC, Choi DK, Lee Y, Seo YJ, et al. Epigallocatechin-3-gallate suppresses IGF-I-induced lipogenesis and cytokine expression in SZ95 sebocytes. J Invest Dermatol. 2012;132:2700–2708. doi: 10.1038/jid.2012.202. [DOI] [PubMed] [Google Scholar]
- 12.Cappel M, Mauger D, Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch Dermatol. 2005;141:333–338. doi: 10.1001/archderm.141.3.333. [DOI] [PubMed] [Google Scholar]
- 13.Zouboulis CC. Is acne vulgaris a genuine inflammatory disease? Dermatology. 2001;203:277–279. doi: 10.1159/000051771. [DOI] [PubMed] [Google Scholar]
- 14.Zouboulis CC, Eady A, Philpott M, Goldsmith LA, Orfanos C, Cunliffe WC, et al. What is the pathogenesis of acne? Exp Dermatol. 2005;14:143–152. doi: 10.1111/j.0906-6705.2005.0285a.x. [DOI] [PubMed] [Google Scholar]
- 15.Webster GF. Inflammation in acne vulgaris. J Am Acad Dermatol. 1995;33:247–253. doi: 10.1016/0190-9622(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 16.Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–1699. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen JE. Diet and acne. Int J Dermatol. 1977;16:488–492. doi: 10.1111/j.1365-4362.1977.tb01861.x. [DOI] [PubMed] [Google Scholar]
- 18.Pappas A. The relationship of diet and acne: a review. Dermatoendocrinol. 2009;1:262–267. doi: 10.4161/derm.1.5.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liakou AI, Theodorakis MJ, Melnik BC, Pappas A, Zouboulis CC. Nutritional clinical studies in dermatology. J Drugs Dermatol. 2013;12:1104–1109. [PubMed] [Google Scholar]
- 20.Smith R, Mann N, Mäkeläinen H, Roper J, Braue A, Varigos G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718–726. doi: 10.1002/mnfr.200700307. [DOI] [PubMed] [Google Scholar]
- 21.Clarke SB, Nelson AM, George RE, Thiboutot DM. Pharmacologic modulation of sebaceous gland activity: mechanisms and clinical applications. Dermatol Clin. 2007;25:137–146. doi: 10.1016/j.det.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Alestas T, Ganceviciene R, Fimmel S, Müller-Decker K, Zouboulis CC. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med (Berl) 2006;84:75–87. doi: 10.1007/s00109-005-0715-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee WJ, Jung HD, Lee HJ, Kim BS, Lee SJ, Kim DW. Influence of substance-P on cultured sebocytes. Arch Dermatol Res. 2008;300:311–316. doi: 10.1007/s00403-008-0854-1. [DOI] [PubMed] [Google Scholar]
- 24.Lee WJ, Jung HD, Chi SG, Kim BS, Lee SJ, Kim DW, et al. Effect of dihydrotestosterone on the upregulation of inflammatory cytokines in cultured sebocytes. Arch Dermatol Res. 2010;302:429–433. doi: 10.1007/s00403-009-1019-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee WJ, Chae SY, Ryu HS, Jang YH, Lee SJ, Kim DW. Inflammatory cytokine expression and sebum production after exposure of cultured human sebocytes to ultraviolet a radiation and light at wavelengths of 650 nm and 830 nm. Ann Dermatol. 2015;27:163–170. doi: 10.5021/ad.2015.27.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirdamadi Y, Thielitz A, Wiede A, Goihl A, Papakonstantinou E, Hartig R, et al. Insulin and insulin-like growth factor-1 can modulate the phosphoinositide-3-kinase/Akt/FoxO1 pathway in SZ95 sebocytes in vitro. Mol Cell Endocrinol. 2015;415:32–44. doi: 10.1016/j.mce.2015.08.001. [DOI] [PubMed] [Google Scholar]