Abstract
Introduction
The Alzheimer's biomarkers in daily practice (ABIDE) project is designed to translate knowledge on diagnostic tests (magnetic resonance imaging [MRI], cerebrospinal fluid [CSF], and amyloid positron emission tomography [PET]) to daily clinical practice with a focus on mild cognitive impairment (MCI)
Methods
ABIDE is a 3-year project with a multifaceted design and is structured into interconnected substudies using both quantitative and qualitative research methods.
Results
Based on retrospective data, we develop personalized risk estimates for MCI patients. Prospectively, we collect MRI and CSF data from 200 patients from local memory clinics and amyloid PET from 500 patients in a tertiary setting, to optimize application of these tests in daily practice. Furthermore, ABIDE will develop strategies for optimal patient-clinician conversations.
Discussion
Ultimately, this will result in a set of practical tools for clinicians to support the choice of diagnostic tests and facilitate the interpretation and communication of their results.
Keywords: Dementia, Alzheimer's disease, Mild cognitive impairment, Biomarkers, Clinical practice
1. Introduction
The advent of magnetic resonance imaging (MRI) and the discovery of cerebrospinal fluid (CSF) biomarkers and amyloid positron emission tomography (PET) are among the greatest successes in Alzheimer's disease (AD) research, allowing an AD diagnosis in an earlier stage of disease [1], [2], [3], [4], [5]. Despite a wealth of literature on AD biomarkers, there is a gap between the published value and the actual utilization of biomarkers in daily clinical practice [6], [7].
Structural MRI biomarkers incorporated in the National Institute on Aging and the Alzheimer's Association diagnostic criteria for AD include atrophy, for example, of the medial temporal lobe, as marker for neurodegeneration [8]. The criteria however lack recommendations for preferred methods to establish atrophy (quantitatively vs. qualitatively), nor do they provide cutoffs [6], [7]. CSF biomarkers, amyloid-beta 1–42 (Aβ1–42), total tau (tau), and phosphorylated tau (p-tau), discriminate AD patients from persons with normal aging with high accuracy and predict dementia in patients with mild cognitive impairment (MCI) [9], [10], [11]. However, there is considerable intervariability and intravariability in the measured levels between the manually operated CSF platforms, which limits comparability between centers and establishing cutoff values [12]. Automated platforms are now being developed, and this is expected to reduce analytical variability [13]. The introduction of amyloid PET in 2004 allowed visualization of amyloid pathology in vivo and the subsequent development of 18F-labeled tracers enabled widespread implementation in memory clinics [1], [14]. Appropriate use criteria for amyloid imaging have been published, but at that time, experience with amyloid PET was limited and based on data from highly selected research populations, so it remains unclear which patients benefit most from costly amyloid PET imaging in the diagnostic tree [15].
Furthermore, MRI, CSF, and amyloid PET predict progression from MCI to dementia, but most available literature is based on group-level data [16], [17], [18], [19]. Translating results to individual patients in daily practice is difficult, as the prognostic value of each biomarker may vary with, for example, age, gender, and cognitive status. Moreover, when combining biomarkers, interpretation of results becomes complicated, especially when they are not clearly positive, negative, or even conflicting. Also, little is known about patients' preferences towards diagnostic testing and best ways of communicating test results with patients and their caregivers. Such conversations about initiating diagnostic testing or communicating test results are challenging for both patients and clinicians. To date, it remains unknown how these conversations are conducted in the clinical routine setting, and how patients and clinicians experience and value these conversations.
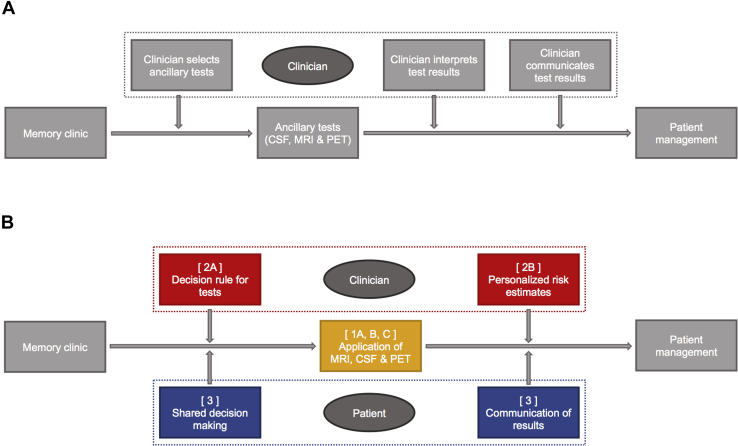
The Alzheimer's biomarkers in daily practice (ABIDE) project has been designed to address the need for a translation of the scientific value of AD biomarkers to actual daily utilization in local memory clinics. Fig. 1 describes the current patient journey of someone attending a regular memory clinic and provides an overview of how ABIDE objectives aim to improve this. ABIDE addresses both the practical use of AD biomarkers by the clinician and how to take patients' preferences towards testing and communication of test results into account. Ultimately, this should lead to a more personalized approach of the patient in their journey toward a diagnosis in memory clinics.
