Abstract
Objectives
This study investigates the effect of surgical margins and radiotherapy, in the presence of individual baseline characteristics, on survival in a large population of high-grade soft tissue sarcoma of the extremities using a multistate model.
Design
A retrospective multicentre cohort study.
Setting
4 tertiary referral centres for orthopaedic oncology.
Participants
687 patients with primary, non-disseminated, high-grade sarcoma only, receiving surgical treatment with curative intent between 2000 and 2010 were included.
Main outcome measures
The risk to progress from ‘alive without disease’ (ANED) after surgery to ‘local recurrence’ (LR) or ‘distant metastasis (DM)/death’. The effect of surgical margins and (neo)adjuvant radiotherapy on LR and overall survival was evaluated taking patients' and tumour characteristics into account.
Results
The multistate model underlined that wide surgical margins and the use of neoadjuvant radiotherapy decreased the risk of LR but have little effect on survival. The main prognostic risk factors for transition ANED to LR are tumour size (HR 1.06; 95% CI 1.01 to 1.11 (size in cm)) and (neo)adjuvant radiotherapy. The HRs for patients treated with adjuvant or no radiotherapy compared with neoadjuvant radiotherapy are equal to 4.36 (95% CI 1.34 to 14.24) and 14.20 (95% CI 4.14 to 48.75), respectively. Surgical resection margins had a protective effect for the occurrence of LR with HRs equal to 0.61 (95% CI 0.33 to 1.12), and 0.16 (95% CI 0.07 to 0.41) for margins between 0 and 2 mm and wider than 2 mm, respectively. For transition ANED to distant metastases/Death, age (HR 1.64 (95% CI 0.95 to 2.85) and 1.90 (95% CI 1.09 to 3.29) for 25–50 years and >50 years, respectively) and tumour size (1.06 (95% CI 1.04 to 1.08)) were prognostic factors.
Conclusions
This paper underlined the alternating effect of surgical margins and the use of neoadjuvant radiotherapy on oncological outcomes between patients with different baseline characteristics. The multistate model incorporates this essential information of a specific patient's history, tumour characteristics and adjuvant treatment modalities and allows a more comprehensive prediction of future events.
Keywords: STATISTICS & RESEARCH METHODS, Margins, Multi-state model, RADIOTHERAPY
Strengths and limitations of this study.
This study employs a multistate model on the largest cohort of patients with soft tissue sarcomas and underlines the importance of individualised cancer care as timing of radiotherapy and intended surgical margins can significantly improve local control but have limited influence on survival.
This is the first study that shows the beneficial effect of neoadjuvant radiotherapy over radiation in an adjuvant setting in improving local control.
This study emphasises how individual patient characteristics and planned surgical resection margins can be used to estimate probabilities of future clinical events such as local recurrence, distant metastasis and death.
This study is limited by the retrospective aspect of the design.
Introduction
Soft tissue sarcomas (STS) are a rare, heterogeneous group of tumours accounting for ∼1% of all adult cancers.1 Approximately 60% of all STS occur in the extremities.2 High-grade STS are a select subgroup (representing 38% of all STS in one series3) of highly aggressive and infiltrative subtypes with an overall poor prognosis.4 5 At present, limb salvage surgery with (neo) adjuvant radiotherapy is the standard of care for most patients, while the role of chemotherapy is more controversial.6 However, locally recurrent disease (LR), distant metastases (DM) and poor survival remain of great concern. Although the risk factors for the occurrence of these adverse events have been the subject of many studies, a solid prognostic profile for individual patients is still lacking.
Considering an individual patient's treatment, two types of prognostic factors can be identified: those that are set at the moment of diagnosis and those that are treatment-related. Prognostic factors such as histology, grade, depth and size3–5 7–13 are generally recognised and set at the moment of diagnosis. At present, surgical resection margin and the administration of (neo) adjuvant radiotherapy/chemotherapy are the only treatment factors that can be influenced. The intended resection margin is part of an intricate balance between the best oncological outcome and maintenance of quality of life, including limb function. Whether limb function should be sacrificed to achieve a negative or wide margin should be based on its effect on the overall prognosis of that specific patient.
Although the increased risk of LR following an intralesional margin resection is generally recognised,8 12 14 the presence of possible associations between margin status and overall survival (OS) or between LR and OS is still under discussion. Results have been published confirming the absence10 15 16 and presence13 17–21 of a prognostic role for margins as well as LR on OS.
Unfortunately, current literature on prognostic factors for STS faces several difficulties: small sample sizes, heterogeneity of study populations and differences in statistical methods applied.7 20 Results from prior studies may, therefore, be misleading when applied to an individual patient with a high-grade STS. In an era where clinicians are moving towards individualised patient treatment, it would be preferable to consider the results of planned resection margins for each patient individually. The great importance of individualised cancer treatment is generally accepted because awareness has been created that certain patients have a higher risk of disease recurrence or death than others, and others are more susceptible to possible adverse effects of treatment.
