Abstract
Introduction
Capillary hypoperfusion is reported in asymptomatic adults at-risk for Alzheimer's disease (AD), but the extent that can be explained by reduced flow in intracranial arteries is unknown.
Methods
One hundred fifty-five asymptomatic adults enriched for AD risk (mean age 61 years) completed arterial spin labeling (pcASL) and 4D-flow MRI sequences. Voxel-wise regression models investigated the relationship between mean flow in bilateral cerebral arteries and capillary perfusion, and tested potential moderators of this relationship.
Results
Mean arterial blood flow through middle cerebral arteries (MCAs) and internal carotid arteries was positively associated with perfusion in large cortical clusters (P < .05, false discovery rate corrected). Trends were observed for the interactions MCA flow × age and MCA flow × cardiovascular risk on cerebral perfusion (P < .001, uncorrected).
Discussion
These findings provide evidence that capillary perfusion measured via pseudocontinuous arterial spin labeling is strongly dependent on inflow from larger cerebral arteries. Further studies are warranted to investigate possible alterations between macrovascular and microvascular flow in advanced age and elevated cardiovascular risk in asymptomatic adults at risk for AD.
Keywords: Cerebral blood flow, Alzheimer's disease, Cerebrovascular disease, Phase-contrast MRI, Arterial spin labeling, Vascular imaging
1. Introduction
Reduced cerebral blood flow (CBF), or hypoperfusion, may be an early marker of neurodegeneration that initiates a cascade of events preceding cognitive decline in Alzheimer's disease (AD) [1]. For example, cardiovascular risk factors may increase risk of cognitive decline by reducing CBF, which may in turn initiate increased production of β-amyloid and capillary hypoperfusion, ultimately inducing neuronal dysfunction [2]. Supporting this assertion, hypoperfusion is observed more frequently in individuals at risk for AD such as older adults [3] and those with a positive family history or genetic risk for AD [3], [4], [5], [6].
Prior studies suggest that cerebral perfusion closely parallels cerebral glucose metabolism and that perfusion measures may be used as surrogate measures for glucose metabolism (e.g., fluorodeoxyglucose [FDG]) without requiring radioactive tracer injection [7]. In favor of this interpretation are consistent patterns of hypoperfusion (measured via pseudocontinuous arterial spin labeling [pcASL] MRI or 15-O-water positron emission tomography [PET]) and hypometabolism (e.g., FDG-PET) among people at risk for AD and with symptomatic disease [8], [9], [10]. However, signal from pcASL is likely influenced by both neurometabolic activity and the health of the supplying arteries. Although prior studies of CBF in asymptomatic adults at risk for AD have investigated parenchymal capillary perfusion, there is little information regarding the effect of macrovascular health on perfusion in this population.
Measuring the relationship between intracranial vessel flow and cerebral perfusion was previously challenging because of practical limitations, including motion artifacts, lengthy scan times, and complex implementation [11]. However, a recently developed 4D-flow MRI technique termed phase-contrast vastly undersampled isotropic projection (PC VIPR) imaging uses radial undersampling, allowing for improved temporal and spatial resolution compared with 2D methods in clinically feasible scan times [12], [13]. Using this technique, lower mean blood flow and higher pulsatility (a marker of vessel stiffness) were observed in individuals with dementia due to AD compared with age-matched cognitively healthy peers, both of which were observed in anterior vessel segments (internal carotid arteries [ICAs] and middle cerebral arteries [MCAs]) [14]. Moreover, across a sample of cognitively healthy and cognitively impaired participants, lower flow in the MCAs and superior ICAs (sICAs) was associated with greater global brain atrophy [15].
Identifying early markers of cerebrovascular dysfunction that contribute to hypoperfusion and cognitive decline will be important for future secondary prevention strategies aiming to slow the impact of AD on cognition. Therefore, we investigated the relationship between cerebral macrovascular and microvascular blood flow in a sample of cognitively asymptomatic adults enriched for AD risk. To reduce the number of comparisons, only vessel segments that exhibited consistent detriments in prior studies were investigated (e.g., sICAs and MCAs). Furthermore, we investigated modifiable and invariable risk factors related to increased dementia risk as potential moderators of the relationship between microvascular and macrovascular CBF. We hypothesized there would be a positive relationship between mean arterial flow in the MCAs and sICAs (as measured via 4D-flow) and cerebral perfusion (as measured via pcASL). We also hypothesized that advanced age, greater cardiovascular risk, and genetic risk for AD would modify the relationship between intracranial arterial flow and cerebral perfusion.