Fig. 1.
Patient journey in memory clinics. (A) Current patient journey in memory clinics. (B) ABIDE patient journey in memory clinics. Abbreviations: ABIDE, Alzheimer's biomarkers in daily practice; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PET, positron emission tomography.
Specific objectives include the following: (1) To decide on the most useful application of specific AD diagnostic tests in clinical practice (Fig. 1B; displayed in orange), including (A) comparison of visual rating and volumetric MRI markers of whole brain atrophy and atrophy of the medial temporal lobe, (B) comparison of manual and automated CSF biomarker platforms, and (C) identify patients that benefit most from amyloid PET. (2) To (A) develop personalized risk estimates in MCI patients for (time to) progression to dementia, and (B) to develop personalized risk estimates in MCI patients for (time to) progression to dementia (displayed in red). (3) To identify optimal strategies, tailored to patients' characteristics, to effectively involve patients in the decision about diagnostic testing (shared decision making) and communicate results of diagnostic tests (displayed in blue). (4) To develop a tool with algorithms for diagnostic testing and personalized risk estimates, and to facilitate communication about test results (not displayed).
2. Methods
2.1. Study design
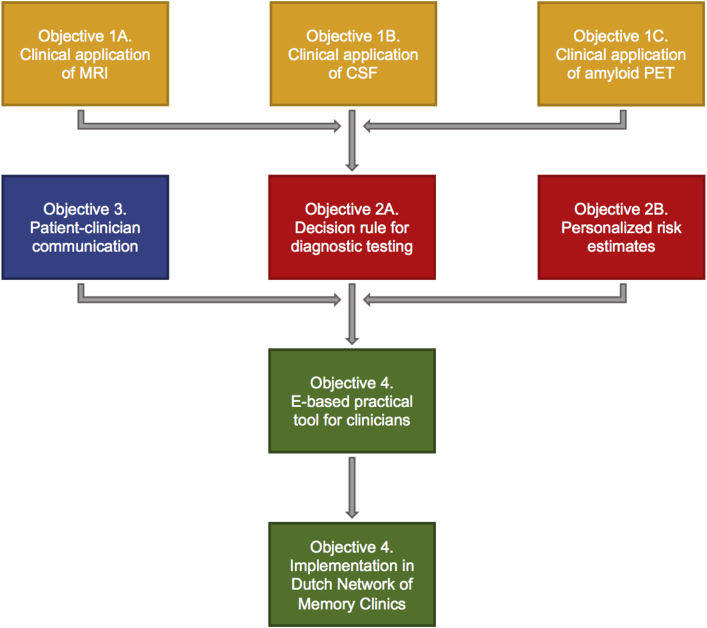
ABIDE is a 3-year project which has been funded in the context of the Dutch national dementia plan (https://www.deltaplandementie.nl/en; project number: 733050201). ABIDE has a multifaceted design and is structured into five substudies each with their own objective, design, patient sample, and data collection. Fig. 2 shows the structure of ABIDE and demonstrates how results from one objective feeds into the next. In the following, we describe the substudies separately. Table 1 provides an overview of the link between objectives, design, patient samples, and outcome measures.
Fig. 2.
Hierarchy of ABIDE objectives. Abbreviation: ABIDE, Alzheimer's biomarkers in daily practice; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PET, positron emission tomography.
Table 1.