This study aims to investigate the effect of margins and radiotherapy, considering individual patient characteristics, on LR and survival in a large population with only high-grade STS of the extremities using a multistate model. Better stratification of risks will lead to better treatment decisions and improved clinical results for patients with high-grade STS.
Patients and methods
A retrospective multicentre analysis of patients surgically treated between 2000 and 2010 for primary, non-disseminated, high-grade (as defined by FNCLCC larger than grade 2) sarcoma, including angiosarcoma, malignant peripheral nerve sheath tumour, synovial sarcoma, spindle cell sarcoma, myxofibrosarcoma and (pleomorphic) STS not-otherwise-specified was performed. All cases were discussed preoperatively in multidisciplinary teams and pretreatment staging was performed with lung CT scans. Postoperative surveillance strategies were comparative between all centres with yearly MRI for local control and chest X-ray/CT scan every 3–4 months according to ESMO guidelines.6
Patients were identified from the local sarcoma databases of the four participating institutions, all tertiary referral centres for orthopaedic oncology. Exclusion criteria were metastatic disease at the time of diagnosis, presentation with recurrent disease, treatment without curative intent (ie, no primary intent of (limb-sparing) surgery with intended sufficient margins), adjuvant treatment other than radiotherapy or chemotherapy and an unknown margin status. Initially, 709 patients received treatment in 1 of 4 participating centres and met the inclusion criteria. Five patients met the exclusion criteria and were excluded. Seventeen patients were excluded because there was insufficient information on all covariates.
Medical records including surgical notes and pathology reports were reviewed and the following information was recorded: age (<25; 25–50; >50 years22), gender, presentation status (no treatment/biopsy only vs incomplete excision elsewhere prior to referral), tumour size (cm), depth (superficial vs deep to investing fascia), location (upper vs lower extremity), surgical margin, (neo) adjuvant therapy (neoadjuvant, adjuvant, no radiotherapy; chemotherapy vs no chemotherapy) and follow-up data.
Experienced musculoskeletal pathologists in each centre defined the closest surgical margin. Owing to the lack of an international consensus on the definition of margin descriptions, the resection margins were categorised quantitatively: tumour at the inked surface of the resection specimen (0 mm); tumour within 2 mm of ink; tumour at more than 2 mm of ink. The 2 mm cut-off point was based on previous research that identified this as the most optimal differentiating distance.16 23 The decision concerning the use of (neo) adjuvant treatment was not uniform during the study period due to variation in management over time and by centre, although the majority of patients (75%) received radiotherapy. The most common radiotherapy regimens were 50 Gy preoperatively (22.4%) or 50–66 Gy postoperatively (52.3%).
LR was defined as the first radiological or pathological manifestation of tumour within or contiguous to the previously treated tumour bed, 2 or more months after primary treatment. DM was defined by clinical or radiological evident systemic spread of tumour outside the primary tumour bed, including nodal metastasis. Dates of death were extracted from the medical records and local or national death registries.
Statistical analysis
Multivariate Cox regression model
The effect of prognostic factors on OS was estimated with a Cox regression model with LR included as a time-dependent covariate. The following risk factors were included in the model: age at diagnosis, presentation, tumour location, size, depth, histopathology subtype, surgical margin, limb sparing and radiotherapy. HRs based on the multivariate Cox regression model and their corresponding 95% CIs were estimated.
Multistate model
Disease progression was investigated with a multistate model.24 A multistate model is a model for time-to-event data, in which all individuals start in one or possibly more starting states (eg, surgery) and eventually may move in one (or more) state(s), for example, progressive distant disease, LR or death. In this approach, transitions are assessed during the course of the disease and prognostic factors for each transition are studied. Figure 1 shows the multistate model applied in this study to describe the disease progression. We propose three possible states in which a patient may be at any time. After surgery, a patient may be alive with no evidence of disease (ANED), alive with LR or may have developed DM and subsequent death (Death). In this analysis, the two states death and DM were pooled into one state (DM/Death) since DM will, with very few exceptions, inevitably lead to death; among the 288 patients who developed metastatic disease, 88% had died. Patients with concurrent LR and DM (diagnosed within 3 months of each other; n=30) were registered as entering the state of DM/Death. The direction of arrows in figure 1 indicates the transitions between states. The time scale used is months since definitive surgery.
Figure 1.
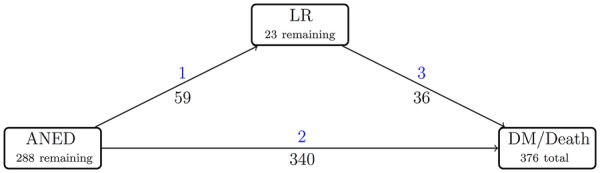
Disease progression of high-grade soft tissue sarcomas represented in a multistate model. Blue, transition number; black, number of patients moving from one state to another. ANED, alive, no evidence of disease; LR, local recurrence; DM, distant metastasis.