2. Methods
2.1. Participants
Participants were enrolled in the Wisconsin Alzheimer's Disease Research Center clinical core, completing a comprehensive neuropsychological battery, physical examination, and fasting lipid panel. Study data were reviewed in a clinical consensus conference, and participants deemed cognitively healthy were included in the present study. One hundred sixty-two cognitively healthy participants underwent PC VIPR scans with complete MCA and sICA flow measurements and pcASL scans. Seven participants were excluded because of poor pcASL scan quality (n = 3), anatomic anomalies (hydrocephalus, cysts, prior neurosurgery, n = 3), or a recent head injury (n = 1). Of the 155 participants included in analyses, 119 were enrolled in the Investigating Memory in People at Risk, Causes and Treatments (ages 40–65 years) cohort and 36 were enrolled in the older adult (age >65 years) control cohort. The sample was enriched for participants at risk for AD (71% with positive parental history of AD). The University of Wisconsin Institutional Review Board approved all study procedures, and each participant provided signed informed consent.
2.2. Magnetic resonance imaging
Magnetic resonance imaging (MRI) scans were completed on a clinical 3T scanner (Discovery MR750; GE Healthcare, Waukesha, WI, USA) using an eight-channel head coil (Excite HD Brain Coil; GE Healthcare). All participants were instructed to abstain from food, tobacco, and caffeine at least 4 hours before the scan. A T1-weighted structural scan (BRAVO) was acquired axially using the following imaging parameters: 3D fast spoiled gradient echo sequence, inversion time = 450 ms; repetition time (TR) = 8.1 ms; echo time (TE) = 3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256; field of view (FOV) = 256 mm; and slice thickness = 1.0 mm. In postprocessing, the T1-weighted volume was segmented into tissue classes using the updated segmentation feature in Statistical Parametric Mapping version 12 (SPM12, www.fil.ion.ucl.ac.uk/spm). The segmentation procedure also produced a deformation field, allowing the T1 image to be mapped to Montreal Neurological Institute (MNI) standard space.
2.3. Intracranial arterial flow—PC VIPR
Blood flow within the bilateral MCAs and sICAs was measured using PC VIPR. The scanning parameters were as follows: velocity encoding (venc) = 80 cm/s, imaging volume = 22 cm3, acquired isotropic spatial resolution = 0.77 mm, TE = 2.7 ms, TR = 7.4 ms, flip angle α = 10°, bandwidth = 83.3 KHz, and 14,000 projection angles. Scan acquisition time was approximately 7 minutes. Hemodynamic evaluation was conducted using the velocity vector data acquired via the PC VIPR scans. Segmentation of the arterial tree was performed in MATLAB (The MathWorks, Natick, MA, USA) from PC angiograms constructed from the PC VIPR data. EnSight (CEI, Apex, NC, USA) was used for interactive flow visualization and manual selection of vessel planes for quantitative analysis. For this analysis, planes were placed 5 mm from the MCA origin and in the distal petrous portion of the ICA. Four-dimensional-flow MRI data were used to generate two-dimensional cine images with through-plane velocities at the selected planes; mean blood flow (mL/min) was calculated. Blood flow through the left and right MCAs and blood flow through the left and right sICAs were added together and divided by 2 to calculate an average blood flow for the MCAs and sICAs [16].
2.4. Cerebral tissue perfusion—pcASL
Cerebral perfusion was measured using background-suppressed pcASL MRI [10]. Images were acquired using a 3D fast spin echo spiral sequence, with a stack of variable density 4-ms readout and eight interleaves. The scan parameters for pcASL were as follows: TR = 6000 ms, TE = 21 ms, FOV = 240 × 240 × 160 mm, matrix size = 128 × 128, slice thickness = 4 mm no gap, number of excitations = 3, and labeling radiofrequency amplitude = 0.24 mG. Postlabeling delay was 2025 ms. A fluid-suppressed proton density (PD) scan was acquired without radiofrequency labeling, but using the same imaging sequence/slab parameters as the pcASL scan. As described previously, the three excitations were averaged to improve signal-to-noise ratio, and the PD sequence was used for flow quantification and image registration [5]. Scan time was approximately 4.5 minutes.