ABIDE substudies and corresponding objectives and patient cohorts
| Substudy | Objective | Cohort | Data collection | Sample |
Measures |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Setting | Population | MRI | CSF | PET | CON | ||||
| 1 | 1A, B | Prospective | Cross-sectional | n = 200 | Local | Mixed | √ | √ | ||
| 2 | 1C | Prospective | Longitudinal | n = 450 | Tertiary | Mixed | √ | √ | √ | |
| 2 | 1C | Prospective | Longitudinal | n = 50 | Tertiary | MCI | √ | √ | √ | |
| 3 | 2A, B | Retrospective | Longitudinal | n = 400 | Tertiary | MCI | √ | √ | ||
| 4 | 3 | Prospective | Cross-sectional | n = 120 | Local | Mixed | (√) | (√) | √ | |
Abbreviations: ABIDE, Alzheimer's biomarkers in daily practice; MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; PET, positron emission tomography; CON, memory clinic consultation between clinician and patient and caregiver(s); MCI, mild cognitive impairment.
2.2. Substudy 1—application of MRI and CSF biomarkers (objectives 1A and 1B)
2.2.1. Patients and design
In this cross-sectional multicenter study designed to compare different measurement methods of MRI and CSF biomarkers, we include 200 patients with subjective cognitive decline (SCD), MCI, or dementia from 10 Dutch local memory clinics. Clinical assessment is according to local practice, including at least medical and informant history, physical examination, and cognitive testing. All patients who are offered MRI and/or lumbar puncture for diagnostic purposes are eligible for the study.
2.2.2. Methods—clinical data
We collect basic demographic and clinical information including age, gender, medical history, use of medication, clinical diagnosis, Mini-Mental State Examination, neuropsychological evaluation, and CDR. We use OpenClinica open source software, version 3.1 (Copyright © OpenClinica LLC and collaborators, Waltham, MA, USA) for data management. In addition, we use The Older Persons and Informal Caregivers Survey Minimal DataSet instrument, which feeds into a uniformly collected public data repository that contains information on the physical and mental health and well-being of older persons and informal caregivers across the Netherlands [20].
2.2.3. Methods—MRI
Structural MRI is performed according to local acquisition protocols and includes at least 3D T1-weighted imaging. Scans are collected centrally at the VU University Medical Center (VUmc). After a quality check, we obtain visual and volumetric measures of medial temporal lobe atrophy, posterior cortical atrophy, and global cortical atrophy [21], [22], [23]. MRIs will also be visually assessed for white-matter hyperintensities, number of infarcts, lacunes and microbleeds [24]. We derive quantitative measures of atrophy via the software package SIENAX (Structural Image Evaluation using Normalization of Atrophy Cross-sectional) [25]. The algorithm FIRST (FMRIBs integrated registration and segmentation tool, FSL 4.15) will be used to estimate volumes of gray-matter structures including the hippocampus.
2.2.4. Methods—CSF
CSF is collected in polypropylene tubes of 10 mL (Sarstedt) after lumbar puncture between L3/L4 or L4/L5 intervertebral space by a needle and syringe. CSF is centrifuged and then divided into two new polypropylene tubes (2.5 mL in one, remainder in other). The tubes are sent to the VUmc Neurochemistry Laboratory, Department of Clinical Chemistry at the VUmc. CSF is frozen at −80°C until analysis of the biomarkers (Aβ1–42, tau, and p-tau). CSF samples will be analyzed using the manually operated Innotest enzyme-linked immunosorbent assay [26], [27]. In addition, we use a novel Roche automated platform, Elecsys immunoassays [28].
2.2.5. Statistics
We will compare (1) visual and volumetric MRI measures and (2) CSF measurements from both platforms using Spearman's rank correlation and concordance measures. For CSF platform comparison, we will use Passing Bablok regression analysis. Accuracy, sensitivity, specificity, and negative and positive predictive value will be compared.
2.3. Sub-study 2—application of amyloid PET (objective 1C)
2.3.1. Patients and design
In this prospective observational study, we offer amyloid PET to all patients visiting our tertiary referral center during 1 year (n = 450) to assess its diagnostic value in an unselected sample of memory clinic patients. Standard diagnostic workup at the memory clinic of the VUmc Alzheimer Center includes medical history, neurological examination, neuropsychological evaluation, basic laboratory testing, and MRI [29]. Additionally, we enroll 50 MCI patients with similar diagnostic workup from the University Medical Center Utrecht (UMCU) to enrich the cohort for MCI. We will follow all patients for one year, to verify diagnosis (dementia) or to assess clinical progression (SCD and MCI). Primary outcome measures are change in diagnosis, change in confidence in diagnosis, and change in planned patient management following amyloid PET results.