To estimate the effect of age at diagnosis, presentation, tumour location, size (in cm), depth, histopathology subtype, surgical margin achieved, limb sparing, and radiotherapy on each transition, a Cox proportional hazards (PH) model was used. For transition 3 (LR to DM/Death), the effects of tumour depth, histopathology subtype, surgery type and radiotherapy could not be estimated due to the relatively small number of patients in this transition. Therefore, these covariates were omitted from the model for this specific transition. The PH assumption in the Cox model was tested for each transition.
Individual risk assessment
Multistate models24 can be used with two different purposes. The first aim is to obtain more biological insight into the disease/recovery process of a patient. It is of interest to determine how certain prognostic factors influence different phases of the evolution of the disease. The second purpose is prediction, as these models help clinicians to obtain more accurate predictions on survival and to adjust predictions by incorporating the occurrence of intermediate events. Predictions are made by estimating the conditional probabilities of future events, given the treatment and patient characteristics.
Patient-specific state occupation probabilities presented in stacked charts provide insight into the effect of margins on the occurrence of events after surgery, given the characteristics of a patient. The stacked charts present a visual aid for surgeons to investigate the effect of margin on the probability of being in different states (LR or DM/Death) at different time points after surgery. The multistate model provides information on the ever-changing nature of a specific patient's history and allows a more comprehensive understanding of the data.
The beginning and end of follow-up corresponded to the date of definitive surgery and the last date of follow-up or death, respectively. The median follow-up was assessed by employing the reverse Kaplan-Meier method.25 The effect of risk factors was estimated by adjusted HRs along with their 95% CIs. p Values at or below 0.05 were considered significant. In the analysis, the variable ‘centre’ was included to account for the presence of heterogeneity between the four treatment centres. All analyses concerning the multistate model were performed using the R-package mstate (R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Vienna, Austria 2011. http://www.r-project.org/).26 27
Results
Table 1 summarises patients' demographics and treatments at baseline for the included 687 patients.
Table 1.
Patient demographics and treatment characteristics
| Characteristic | |
|---|---|
| Age, mean (SD), years | 57.9 (19.8) |
| Age, no. (%) | |
| <25 | 49 (7.1) |
| 25–50 | 170 (24.7) |
| >50 | 468 (68.1) |
| Gender, no. (%) | |
| Male | 389 (56.6) |
| Female | 298 (43.4) |
| Tumour presentation, no. (%) | |
| Primary | 555 (80.8) |
| ‘Whoops’* | 132 (19.2) |
| Tumour location, no. (%) | |
| Upper extremity | 162 (23.6) |
| Lower extremity | 525 (76.4) |
| Tumour size, mean (SD), cm | 10.0 (6.2) |
| Depth, no. (%) | |
| Deep | 531 (77.3) |
| Superficial | 115 (16.7) |
| Deep and superficial | 41 (6) |
| Histopathology, no. (%) | |
| Angiosarcoma | 19 (2.8) |
| MPNST† | 81 (11.8) |
| Myxofibrosarcoma | 217 (31.6) |
| Synovial sarcoma | 134 (19.5) |
| Spindle cell sarcoma | 165 (24.0) |
| Sarcoma NOS‡ | 17 (2.5) |
| MFH/UPS§ | 54 (7.9) |
| Surgical margin, no. (%) | |
| 0 mm | 114 (16.6) |
| ≤2 mm | 325 (47.3) |
| >2 mm | 248 (36.1) |
| Type of surgery, no. (%) | |
| Limb-sparing | 611 (88.9) |
| Amputation | 76 (11.1) |
| Radiotherapy, no. (%) | |
| Neoadjuvant | 154 (22.4) |
| Adjuvant | 359 (52.3) |
| No radiotherapy | 174 (25.3) |
| (Neo)Adjuvant chemotherapy, no. (%) | |
| Yes | 82 (11.9) |
| No | 605 (88.1) |
*Incomplete excision elsewhere prior to referral.
†Malignant peripheral nerve sheath tumour.
‡Not otherwise specified.
§Malignant fibrous histiocytoma/undifferentiated pleomorphic sarcoma.
The estimated 5-year OS was 52.7% (95% CI 48.8% to 56.6%) with a median follow-up of 71 (95% CI 67 to 75) months. In total, 106 patients (15%) developed LR; however, only 59 patients (9%) developed isolated LR, while the other 47 patients (6%) developed LR synchronous or following DM. In total, 288 (42%) developed DM. Seventy-two patients (10%) died without known DM or LR.
A traditional multivariate Cox regression model with LR as a time-dependent covariate showed a significant effect of age (HR 2.22; 95% CI 1.25 to 3.92 for >50 years compared with <25 years), tumour size (HR 1.06 for every cm; 95% CI 1.04 to 1.08) and actual LR (HR 3.42; 95% CI 2.55 to 4.60) on OS (table 2). Note that tumour size is given in centimetre, implying that a ‘k’ cm change in size multiplies the hazard by HRk. For example, an HR equal to 1.34 (95% CI 1.22 to 1.47) and 1.79 (95% CI 1.48 to 2.16) are associated with a tumour of size 5 and 10 cm, respectively. Estimated HRs for histopathology with respect to the reference group angiosarcoma are shown in table 2. Radiotherapy violated the PH assumption and was incorporated in the analysis by fitting a stratified Cox model in which a separate baseline hazard is used for patients with and without (neo) adjuvant radiotherapy.