Postacquisition processing of the pcASL scans was performed using SPM12. Each participant's PD image was colocalized to their T1 image, and the derived transformation matrix was applied to the CBF map. Subsequently, the coregistered CBF map was spatially normalized to the MNI template with resampling to a 2 × 2 × 2 mm voxel size via the deformation field produced during tissue segmentation. The normalized CBF maps were then smoothed using an 8-mm full-width at half-maximum Gaussian kernel. Grand mean scaling to a value of 50 and proportional normalization were applied in all voxel-wise regression analyses [5]. A gray matter mask (created with SPM12 ImCalc, thresholded at 0.2) was applied to the resulting SPM.mat file to limit analyses to voxels with greater than 20% probability of containing gray matter.
2.5. Atherosclerotic Cardiovascular Disease risk score
The Atherosclerotic Cardiovascular Disease (ASCVD) risk score, developed by the American College of Cardiology and the American Heart Association, estimates an individual's risk of a major cardiac event (myocardial infarction and stroke) in the next 10 years [17]. The score is comprised of systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, age, sex, race, diabetes status, smoking status, and antihypertensive medication status. These data were acquired at the study visit closest to the MRI date. Analyses of ASCVD risk included 154 participants because one participant was missing complete ASCVD data. Owing to a nonnormal distribution, this score was log-transformed before inclusion in statistical models.
2.6. Genetic risk
Genotyping was conducted by LGC Genomics (Beverly, MA, USA) using competitive allele-specific PCR-based KASP genotyping assays, and quality control was performed as described previously [18]. Apolipoprotein E (APOE) risk was calculated as a polygenic risk score according to the odds ratio (OR) of the ε2/ε3/ε4 genotype, as indicated in the meta-analysis of APOE genotype frequencies reported in AlzGene [19]. The specific ORs used were based on studies of Caucasian individuals for consistency with the current sample and were calculated using the ε2/ε2 genotype as the reference (ε2/ε2 OR = 1) as follows: ε2/ε3 OR = 1.38, ε3/ε3 OR = 2.00, ε2/ε4 OR = 4.45, ε3/ε4 OR = 6.78, ε4/ε4 OR = 25.84 [18]. Analyses of APOE risk included 151 participants because four participants were missing genetic data.
2.7. Statistical analyses
SPSS statistics (version 23, IBM) was used to conduct analyses investigating relationships between perfusion, arterial flow, and hypothesized moderators (age, ASCVD risk, and APOE risk). Statistical significance was defined as P < .05. Multiple linear regression models tested the relationship between mean arterial flow (MCAs or sICAs) as the predictor variable and mean perfusion as the outcome variable, with age and sex as covariates. Bivariate correlations were conducted to assess the relationship between age and flow (perfusion, arterial flow). Multiple linear regression models (including age and sex covariates) were conducted to examine the relationship between blood flow (e.g., mean perfusion, mean arterial flow) and (1) ASCVD risk score or (2) APOE risk score.
Voxel-wise analyses were conducted using SPM12. Whole-brain voxel-wise multiple linear regression models included mean arterial flow (MCAs or sICAs) as the predictor variable, age and sex as covariates, and whole-brain cerebral perfusion as the outcome variable. Three subsequent multiple linear regression analyses added the following terms to the aforementioned model: (1) interaction of age × mean arterial flow, (2) main effect of ASCVD risk score and interaction of ASCVD risk score × mean arterial flow, and (3) main effect of APOE risk score and interaction of APOE risk score × mean arterial flow. A false discovery rate (FDR) correction at the P < .05 level and a cluster extent of >100 voxels was applied to adjust for multiple comparisons. For exploratory purposes, an uncorrected P < .001 threshold at the voxel level was also applied, and results are reported as trends.
3. Results
3.1. Sample characteristics
The sample was on average late middle-aged, highly educated, female, and Caucasian (see Table 1). The sample was enriched for AD risk with 71% with parental history of AD and 40% at genetic risk for AD. Mean 10-year risk of an atherosclerotic cardiovascular event was 9%.
Table 1.