2.3.2. Methods
At the VUmc, amyloid PET scans are made with 3-Tesla Philips Ingenuity TF PET/MR, Philips Ingenuity TF PET/CT and Philips Gemini TF PET/CT scanners. UMCU uses a Siemens Biograph 40 MCT scanner. Before and after scanning, patients fill out a questionnaire regarding their expectations and perceptions of amyloid PET. Patients are injected with a tracer dose of approximately 300 MBq ± 20% 18F-florbetaben (NeuraceqTM). The image acquisition window extends from 90 to 110 minutes (4 × 5-minute frames) after dose injection. PET scans are collected centrally at the VUmc and visually assessed by an experienced nuclear physician (B.V.B.) for amyloid positivity (yes/no).
For each patient, a preamyloid and postamyloid PET diagnosis is obtained with a level of confidence indicated by the neurologist (F.B. at the VUmc and G.J.B. at the UMCU) varying from 0%–100%. Also, the neurologists are asked about patient management before and after amyloid PET scan results are revealed, to assess a change in ancillary investigations, care, and medication.
2.3.3. Statistics
We will assess change of syndrome diagnosis and suspected underlying pathophysiology. To assess differences in change of diagnostic confidence among diagnostic groups, we will use analysis of variance for repeated measures with group as between-subjects variable and (change in) diagnostic confidence as within-subjects variable. Finally, we will evaluate whether the appropriate use criteria for amyloid imaging select those patients that are affected most by amyloid PET, in terms of change in diagnosis and change of planned management.
2.4. Substudy 3—algorithms for diagnostic testing and personalized risk estimates (objectives 2A and 2B)
2.4.1. Patients and design
In this retrospective, longitudinal study designed to develop individualized risk estimates, we include MCI patients with baseline MRI and/or CSF and at least one year of clinical follow-up from the Amsterdam Dementia Cohort [29]. Clinical diagnoses of MCI were made in a multidisciplinary team according to international guidelines [6], [31]. Progression to AD dementia at clinical follow-up is the outcome measure [8].
2.4.2. Methods—personalized risk estimates
We will use Cox proportional hazards analyses to develop prognostic models for MRI biomarkers (ordinal), for CSF biomarkers (continuous), and for the two combined. To account for patient diversity, we will explicitly take gender, age, and MMSE into account [32], [33]. The resulting models will allow to derive individual risk estimates and 95% confidence intervals for a patient with any given age, gender, MMSE, and biomarkers result (MRI and/or CSF). In addition, we will obtain estimates of probability of progression, within 1 year and within 3 years, facilitating translation of these risk scores to the clinical setting. Five-fold cross-validated Harrell's concordance index will be used to validate the models.
2.4.3. Methods—algorithms for diagnostic testing
To develop algorithms for selection of diagnostic tests, that is, MRI and CSF, we will perform an extensive literature search on the diagnostic and prognostic value of individual and combined tests, taking special care to account for patient diversity [34]. We will combine the literature search results with the retrospective cohort data to develop algorithms for selection of tests. Subsequently, we will validate these rules in the prospective sample of local memory clinics (see objective 1).
2.5. Substudy 4—identifying optimal strategies for shared decision making and communication of test results (objective 3)
2.5.1. Patients, design, and methods
We will use qualitative and quantitative research methods to study shared decision making and patient–clinician communication. First, to identify which diagnostic dilemmas occur in the consultation room, we will organize separate focus groups for clinicians, patients, and caregivers (n = 10 per focus group). Participants will be asked to share their views, experiences, and perceived dilemmas regarding diagnostic testing and communication of test results. Focus group discussions will be audiotaped, transcribed, and coded using MAXQDA software [30].
Second, we will perform an observational audiotaping study in the routine diagnostic workup of dementia. We will assess patient–clinician communication in both prediagnostic and postdiagnostic testing consultations in 12 memory clinics (n = 10 per clinician, n = 240 consultations in total). After each consultation, patients and their caregivers will receive a brief questionnaire on their views and experiences. Based on insights gained from the consultations and focus groups, we will extract a set of recommendations on how to effectively involve patients and caregivers in deciding about diagnostic testing, and on how to best discuss the results of such diagnostic tests.
2.6. Substudy 5—practical e-based tools and implementation (objective 4)
Ultimately, to facilitate communication about diagnostic tests in the daily routine of memory clinics, we aim to develop tools that can be used by clinicians in daily practice. We will combine our results from the best application of MRI, CSF, and amyloid PET biomarkers (objective 1) with the developed algorithms for diagnostic testing, personalized risk estimates (objective 2), and recommendations for patient–clinician communication (objective 3) to create practical tools that can be used in the care process (Fig. 2).