Table 2.
Cox regression analysis for overall survival
| Variable | p Value | HR | 95% CI |
|---|---|---|---|
| Age | |||
| <25 | 1 | ||
| 25–50 | 0.115 | 1.59 | 0.89 to 2.82 |
| >50 | 0.006 | 2.22 | 1.25 to 3.92 |
| Tumour presentation (‘whoops’* vs primary) | 0.828 | 1.04 | 0.75 to 1.43 |
| Tumour location (lower vs upper) | 0.336 | 1.14 | 0.87 to 1.50 |
| Tumour size, cm | 0.000 | 1.06 | 1.04 to 1.08 |
| Depth | |||
| Deep | 1 | ||
| Superficial | 0.561 | 0.90 | 0.64 to 1.28 |
| Deep and superficial | 0.877 | 1.04 | 0.63 to 1.71 |
| Histopathology | |||
| Angiosarcoma | 1 | ||
| MPNST† | 0.005 | 3.29 | 1.43 to 7.54 |
| Myxofibrosarcoma | 0.060 | 2.15 | 0.97 to 4.78 |
| Synovial sarcoma | 0.027 | 2.59 | 1.12 to 6.02 |
| Spindle cell sarcoma | 0.030 | 2.51 | 1.09 to 5.77 |
| Sarcoma NOS‡ | 0.057 | 2.66 | 0.97 to 7.27 |
| MFH/UPS§ | 0.025 | 2.68 | 1.13 to 6.37 |
| Surgical margin (mm) | |||
| 0 | 1 | ||
| ≤2 | 0.433 | 0.89 | 0.66 to 1.20 |
| >2 | 0.319 | 0.83 | 0.58 to 1.20 |
| Type of surgery (limb-sparing vs amputation) | 0.478 | 0.86 | 0.56 to 1.31 |
| Local recurrence (yes vs no)¶ | 0.000 | 3.42 | 2.55 to 4.60 |
*Incomplete excision elsewhere prior to referral.
†Malignant peripheral nerve sheath tumour.
‡Not otherwise specified.
§Malignant fibrous histiocytoma/undifferentiated pleomorphic sarcoma.
¶Time-dependent variable.
In the multistate model depicted in figure 1, the number of patients moving from one state to the other is illustrated. The majority moved from the state ANED to DM/Death directly (n=340; 49%). In 42% of the patients (n=288), no further disease was detected; therefore, they remained in their postoperative state ANED. A small group (n=59; 9%) developed LR first, after which 36 of these 59 patients (61%) moved to the final state DM/Death. To estimate the adjusted HRs for each transition, a multivariate Cox proportional hazard regression model was employed (table 3). The main prognostic risk factors for transition 1 (ANED to LR) are tumour size (HR 1.06; 95% CI 1.01 to 1.11 with size in cm) and (neo) adjuvant radiotherapy. The HRs for patients treated with adjuvant or no radiotherapy compared with neoadjuvant radiotherapy are equal to 4.36 (95% CI 1.34 to 14.24) and 14.20 (95% CI 4.14 to 48.75), respectively (table 3). Surgical resection margins had a protective effect on the occurrence of LR with HRs equal to 0.61 (95% CI 0.33 to 1.12) and 0.16 (95% CI 0.07 to 0.41) for margins between 0 and 2 mm and wider than 2 mm, respectively. No statistically significant effect of margins was detected when patients move directly to the state DM/Death from ANED (transition 2). The effect of age on the transition between ANED and DM/Death (transition 2) is equal to 1.64 (95% CI 0.95 to 2.85) and 1.90 (95% CI 1.09 to 3.29) for patients aged 25–50 years and >50 years, respectively, compared with patients <25 years of age. The HR for tumour size (in cm) is equal to 1.06 (95% CI 1.04 to 1.08). There was no significant effect of prognostic factors on the transition hazards between LR and DM/Death (transition 3). There was no significant difference between the centres for each outcome in the classical Cox model and the multistate model.
Table 3.