Sample characteristics
| Characteristic | Value |
|---|---|
| N | 155 |
| Age (mean years; SD; range) | 61.1 (8.5; 45–86) |
| Sex (N; % female) | 111; 72% |
| Education (mean years; SD) | 16.46; 2.356 |
| Race/ethnicity | 97.4% Caucasian |
| Positive parental history of dementia (N; %) | 110; 71% |
| APOE ε4 positive (N; %) | 61; 39.4% |
| Mini-Mental State Examination (mean; SD; range) | 29.31; 0.883; 26–30 |
| Mean perfusion, mL/g/min tissue (mean; SD; range) | 33.77; 5.90; 15.22–51.38 |
| MCA mean flow mL/min (mean; SD; range) | 160.2; 32.4; 67.2–265.7 |
| sICA (petrous portion) mean flow mL/min (mean; SD; range) | 270.1; 49.9; 173.05–497.01 |
| ASCVD 10-y risk score % (mean; SD; range) | 9.1%; 11.7; 0.3%–69% |
| Diagnosed with diabetes (N; %) | 12; 7.7% |
| Systolic blood pressure, mmHg (mean; SD) | 127.5; 14.2 |
| Diastolic blood pressure, mmHg (mean; SD) | 73.7; 9.2 |
| Total cholesterol (mean; SD) | 195.5; 35.9 |
| HDL cholesterol (mean; SD) | 63.2; 20.1 |
| Taking blood pressure–lowering medication (N; %) | 46; 29.7% |
Abbreviations: APOE, apolipoprotein; ASCVD, Atherosclerotic Cardiovascular Disease; MCA, middle cerebral artery; sICA, superior portion of the internal carotid artery.
3.2. Relationship between arterial flow and cerebral perfusion
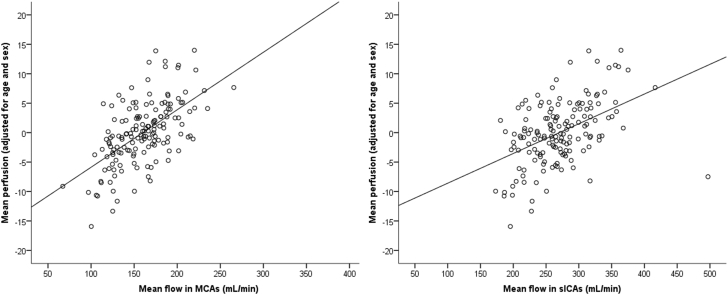
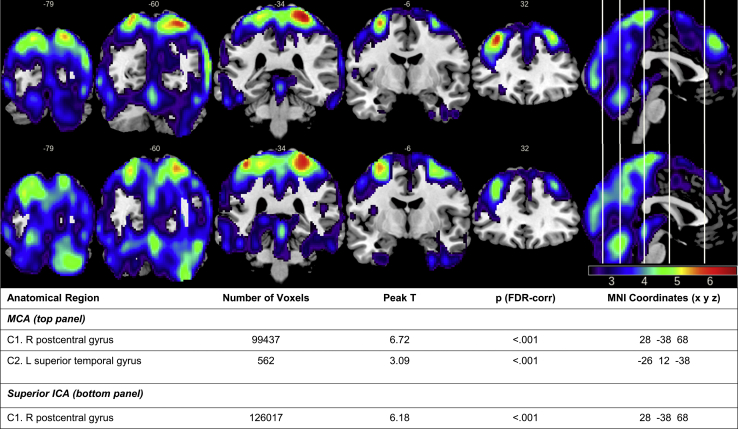
Mean flow in the MCAs and sICAs accounted for a significant amount of variance in mean perfusion, after adjusting for covariates of age and sex (MCA: β = 6.96, t = 10.23, P < .001; sICA: β = 3.32, t = 6.82, P < .001; see Fig. 1). A voxel-wise analysis revealed a statistically significant positive relationship between MCA flow and perfusion in a large cluster that encompassed several cortical regions, with peak voxels in the superior parietal lobe (postcentral gyrus) and lateral temporal lobe (superior temporal gyrus). Similarly, significant positive relationships were observed between sICA flow and cerebral perfusion in bilateral parietal and occipital regions, with a peak voxel also in the superior parietal lobe (postcentral gyrus). Regions exhibiting positive relationships between MCA and sICA flow and cerebral perfusion are displayed in Fig. 2. No negative relationships between sICA or MCA flow and perfusion were observed.
Fig. 1.
Significant positive relationship between mean perfusion and mean flow in MCAs (left) and sICAs (right), adjusted for age and sex in cognitively healthy middle-aged and older adults enriched for Alzheimer's disease risk (n = 155).