To prepare for the implementation and test phase of these tools, we will develop training sessions for clinicians. We will prospectively pilot and validate the tools in the panel of participating local memory clinics. To provide an infrastructure for nationwide implementation of ABIDE results in the Netherlands, we recently established the Dutch Memory Clinic Network. This network aims to provide a platform for clinicians working at the more than 90 memory clinics in the Netherlands, enabling them to share new knowledge, harmonize diagnostic and treatment protocols, and facilitate participation in research.
3. Expected results
ABIDE officially started on December 1, 2014 and is a 4-year project (end date: November 30, 2018). In the first year, we obtained institutional review board approval for the different substudies. Subsequently, we started with the inclusion of patients for the different substudies (Table 1). To date, at the end of the second project year, inclusion targets for substudies 1, 2, and 3 have largely been reached. In substudy 1, we collected n = 202 patients with MRI (target: n = 200) and n = 135 with CSF (target: n = 200) from local memory clinics. In substudy 2, we collected n = 495 memory clinic patients with amyloid PET (target: n = 450 mixed memory clinic + n = 50 MCI). The retrospective data collected from the Amsterdam Dementia Cohort for substudy 3 includes n = 525 MCI patients (mean age 67 [±8] years; 60% males; mean MMSE 27 [±2]; mean follow-up duration 2.4 [±1.6] years). Finally, for substudy 4, we held focus groups to identify and discuss diagnostic dilemmas with professionals, patients, and caregivers and conducted an online survey among almost 100 memory clinic professionals. We are currently conducting the audiotape study and have included n = 65 of prediagnostic and postdiagnostic audiotaped consultations from local memory clinics (target n = 120). Data lock for substudies 1 and 2 is expected in Q1 of 2017 and for substudy 4 in Q3 2017.
In the third project year, we will finish data collection, perform statistical analyses, create a prototype of the practical e-based tool (substudy 5), and publish first results. The fourth and final year will consist of finalizing data analyses, publishing results, and pilot and validate the practical e-based tool in local memory clinics.
An important milestone was the launch of the Dutch Memory Clinics Network (Nederlands Geheugenpoli Netwerk [NGN]; www.geheugenpoliklinieken.nl), linking the more than 90 memory clinics in the Netherlands. This network will facilitate the exchange of knowledge and resources, harmonize diagnostic and treatment protocols, and facilitate participation in research. In addition, we will use this network for dissemination of ABIDE results.
4. Discussion
The major research advances in AD biomarkers (MRI, CSF, and amyloid PET) have led to earlier and more accurate diagnoses, but these developments come with new challenges. First, the availability of biomarker tests poses the clinician for the challenge to select the right tests for each patient. Second, effectively communicating with patients and deciding mutually whether to use certain tests is difficult, especially in view of the cognitive deficits that come with (prodromal) AD. Third, biomarker results could affect (future) choices made by patients and their caregivers, especially when it concerns nondemented patients. With the advent of AD biomarkers, their disclosure to nondemented patients in clinical practice is therefore an emerging topic in research. Fourth, from an economic perspective, biomarkers should only be applied if the results are useful, for example, when changing patient management or preventing crises later in the disease process. The ABIDE study aims to address the practical use of AD biomarkers by the clinician and how to take patients' preferences toward testing and communication of test results into account.
In contrast to amyloid PET, MRI and CSF biomarkers are already widely used in clinical practice. Nonetheless, even for these tests, a lot of work still needs to be done to translate research findings to daily practice as clinicians vary greatly in their knowledge of these markers. In this context of clinical use of AD biomarkers, ABIDE is aligned with several ongoing research initiatives. For example, the Geneva Task Force for the Roadmap of Alzheimer's biomarkers works on a plan of actions that are needed to accelerate the implementation of AD biomarkers in daily practice [35], [36]. ABIDE adds to this study by prospectively evaluating the use of MRI and CSF in local memory clinics. When it comes to amyloid PET, its use is self-evident with respect to clinical trials in AD (e.g., generation [NCT02565511], early [NCT02569398], and A4 [NCT02008357]), but clinical utility still has to be established [37], [38]. Currently, the Imaging Dementia–Evidence for Amyloid Scanning study (www.ideas-study.org) in the United States and the Amyloid Imaging to Prevent Alzheimer's Disease study (http://www.amypad.eu) in Europe assess the clinical utility of amyloid PET in the diagnostic workup of AD. ABIDE adds to these initiatives by including an unselected memory clinic cohort. This will allow us to empirically evaluate the appropriate use criteria for amyloid imaging.