HRs and 95% CIs for all prognostic factors and all transitions in the multistate model
| Trans 1: ANED → LR |
Trans 2: ANED → DM/Death |
Trans 3: LR → DM/Death |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI |
| Age | |||||||||
| <25 | 1 | 1 | 1 | ||||||
| 25–50 | 0.649 | 0.76 | 0.23 to 2.50 | 0.077 | 1.64 | 0.95 to 2.85 | 0.413 | 0.50 | 0.10 to 2.60 |
| >50 | 0.955 | 1.03 | 0.32 to 3.31 | 0.023 | 1.90 | 1.09 to 3.29 | 0.302 | 0.47 | 0.11 to 1.97 |
| Tumour presentation (‘whoops’* vs primary) | 0.344 | 1.43 | 0.68 to 3.03 | 0.586 | 0.91 | 0.66 to 1.26 | 0.539 | 1.39 | 0.48 to 4.03 |
| Tumour location (lower vs upper) | 0.116 | 0.61 | 0.33 to 1.13 | 0.919 | 1.01 | 0.78 to 1.32 | 0.474 | 1.43 | 0.54 to 3.83 |
| Tumour size, cm | 0.018 | 1.06 | 1.01 to 1.11 | 0.000 | 1.06 | 1.04 to 1.08 | 0.114 | 1.05 | 0.99 to 1.12 |
| Depth | |||||||||
| Deep | 1 | 1 | |||||||
| Superficial | 0.093 | 0.51 | 0.23 to 1.12 | 0.653 | 0.92 | 0.66 to 1.30 | |||
| Deep and superficial | 0.226 | 0.26 | 0.03 to 2.33 | 0.253 | 1.31 | 0.82 to 2.09 | |||
| Histopathology | |||||||||
| Angiosarcoma | 1 | 1 | |||||||
| MPNST† | 0.034 | 0.23 | 0.06 to 0.90 | 0.845 | 1.08 | 0.51 to 2.26 | |||
| Myxofibrosarcoma | 0.085 | 0.34 | 0.10 to 1.16 | 0.777 | 0.90 | 0.44 to 1.84 | |||
| Synovial sarcoma | 0.023 | 0.21 | 0.05 to 0.80 | 0.972 | 0.99 | 0.47 to 2.07 | |||
| Spindle cell sarcoma | 0.078 | 0.32 | 0.09 to 1.14 | 0.910 | 0.96 | 0.46 to 2.01 | |||
| Sarcoma NOS‡ | 0.918 | 0.90 | 0.13 to 6.14 | 0.702 | 0.82 | 0.31 to 2.22 | |||
| MFH/UPS§ | 0.032 | 0.19 | 0.04 to 0.87 | 0.560 | 1.26 | 0.58 to 2.76 | |||
| Surgical margin (mm) | |||||||||
| 0 | 1 | 1 | 1 | ||||||
| ≤2 | 0.113 | 0.61 | 0.33 to 1.12 | 0.211 | 0.82 | 0.61 to 1.12 | 0.746 | 1.15 | 0.50 to 2.62 |
| >2 | 0.000 | 0.16 | 0.07 to 0.41 | 0.193 | 0.80 | 0.56 to 1.12 | 0.949 | 1.04 | 0.32 to 3.36 |
| Type of surgery (limb-sparing vs amputation) | 0.486 | 1.55 | 0.45 to 5.32 | 0.717 | 0.93 | 0.61 to 1.40 | |||
| Radiotherapy | |||||||||
| Neoadjuvant | 1 | 1 | |||||||
| Adjuvant | 0.015 | 4.36 | 1.34 to 14.24 | 0.840 | 0.96 | 0.63 to 1.46 | |||
| No radiotherapy | 0.000 | 14.20 | 4.14 to 48.75 | 0.340 | 1.24 | 0.80 to 1.91 | |||
*Incomplete excision elsewhere prior to referral.
†Malignant peripheral nerve sheath tumour.
‡Not otherwise specified.
§Malignant fibrous histiocytoma/undifferentiated pleomorphic sarcoma.
ANED, alive, no evidence of disease; DM, distant metastasis; LR, local recurrence; Trans., transition.
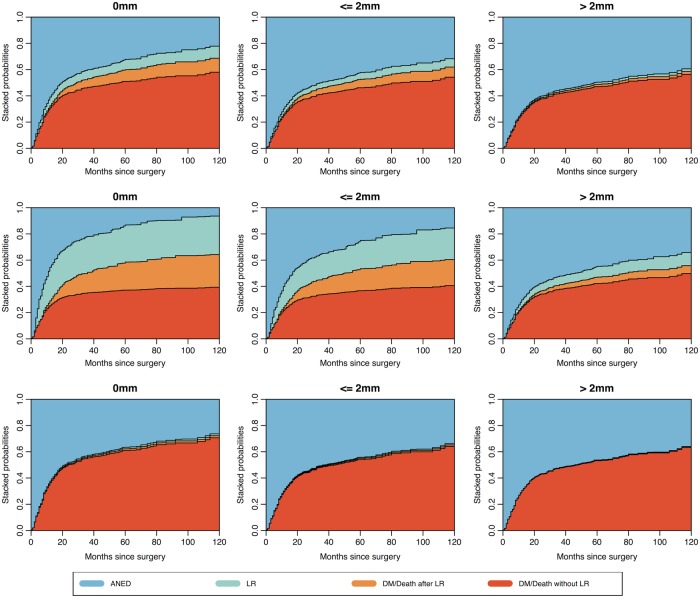
The estimated multistate model was used to predict outcome probabilities for each specific patient. Estimates of these probabilities are based on the results obtained from the Cox model on the transition hazards between the states. Different resection margins and patient characteristics are considered. The patient-specific state occupation probabilities at different time points after surgery are visualised in stacked charts (figure 2). For any individual patient, three separate charts show the effect of resection margins, in the presence of patient, tumour and (neo) adjuvant treatment characteristics. The distance between two curves represents the probability of being in a specific state (ANED, or LR or DM/Death) at a specific time point. Figure 2 illustrates the three margin scenarios for three different patients. After surgery, the probability of occupying the state ‘LR’ (green area) decreases as margins increase in the two patients receiving adjuvant radiotherapy, while the probability of occupying the state ‘ANED’ (light blue area) increases as margins increase. The probability of occupying the state ‘DM/Death without LR’ (red area) decreases slightly for patient A (upper panels) as margins increase, while for patient B (middle panels), the probability remains almost the same for the first two margin scenarios and even increases for a margin wider than 2 mm. The probability of occupying the state ‘DM/Death after LR’ (orange area) decreases as the margin increases in patients A and B. Patient C received neoadjuvant radiotherapy and for this patient, the probability of occupying the state ‘LR’ (green area) is very low and it is not affected by the margin. A wider margin also appears to have little effect on the probability of occupying the state ‘DM/Death without LR’ (red area).