Fig. 2.
Results of voxel-wise analyses (t statistic map) demonstrating a positive relationship between mean flow in MCAs (top) and sICAs (bottom) and regional perfusion in cognitively healthy middle-aged and older adults (n = 155; P < .05 FDR corrected; cluster extent = 100).
3.3. Potential moderators of relationship between arterial flow and cerebral perfusion
3.3.1. Age
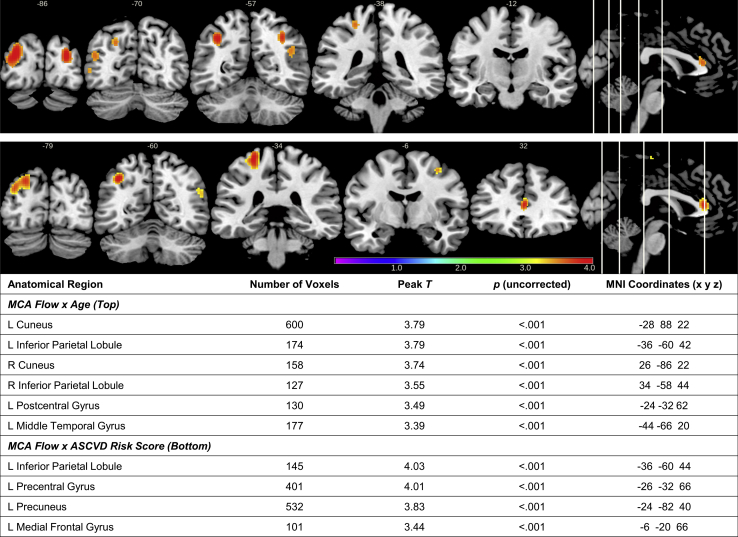
Age was negatively associated with mean flow in the MCAs (r = −.39, P < .001), sICAs (r = −.31, P < .001), and mean cerebral perfusion (r = −.39, P < .001). In voxel-wise analyses, the interaction term age × MCA flow or age × sICA flow on whole-brain perfusion was not statistically significant at the FDR-corrected threshold. Trends in the positive direction were observed for the interaction term of age × MCA mean flow on perfusion in parietal regions (inferior parietal lobule, postcentral gyrus), cuneus, and middle temporal gyrus (uncorrected P < .001; see Fig. 3). These findings suggest a potentially stronger relationship between MCA flow and perfusion in older age. In contrast, there was no relationship between age and sICA flow on perfusion (FDR corrected P < .05 or uncorrected P < .001).
Fig. 3.
Results of voxel-wise analyses (t statistic map) displaying clusters in which perfusion was positively associated with the age × MCA mean flow interaction term (top panel; n = 155; P < .001 uncorrected; cluster extent = 100) or the atherosclerotic cardiovascular disease (ASCVD) risk score × MCA mean flow interaction term (bottom panel; n = 154; P < .001 uncorrected; cluster extent = 100).
3.3.2. Cardiovascular risk
ASCVD risk score accounted for a nearly significant amount of variance in mean MCA flow (β = −0.4, t = −1.85, P = .067) and mean sICA flow (β = −0.6, t = −1.93, P = .056), and a statistically significant amount of variance in mean cerebral perfusion (β = −8.8, t = −4.46, P < .001), after accounting for variance associated with covariates of age and sex. The interaction of ASCVD risk score and MCA or sICA flow on perfusion was not statistically significant at the FDR-corrected threshold. Trends in the positive direction were observed, suggesting that higher ASCVD risk scores were associated with a stronger relationship between MCA flow and perfusion in left parietal and frontal regions (uncorrected P < .001; see Fig. 3). The interaction between ASCVD risk score and sICA mean flow was not significant at both FDR-corrected and uncorrected P < .001 thresholds.
3.3.3. Genetic risk
There were no significant relationships between the APOE risk score and mean flow in MCAs (P = .62), sICAs (P = .997), or mean perfusion (P = .78), after accounting for variance associated with age and sex. Similarly, voxel-wise analyses resulted in no significant interactions between APOE risk score and mean flow in MCAs or sICAs on perfusion, as well as no main effect of APOE risk score on perfusion.