The decision for clinicians whether to initiate diagnostic testing and choosing a test is still quite novel, so it is not yet common practice to involve patients and their caregivers in their diagnostic dilemmas. ABIDE will provide insight in the degree of shared decision making during the diagnostic dementia workup and will help identify roadblocks for the involvement of patients and their caregivers. By translating this knowledge into recommendations for best practice, we can facilitate patients and clinicians to engage in a conversation and work together in choosing care that fits the individual patient. This supports their autonomy and ensures personalized care and management strategies.
In the context of preclinical AD trials, some work has been done on risk disclosure to nondemented individuals, based on APOE genotype and/or amyloid status [39], [40], [41], [42]. Also, the European Prevention of Alzheimer's Dementia will address ethical aspects of risk disclosure [43]. However, none of these studies focuses on disclosure of test results to nondemented patients in the clinical setting. ABIDE adds to these ongoing initiatives, as we focus on diagnostic dilemma's in daily clinical routine and will deliver practical support to facilitate communication of test results in the clinical setting.
The worldwide costs of dementia care keep rising, and treatments that prevent or delay AD are not yet available so far [44]. Diagnosing patients in an early stage of disease is important instrument to manage the impact of dementia but also comes with costs [45]. Early diagnosis might reduce costs later on in the disease process, as a well-informed patient is less likely to experience crisis situations or premature institutionalization. ABIDE attempts to harmonize and improve the diagnostic workup with the ultimate goal of patient-centered diagnostic care, based on current scientific knowledge, clinician expertise, and patient preferences. Evaluation whether such strategies are cost-efficient will be a necessary next step [46].
ABIDE attempts to take the next step in the dementia workup by considering what specific test results imply for individual patients. We will incorporate the developed individualized risk models in practical tools, which will facilitate the use of these diagnostic tests by clinicians in daily practice. By involving the main stakeholders of these novel diagnostic tests, that is, patients, caregivers, and professionals working in local memory clinics, ABIDE attempts to truly translate findings from research to the clinic, with the ultimate goal to improve the quality of diagnostic care.
Research in context.
-
1.
Systematic review: We searched PubMed for literature on diagnostic value and practical use of Alzheimer's disease (AD) biomarkers and guidelines. We concluded that guidelines on which test to use, in which setting, for which patient and how to weigh and communicate biomarker results to patients are lacking.
-
2.
Interpretation: Alzheimer's biomarkers in daily practice (ABIDE) has been designed to address the need for a translation of the scientific value of AD biomarkers to actual daily utilization by local memory clinic physicians. ABIDE is aligned with several ongoing research initiatives (Imaging Dementia–Evidence for Amyloid Scanning, Amyloid Imaging to Prevent Alzheimer's Disease, Geneva Task Force for the Roadmap of Alzheimer's Biomarkers, European Prevention of Alzheimer's Dementia) in that it studies clinical utility of AD biomarkers. ABIDE takes the next step in the dementia diagnostic workup by considering what specific test results imply for individual patients.
-
3.
Future directions: ABIDE attempts to develop patient-centered diagnostic care. To achieve this, we will develop e-based tools to support the diagnostic process.
Acknowledgments
Research of the VUmc Alzheimer Center is part of the neurodegeneration research program of Amsterdam Neuroscience. The VUmc Alzheimer Center is supported by Alzheimer Nederland and Stichting VUmc fonds. This study is funded by ZonMW-Memorabel (ABIDE; project no 733050201), a project in the context of the Dutch Deltaplan Dementie.
Arno de Wilde, Ingrid S. van Maurik, Marleen Kunneman, Femke Bouwman, Marissa Zwan, Eline A.J. Willemse, Geert Jan Biessels, Mirella Minkman, Ruth Pel, Niki S.M. Schoonenboom, Ellen M.A. Smets, Mike P. Wattjes, Charlotte E. Teunissen, and Bart N.M. van Berckel declare no conflict of interests. Frederik Barkhof serves as a consultant for Biogen-Idec, Janssen Alzheimer Immunotherapy, Bayer-Schering, Merck-Serono, Roche, Novartis, Genzume, and Sanofi-aventis and has received sponsoring from EU-H2020, NOW, SMSR, TEVA, Novartis, Toshiba, and serves on the editorial boards of Radiology, Brain, Neuroradiology, MSJ, and Neurology. Andrew Stephens is a full-time employee of Piramal Imaging GmbH and owns stock. Erik J. van Lier is a full-time employee of BV Cyclotron VU. Richard Batrla-Utermann is a full-time employee of Roche Diagnostics. Philip Scheltens has acquired grant support (for the institution) from GE Healthcare, Danone Research, Piramal, and MERCK. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, GE Healthcare, Novartis, Sanofi, Nutricia, Probiodrug, Biogen, Roche, Avraham, and EIP Pharma. Wiesje M. van der Flier performs contract research for Boehringer Ingelheim and has been an invited speaker at Boehringer Ingelheim. Research programs of W.M.v.d.F. have been funded by ZonMW, NWO, EU-FP7, Alzheimer Nederland, Cardiovasculair Onderzoek Nederland, stichting Dioraphte, Gieskes-Strijbis fonds, Boehringer Ingelheim, Piramal Neuroimaging, Roche BV, Janssen Stellar, and Combinostics. All funding is paid to her institution.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2017.01.003.