Figure 2.
Stacked state occupation probabilities for patients for different margins after surgery, based on the model in figure 1. Upper panels: patient A: a woman aged 74 years with a large (>10 cm), high-grade myxofibrosarcoma of the upper leg, resection with adjuvant radiotherapy. Middle panels: patient B: a man aged 60 years with a 7 cm angiosarcoma of the arm, resection with adjuvant radiotherapy. Lower panels: patient C: a woman aged 70 years with a large (>10) synoviosarcoma of the upper leg, resection after neoadjuvant radiotherapy. From left to right: Left panels: a 0 mm margin. Middle panels: margins smaller than or equal to 2 mm. Right panels: margins wider than 2 mm.
Discussion
High-grade STS are associated with frequent LRs and poor survival. Since several prognostic factors are set at baseline (ie, tumour size, grade), the resection margin and indication or timing of radiotherapy might be the only prognostic factors that can be affected by the multidisciplinary team. The results of this study stress the importance of individual prediction of survival, considering the different prognostic effects of radiotherapy and surgical margins between patients.
This study brings a new element into the discussion of the effect of margins by using a multistate model. The estimated state occupation probabilities based on the multistate model show the different effect of margins on outcomes between patients with different baseline characteristics and adjuvant treatment modalities. This implies that, in the discussion of the effect of margins, margins cannot be considered as a single entity, but only in combination with patient-specific baseline characteristics and additional radiotherapy. Although previous studies on the effect of margins take patient characteristics into account in their multivariate analysis, it has not earlier been emphasised and visualised how much these characteristics influence the effect of margins. To the best of our knowledge, the stacked charts presented here are the first visualisation of the complex relationship between prognostic factors and probabilities of disease progression for individual patients. An additional asset of the multistate model is that future disease progression can be estimated based on the baseline characteristics of a patient at diagnosis, as well as on his known disease progression after surgery. This enables real-time updates of future outcomes when additional information becomes available over time.
The results from this study can be applied in clinical practice by taking the probabilities of future state occupation for a specific patient into account when weighing invasive surgery against maintaining quality of life, especially in cases with limited expected survival. However, the authors acknowledge that the presented data are too intricate to directly apply in daily practice. Therefore, a user-friendly web-based tool based on the multistate model presented in this study will be developed.
This study presents new knowledge on the effect of neoadjuvant radiotherapy in patients with high-grade STS. In clinical practice, the difference in the effect of preoperative and postoperative use of radiotherapy on LR, DM and survival of patients with high-grade STS of the extremities remains the subject of discussion. Surgery is delayed ∼3 months in patients receiving preoperative radiotherapy, compared with patients receiving no or postoperative radiotherapy. Therefore, it is important to assess the effect of our surgical planning and the use of radiotherapy on the course of the disease. The current results show that patients receiving neoadjuvant radiotherapy were less likely to develop LR when compared with patients with no or adjuvant radiotherapy, even though the 95% CI was large. This is consistent with previously published results,28 although others did not find a true difference in the risk of LR.29 30 One recent large retrospective database study showed that neoadjuvant radiotherapy was associated with improved survival.31 This is in contrast to several other studies that showed no significant effect of timing of radiotherapy on overall survival.32 33 Since all these trials face the limitations of retrospective studies, a firm conclusion is still not possible. Possibly, a larger randomised trial will be able to provide a decisive answer on which sequence is superior.