4. Discussion
In this cognitively healthy late middle-aged sample enriched for AD risk, mean blood flow in the MCAs and sICAs was positively associated with cerebral perfusion across a majority of the cortex after adjusting for age and sex, supporting our hypothesis. Flow in the MCAs and sICAs was associated with perfusion in similar brain regions (postcentral gyrus); however, MCA flow was uniquely associated with perfusion in the superior temporal gyrus and frontal regions. Furthermore, results suggest that the relationship between macrovascular and microvascular blood flow may be disrupted by age and cardiovascular risk factors. In contrast, macrovascular or microvascular blood flow was not associated with the APOE status.
Prior studies have reported that increased age and greater cardiovascular risk factors are associated with lower perfusion in middle-aged at-risk adults [20]. This study extended prior results by demonstrating that elevated age and cardiovascular risk are associated with lower flow in the MCAs and ICAs. Furthermore, trends (e.g., uncorrected P < .001) in the positive direction were observed, suggesting a stronger relationship between flow in the MCAs and perfusion in parietal-occipital regions with older age and in frontal-parietal regions with higher cardiovascular risk. These findings suggest that with increasing age and greater cardiovascular risk, perfusion rate in the capillary beds may be more dependent on inflow from the MCAs; however, these results should be considered with caution and require replication. One potential confounder is that validated composite measures of vascular risk burden, including ASCVD, include age as a component of the risk score calculation. Therefore, it is possible that the associations with vascular risk are driven by age, rather than other factors such as hypertension or cholesterol levels. However, the regions in which age versus vascular risk score moderated the relationship between large-vessel flow and perfusion did not completely overlap, suggesting that there may be a component of the ASCVD score that independently affects this relationship above and beyond age. Future studies will continue to explore these questions through analyses investigating relationships between specific vascular risk factors and CBF. Although no similar relationships were observed in the sICAs in the present study, a recent study conducted by our group demonstrated a similar uncoupling of macrovasculature and microvasculature in the ICAs in cognitively healthy at-risk adults with higher insulin resistance [16].
Overall, these findings suggest that perfusion measured via pcASL is strongly dependent on inflow from larger cerebral arteries and, therefore, may not be an optimal functional imaging technique of neurometabolism in populations in which large-vessel flow is suspected to be compromised. Mechanisms underlying the development of hypoperfusion and potential alterations in macrovasculature and microvasculature require further investigation. Changes in vasculature that become more common with advanced age, such as atherosclerosis and vessel stiffening, can disrupt blood flow–based delivery of essential nutrients to neurons. In addition, elevated cerebrovascular resistance is commonly observed in individuals diagnosed with dementia due to AD [21], [22] and is postulated to contribute to pathologic changes in the cerebrovascular system, including atherosclerosis within intracranial vessels [23]. In turn, stenosis of cerebral arteries may partially cause reduced cerebral perfusion in AD [23]. In addition to large-vessel changes, growing evidence indicates that progressive disturbances in capillary flow may be a response to maintain adequate oxygenation of tissue and that decoupling of blood flow with metabolism occurs across both presymptomatic and postsymptomatic disease phases [24]. Furthermore, age and vascular risk factors are associated with altered capillary morphology, including increased permeability of the blood-brain barrier, which may contribute to capillary dysfunction [24], hippocampal atrophy, and early cognitive impairment [25]. Further studies are needed to examine relationships among vessel diameter, stiffness, and flow to clarify if hypoperfusion in preclinical AD can be explained by cerebrovascular pathology. In addition, while the current results demonstrate a strong positive relationship between perfusion and arterial blood flow, more investigation is needed on the direct effect of intracranial vascular health on glucose metabolism, as FDG-PET data were not available for these participants.
Limitations of this study should be noted. Blood flow within the MCAs and sICAs was examined specifically because of hypotheses based on prior reported associations with global atrophy and dementia. However, it is possible that collateral flow in the circle of Willis that was not examined (e.g., basilar artery) may moderate relationships between the vessels examined and perfusion in aging and vascular risk. In addition, future studies assessing contributions of AD biomarkers, blood-brain barrier integrity, vessel stiffness, mean arterial pressure, and cerebrovascular resistance are needed to provide greater context for the current results. This study also did not collect data on extracranial carotid artery status or cardiac dysfunction which may affect CBF, and future studies investigating the relationship between extracranial and intracranial flow in this population are needed. Furthermore, the sample is enriched for AD risk, and these results may not generalize to other cognitively healthy middle-aged populations. It is also important to acknowledge that the current sample is primarily composed of Caucasian participants (97.4%), and thus, findings may lack generalizability to broader populations. With a concerted effort to enroll more participants in our studies from groups traditionally underrepresented in research, future studies will be able to investigate cerebrovascular function in a more generalizable and diverse sample. Despite these limitations, our study provides evidence of a strong relationship between intracranial arterial flow and capillary perfusion and suggests future directions to explore potential breakdown in this connection in the middle age.