Supplementary data
References
- 1.Herholz K., Ebmeier K. Clinical amyloid imaging in Alzheimer's disease. Lancet Neurol. 2011;10:667–670. doi: 10.1016/S1474-4422(11)70123-5. [DOI] [PubMed] [Google Scholar]
- 2.Frisoni G.B., Fox N.C., Jack C.R., Scheltens P., Thompson P.M. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 6.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperling R., Johnson K. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn) 2013;19:325–338. doi: 10.1212/01.CON.0000429181.60095.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoonenboom N.S., Reesink F.E., Verwey N.A., Kester M.I., Teunissen C.E., van de Ven P.M. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 10.Mulder C., Verwey N.A., van der Flier W.M., Bouwman F.H., Kok A., van Elk E.J. Amyloid-beta(1-42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clin Chem. 2010;56:248–253. doi: 10.1373/clinchem.2009.130518. [DOI] [PubMed] [Google Scholar]
- 11.Mattsson N., Zetterberg H., Hansson O., Andreasen N., Parnetti L., Jonsson M. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 12.Mattsson N., Andreasson U., Persson S., Carrillo M.C., Collins S., Chalbot S. CSF biomarker variability in the Alzheimer's Association quality control program. Alzheimers Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blennow K., Zetterberg H. The past and the future of Alzheimer's disease CSF biomarkers-a journey toward validated biochemical tests covering the whole spectrum of molecular events. Front Neurosci. 2015;9:345. doi: 10.3389/fnins.2015.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 15.Johnson K.A., Minoshima S., Bohnen N.I., Donohoe K.J., Foster N.L., Herscovitch P. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement. 2013;9 doi: 10.1016/j.jalz.2013.01.002. e-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prestia A., Caroli A., Wade S.K., van der Flier W.M., Ossenkoppele R., Van Berckel B. Prediction of AD dementia by biomarkers following the NIA-AA and IWG diagnostic criteria in MCI patients from three European memory clinics. Alzheimers Dement. 2015;11:1191–1201. doi: 10.1016/j.jalz.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Landau S.M., Harvey D., Madison C.M., Reiman E.M., Foster N.L., Aisen P.S. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vos S.J.B., Verhey F., Frölich L., Kornhuber J., Wiltfang J., Maier W. Prevalence and prognosis of Alzheimer's disease at the mild cognitive impairment stage. Brain. 2015;138:1327–1338. doi: 10.1093/brain/awv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen R.C., Roberts R.O., Knopman D.S., Boeve B.F., Geda Y.E., Ivnik R.J. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutomski J.E., Baars M.A., Schalk B.W., Boter H., Buurman B.M., Elzen den W.P. The development of the Older Persons and Informal Caregivers Survey Minimum DataSet (TOPICS-MDS): a large-scale data sharing initiative. PLoS One. 2013;8:e81673. doi: 10.1371/journal.pone.0081673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheltens P., Leys D., Barkhof F., Huglo D., Weinstein H.C., Vermersch P. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koedam E.L., Lehmann M., van der Flier W.M., Scheltens P., Pijnenburg Y.A., Fox N. Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol. 2011;21:2618–2625. doi: 10.1007/s00330-011-2205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquier F., Leys D., Weerts J.G., Mounier-Vehier F., Barkhof F., Scheltens P. Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol. 1996;36:268–272. doi: 10.1159/000117270. [DOI] [PubMed] [Google Scholar]
- 24.Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 25.Smith S.M., Zhang Y., Jenkinson M., Chen J., Matthews P.M., Federico A. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 26.Vanderstichele H., Van Kerschaver E., Hesse C., Davidsson P., Buyse M.A., Andreasen N. Standardization of measurement of beta-amyloid(1-42) in cerebrospinal fluid and plasma. Amyloid. 2000;7:245–258. doi: 10.3109/13506120009146438. [DOI] [PubMed] [Google Scholar]