Undeniably, the question of the definition of a marginal or wide margin remains. Multiple different descriptions are used in the literature.34 In contrast to its continued use, the Enneking classification35 is not considered detailed enough in respect of (large) STS with close involvement of essential structures such as vessels, nerves and bone.21 In addition, the use of (neo) adjuvant radiotherapy has decreased the necessity for radical or even wide margins.21 The dichotomous classification proposed by Trovik et al9 may be too simplistic regarding adequate or inadequate margins. While the poor prospect associated with macroscopically intralesional resections is evident, the implications of microscopically positive or marginal resections should not be regarded as identical.21 23 The quantitative measurement as applied in this study did not take into account the type of tissue of which the margin consisted (eg, fascia, fat), which might also influence the required minimum width of a margin.21 36 As Hoang et al34 recently proposed, a universally updated surgical margin reporting system would improve communication and understanding regarding surgical treatment of STS. To create a broad basis for such a global system, international collaboration is needed.
The main strengths of this study are its large cohort of high-grade extremity STS only and the use of a multistate model to investigate the evolution of the disease and to estimate the probabilities of clinical future events, given a set of individual patient characteristics. The estimates of these probabilities are based on the results obtained from the Cox model on the transition hazards between the states. The study population is limited to high-grade extremity tumours of the most common sarcoma types, and thus, the results are not attenuated by a diversity of low-grade, low-impact STS. Finally, this study introduces the possibility of a practical aid for clinical practice that would allow for individually tailored treatments, in contrast to many previous studies that provide general prognostic factors for treatment decisions based on groups of patients. Several limitations exist in this study. First, the inherent effects of a retrospective study design, such as selection bias, are present. Second, owing to the multicentre aspect of the study, a revision of all histological data was not possible. However, all centres reported pathology results in the same manner. Margin width as stated in the pathology reports was used for the analyses instead of descriptive results. Additionally, all analyses were corrected for centre effect and there was no significant difference between centres. Despite the limitations, the current analysis is the largest investigation into the effect of margins on LR and OS for patients with high-grade extremity STS.
This study stresses the importance of patient-specific characteristics when evaluating the effect of surgical margins and (neo) adjuvant radiotherapy. On the basis of the estimated state occupation probabilities, the effect of margin differs significantly in individual cases depending on baseline characteristics and the administration of (neo) adjuvant radiotherapy. To use prognostic factors for LR and DM/Death in daily practice and thereby enable personalised care, a user-friendly web-based tool (application) based on the model presented in this study will be validated and published.
Footnotes
Contributors: MAJvdS, MF, LMJ, JSW and JJW are responsible for conceptualisation; MAJvdS, MF and JJW are responsible for methodology; AJR-B and MF are responsible for formal analysis; JJW and MAJvdS are responsible for investigation; JJW, WA, ML and AMG are responsible for resources; JJW, AJR-B, MAJvdS and MF are responsible for writing—original draft; JJW, LJ, ML, RP, WA, PDSD, PCF, AMG, JSW, MF and MAJvdS are responsible for writing—review and editing; MAJvdS is responsible for supervision and guarantor.
Funding: This work was supported by the Dutch Cancer Society and Alpe d'HuZes, grant number UL 2015-8028.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: MAJvdS received financial support from the Dutch Cancer Society and Alpe d'HuZes for the submitted work.
Ethics approval: Medical Ethical Committee Leiden University Medical Center.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.Coindre J, Terrier P, Bui N et al. . Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 1996;14:869–77. [DOI] [PubMed] [Google Scholar]
- 3.Italiano A, Le Cesne A, Mendiboure J et al. . Prognostic factors and impact of adjuvant treatments on local and metastatic relapse of soft-tissue sarcoma patients in the competing risks setting. Cancer 2014;120:3361–9. 10.1002/cncr.28885 [DOI] [PubMed] [Google Scholar]
- 4.Pisters P, Leung D, Woodruff J et al. . Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89. [DOI] [PubMed] [Google Scholar]
- 5.Zagars GK, Ballo MT, Pisters PW et al. . Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43. 10.1002/cncr.11365 [DOI] [PubMed] [Google Scholar]
- 6.ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl 7):vii92–9. [DOI] [PubMed] [Google Scholar]
- 7.Eilber F, Rosen G, Nelson S et al. . High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg 2003;237:218–26. 10.1097/01.SLA.0000048448.56448.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronchi A, Casali PG, Mariani L et al. . Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol 2005;23:96–104. 10.1200/JCO.2005.04.160 [DOI] [PubMed] [Google Scholar]