A recent study based on a large sample of older adults enrolled in the Alzheimer's Disease Neuroimaging Initiative reported that reduced CBF may be the first abnormality that is detectable in the progression of AD, even before amyloid and tau alterations [26]. Therefore, developing and applying sensitive measures of cerebrovascular function (PC VIPR and pcASL) in the earliest stages of disease before development of clinical symptoms will be essential to improving understanding of mechanisms underlying hypoperfusion. In addition, as vascular health during midlife is associated with dementia risk in late life [27], understanding alterations in CBF in cognitively healthy adults at risk for AD is important for development of effective secondary prevention strategies to improve cerebrovascular health and ultimately slow cognitive decline.
Research in Context.
-
1.
Systematic review: We examined relevant literature via PubMed. Although MRI methods of arterial spin labeling and intracranial 4D-flow imaging have been used independently to study cerebral blood flow in individuals at risk for Alzheimer's disease (AD), information was limited on the comparison between these modalities and the relationship between large-vessel blood flow and tissue perfusion in this population.
-
2.
Interpretation: Our findings suggest that there is a strong relationship between intracranial arterial vessel flow and local tissue perfusion in cognitively healthy adults at risk for AD. These relationships are moderated at a trend level by age and cardiovascular disease risk.
-
3.
Future directions: Follow-up studies should include extension to a larger sample, examination of longitudinal relationships between large-vessel blood flow and perfusion alterations, and contributions of AD biomarkers to hypoperfusion. In addition, studies exploring the impact of cerebrovascular health on measures of neuronal function in asymptomatic at-risk adults are warranted.
Acknowledgments
The project described was supported by the Clinical Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS), and grant UL1TR00427. Funding support was also provided by NIH grants ADRC P50 AG033514 (S.A.), F30 AG054115 (S.E.B.), T32 GM007507 (S.E.B.), and T32 GM008692 (S.E.B.). Funding support was also provided by grants from the Foundation of the American Society of Neuroradiology (S.C.J.), the University of Wisconsin School of Medicine and Public Health Rath Distinguished Graduate Research Fund (S.E.B.), and the Wisconsin Alzheimer's Institute Lou Holland Research Fund (L.R.C.). The content is solely the responsibility of the authors and does not represent the official views of NIH. The authors deny any conflicts of interest related to this project. We gratefully acknowledge the assistance of Amy Hawley, Laura Hegge, Jennifer Oh, Chuck Illingworth, and researchers and staff at the Wisconsin Institutes for Medical Research for assistance in recruitment, data collection, and analysis. Most importantly, we thank the dedicated participants of the ADRC for their continued support and participation in this research.