- 27.Vanmechelen E., Vanderstichele H., Davidsson P., Van Kerschaver E., Van Der Perre B., Sjogren M. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000;285:49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- 28.Bittner T., Zetterberg H., Teunissen C.E., Ostlund R.E., Militello M., Andreasson U. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12:517–526. doi: 10.1016/j.jalz.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 29.van der Flier W.M., Pijnenburg Y.A., Prins N., Lemstra A.W., Bouwman F.H., Teunissen C.E. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 30.MAXQDA, software for data analysis, 1989-2016, VERBI software - Consult. Sozialforschung GmbH; Berlin, Germany: 2016. [Google Scholar]
- 31.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 32.Bouwman F.H., Verwey N.A., Klein M., Kok A., Blankenstein M.A., Sluimer J.D. New research criteria for the diagnosis of Alzheimer's disease applied in a memory clinic population. Dement Geriatr Cogn Disord. 2010;30:1–7. doi: 10.1159/000315542. [DOI] [PubMed] [Google Scholar]
- 33.Richard E., Schmand B.A., Eikelenboom P., Van Gool W.A., The Alzheimer's Disease Neuroimaging Initiative MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer's disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bloudek L.M., Spackman D.E., Blankenburg M., Sullivan S.D. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer's disease. J Alzheimers Dis. 2011;26:627–645. doi: 10.3233/JAD-2011-110458. [DOI] [PubMed] [Google Scholar]
- 35.Teipel S., Drzezga A., Grothe M.J., Barthel H., Chételat G., Schuff N. Multimodal imaging in Alzheimer's disease: validity and usefulness for early detection. Lancet Neurol. 2015;14:1037–1053. doi: 10.1016/S1474-4422(15)00093-9. [DOI] [PubMed] [Google Scholar]
- 36.The Lancet Neurology Bringing forward the diagnosis of Alzheimer's disease. Lancet Neurol. 2014;13:961. doi: 10.1016/S1474-4422(14)70211-X. [DOI] [PubMed] [Google Scholar]
- 37.Ossenkoppele R., Prins N.D., Pijnenburg Y.A., Lemstra A.W., van der Flier W.M., Adriaanse S.F. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013;9:414–421. doi: 10.1016/j.jalz.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Boccardi M., Altomare D., Ferrari C., Festari C., Guerra U.P., Paghera B. Assessment of the incremental diagnostic value of florbetapir F 18 imaging in patients with cognitive impairment. JAMA Neurol. 2016;73:1417–1424. doi: 10.1001/jamaneurol.2016.3751. [DOI] [PubMed] [Google Scholar]
- 39.Stockman J.A., III Disclosure of APOE genotype for risk of Alzheimer's disease. Yearb Pediatr. 2011;2011:406–407. [Google Scholar]
- 40.Green R.C., Christensen K.D., Cupples L.A., Relkin N.R., Whitehouse P.J., Royal C.D. A randomized noninferiority trial of condensed protocols for genetic risk disclosure of Alzheimer's disease. Alzheimers Dement. 2015;11:1222–1230. doi: 10.1016/j.jalz.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harkins K. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther. 2015;7:1–9. doi: 10.1186/s13195-015-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie C.W., Molinuevo J.L., Truyen L., Satlin A., Van der Geyten S., Lovestone S. Development of interventions for the secondary prevention of Alzheimer's dementia: the European Prevention of Alzheimer's Dementia (EPAD) project. Lancet Psychiatry. 2016;3:179–186. doi: 10.1016/S2215-0366(15)00454-X. [DOI] [PubMed] [Google Scholar]
- 44.Wimo A., Guerchet M., Ali G.C., Wu Y.T., Prina A.M., Winblad B. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13:1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handels R.L., Wolfs C.A., Aalten P., Joore M.A., Verhey F.R., Severens J.L. Diagnosing Alzheimer's disease: a systematic review of economic evaluations. Alzheimers Dement. 2014;10:225–237. doi: 10.1016/j.jalz.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Wimo A., Religa D., Spångberg K., Edlund A.K., Winblad B., Eriksdotter M. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039–1044. doi: 10.1002/gps.3925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.