- 9.Trovik C, Bauer H, Alvegård T et al. . Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer 2000;36:710–16. 10.1016/S0959-8049(99)00287-7 [DOI] [PubMed] [Google Scholar]
- 10.Tanabe K, Pollock R, Ellis L et al. . Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer 1994;73:1652–9. [DOI] [PubMed] [Google Scholar]
- 11.Lewis J, Leung D, Heslin M et al. . Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 1997;15:646–52. 10.1200/jco.1997.15.2.646 [DOI] [PubMed] [Google Scholar]
- 12.Stojadinovic A, Leung D, Hoos A et al. . Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg 2002;235:424–34. 10.1097/00000658-200203000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biau DJ, Ferguson PC, Chung P et al. . Local recurrence of localized soft tissue sarcoma: a new look at old predictors. Cancer 2012;118:5867–77. 10.1002/cncr.27639 [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi N, Matumoto S, Manabe J. New method of evaluating the surgical margin and safety margin for musculoskeletal sarcoma, analysed on the basis of 457 surgical cases. J Cancer Res Clin Oncol 1995;121:555–63. 10.1007/BF01197769 [DOI] [PubMed] [Google Scholar]
- 15.McKee MD, Liu DF, Brooks JJ et al. . The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol 2004;85:68–76. 10.1002/jso.20009 [DOI] [PubMed] [Google Scholar]
- 16.Willeumier JJ, Fiocco M, Nout R et al. . High-grade soft tissue sarcomas of the extremities: surgical margins influence only local recurrence not overall survival. Int Orthop 2015;39:935–41. 10.1007/s00264-015-2694-x [DOI] [PubMed] [Google Scholar]
- 17.Atean I, Pointreau Y, Rosset P et al. . Prognostic factors of extremity soft tissue sarcoma in adults. A single institutional analysis. Cancer Radiother 2012;16:661–6. 10.1016/j.canrad.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 18.Gronchi A, Lo Vullo S, Colombo C et al. . Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg 2010;251:506–11. 10.1097/SLA.0b013e3181cf87fa [DOI] [PubMed] [Google Scholar]
- 19.Novais EN, Demiralp B, Alderete J et al. . Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res 2010;468:3003–11. 10.1007/s11999-010-1471-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A et al. . Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: a cohort study of 922 consecutive patients. Acta Orthop 2014;85:323–32. 10.3109/17453674.2014.908341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Donnell PW, Griffin AM, Eward WC et al. . The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer 2014;120:2866–75. 10.1002/cncr.28793 [DOI] [PubMed] [Google Scholar]
- 22.van Gaal JC, Bastiaannet E, Schaapveld M et al. . Cancer in adolescents and young adults in north Netherlands (1989–2003): increased incidence, stable survival and high incidence of second primary tumours. Ann Oncol 2009;20:365–73. 10.1093/annonc/mdn588 [DOI] [PubMed] [Google Scholar]
- 23.Kainhofer V, Smolle MA, Szkandera J et al. . The width of resection margins influences local recurrence in soft tissue sarcoma patients. Eur J Surg Oncol 2016;42:899–906. 10.1016/j.ejso.2016.03.026 [DOI] [PubMed] [Google Scholar]
- 24.Putter H, Fiocco M. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 25.Schemper M, Smith T. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–6. 10.1016/0197-2456(96)00075-X [DOI] [PubMed] [Google Scholar]
- 26.de Wreede LC, Fiocco M, Putter H. mstate: An R Package for the Analysis of Competing Risks and Multi-State Models. J Stat Softw 2011;38. [Google Scholar]
- 27.de Wreede L, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed 2010;99:261–74. 10.1016/j.cmpb.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 28.Al-Absi E, Farrokhyar F, Sharma R et al. . A systematic review and meta-analysis of oncologic outcomes of pre- versus postoperative radiation in localized resectable soft-tissue sarcoma. Ann Surg Oncol 2010;17:1367–74. 10.1245/s10434-009-0885-7 [DOI] [PubMed] [Google Scholar]
- 29.O'Sullivan B, Davis A, Turcotte R et al. . Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002;359:2235–41. 10.1016/S0140-6736(02)09292-9 [DOI] [PubMed] [Google Scholar]
- 30.Müller DA, Beltrami G, Scoccianti G et al. . Combining limb-sparing surgery with radiation therapy in high-grade soft tissue sarcoma of extremities—is it effective? Eur J Surg Oncol 2016;42:1057–63. 10.1016/j.ejso.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 31.Sampath S, Schultheiss TE, Hitchcock YJ et al. . Preoperative versus postoperative radiotherapy in soft-tissue sarcoma: multi-institutional analysis of 821 patients. Int J Radiat Oncol Biol Phys 2011;81:498–505. 10.1016/j.ijrobp.2010.06.034 [DOI] [PubMed] [Google Scholar]
- 32.Zagars GK, Ballo MT, Pisters PWT et al. . Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys 2003;56:482–8. [DOI] [PubMed] [Google Scholar]
- 33.Kuklo TR, Temple HT, Owens BD et al. . Preoperative versus postoperative radiation therapy for soft-tissue sarcomas. Am J Orthop (Belle Mead NJ) 2005;34:75–80. [PubMed] [Google Scholar]
- 34.Hoang K, Gao Y, Miller BJ. The variability in surgical margin reporting in limb salvage surgery for sarcoma. Iowa Orthop J 2015;35:181. [PMC free article] [PubMed] [Google Scholar]
- 35.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980;153:106–20. [PubMed] [Google Scholar]
- 36.Kawaguchi N, Ahmed A, Matsumoto S et al. . The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res 2004;419:165–72. 10.1097/00003086-200402000-00027 [DOI] [PubMed] [Google Scholar]