References
- 1.Wolk D.A., Detre J.A. Arterial spin labeling MRI: an emerging biomarker for Alzheimer's disease and other neurodegenerative conditions. Curr Opin Neurol. 2012;25:421–428. doi: 10.1097/WCO.0b013e328354ff0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw T.G., Mortel K.F., Meyer J.S., Rogers R.L., Hardenberg J., Cutaia M.M. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34:855–862. doi: 10.1212/wnl.34.7.855. [DOI] [PubMed] [Google Scholar]
- 4.Kim S.M., Kim M.J., Rhee H.Y., Ryu C.W., Kim E.J., Petersen E.T. Regional cerebral perfusion in patients with Alzheimer's disease and mild cognitive impairment: effect of APOE epsilon4 allele. Neuroradiology. 2013;55:25–34. doi: 10.1007/s00234-012-1077-x. [DOI] [PubMed] [Google Scholar]
- 5.Okonkwo O.C., Xu G., Oh J.M., Dowling N.M., Carlsson C.M., Gallagher C.L. Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer's disease. Cereb Cortex. 2014;24:978–988. doi: 10.1093/cercor/bhs381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wierenga C.E., Clark L.R., Dev S.I., Shin D.D., Jurick S.M., Rissman R.A. Interaction of age and APOE genotype on cerebral blood flow at rest. J Alzheimers Dis. 2013;34:921–935. doi: 10.3233/JAD-121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musiek E.S., Chen Y., Korczykowski M., Saboury B., Martinez P.M., Reddin J.S. Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer's disease. Alzheimers Dement. 2012;8:51–59. doi: 10.1016/j.jalz.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander G.E., Chen K., Pietrini P., Rapoport S.I., Reiman E.M. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer's disease treatment studies. Am J Psychiatry. 2002;159:738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 9.Reiman E.M., Caselli R.J., Yun L.S., Chen K., Bandy D., Minoshima S. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 10.Xu G., Rowley H.A., Wu G., Alsop D.C., Shankaranarayanan A., Dowling M. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer's disease. NMR Biomed. 2010;23:286–293. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turski P., Scarano A., Hartman E., Clark Z., Schubert T., Rivera L. Neurovascular 4DFlow MRI (phase contrast MRA): emerging clinical applications. Neurovascular Imaging. 2016;2:1–11. [Google Scholar]
- 12.Gu T., Korosec F.R., Block W.F., Fain S.B., Turk Q., Lum D. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol. 2005;26:743–749. [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson K.M., Lum D.P., Turski P.A., Block W.F., Mistretta C.A., Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med. 2008;60:1329–1336. doi: 10.1002/mrm.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivera-Rivera L.A., Turski P., Johnson K.M., Hoffman C., Berman S.E., Kilgas P. 4D flow MRI for intracranial hemodynamics assessment in Alzheimer's disease. J Cereb Blood Flow Metab. 2016;36:1718–1730. doi: 10.1177/0271678X15617171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berman S.E., Rivera-Rivera L.A., Clark L.R., Racine A.M., Keevil J.G., Bratzke L.C. Intracranial arterial four-dimensional flow is associated with metrics of brain health and Alzheimer's disease. Alzheimers Dement (Amst) 2015;1:420–428. doi: 10.1016/j.dadm.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoscheidt S.M., Kellawan J.M., Berman S.E., Rivera-Rivera L.A., Krause R.A., Oh J.M. Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16663214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Gibbons R. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 18.Darst B.F., Koscik R.L., Racine A.M., Oh J.M., Krause R.A., Carlsson C.M. Pathway-specific polygenic risk scores as predictors of amyloid-beta deposition and cognitive function in a sample at increased risk for Alzheimer's disease. J Alzheimers Dis. 2017;55:473–484. doi: 10.3233/JAD-160195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AlzGene. Available at: alzgene.org. Accessed July 15, 2015.
- 20.Birdsill A.C., Carlsson C.M., Willette A.A., Okonkwo O.C., Johnson S.C., Xu G. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring) 2013;21:1313–1320. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gommer E.D., Martens E.G., Aalten P., Shijaku E., Verhey F.R., Mess W.H. Dynamic cerebral autoregulation in subjects with Alzheimer's disease, mild cognitive impairment, and controls: evidence for increased peripheral vascular resistance with possible predictive value. J Alzheimers Dis. 2012;30:805–813. doi: 10.3233/JAD-2012-111628. [DOI] [PubMed] [Google Scholar]
- 22.Nation D.A., Wierenga C.E., Clark L.R., Dev S.I., Stricker N.H., Jak A.J. Cortical and subcortical cerebrovascular resistance index in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2013;36:689–698. doi: 10.3233/JAD-130086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roher A.E., Tyas S.L., Maarouf C.L., Daugs I.D., Kokjohn T.A., Emmerling M.R. Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimers Dement. 2011;7:436–444. doi: 10.1016/j.jalz.2010.08.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Østergaard L., Aamand R., Gutiérrez-Jiménez E., Ho Y.C., Blicher J.U., Madsen S.M. The capillary dysfunction hypothesis of Alzheimer's disease. Neurobiol Aging. 2013;34:1018–1031. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iturria-Medina Y., Sotero R.C., Toussaint P.J., Mateos-Perez J.M., Evans A.C. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng X.F., Yu J.T., Wang H.F., Tan M.S., Wang C., Tan C.C. Midlife vascular risk factors and the risk of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42:1295–1310. doi: 10.3233/JAD-140954. [DOI] [PubMed] [Google Scholar]