Abstract
Background
In 2014, guidelines from the National Institute for Health and Care Excellence (NICE) provided updated recommendations on lipid-modifying therapy (LMT). We assessed clinical practice contemporaneous to release of these guidelines in a UK general practice setting for secondary and high-risk primary-prevention populations, and extrapolated the findings to UK nation level.
Methods
Patients from The Health Improvement Network database with the following criteria were included: lipid profile in 2014 (index date); ≥20 years of age; ≥2 years representation in database prior to index; ≥1 statin indication either for atherosclerotic cardiovascular disease (ASCVD) or the non-ASCVD conditions high-risk diabetes mellitus and/or chronic kidney disease.
Results
Overall, 183 565 patients met the inclusion criteria (n=91 479 for ASCVD, 92 086 for non-ASCVD). In those with ASCVD, 79% received statin treatment and 31% received high-intensity statin. In the non-ASCVD group, 62% were on a statin and 57% received medium-intensity or high-intensity statin. In the ASCVD and non-ASCVD cohorts, 6% and 15%, respectively, were already treated according to dosing recommendations as per updated NICE guidelines. Extrapolation to the 2014 UK population indicated that, of the 3.3 million individuals with ASCVD, 2.4 million would require statin uptitration and 680 000 would require statin initiation (31% de novo initiation, 60% reinitiation, 9% addition to non-statin LMT) to achieve full concordance with updated guidelines. Of the 3.5 million high-risk non-ASCVD individuals, 1.6 million would require statin uptitration and 1.4 million would require statin initiation (59% de novo initiation, 36% reinitiation, 5% addition to non-statin LMT).
Conclusions
A large proportion of UK individuals with ASCVD and high-risk non-ASCVD received statin treatment (79% and 62%, respectively) during the year of NICE 2014 guidelines release. Up to 94% of patients with ASCVD and 85% of high-risk non-ASCVD individuals, representing ∼3 million individuals in each group, would require statin uptitration or initiation to achieve full concordance with updated guidelines.
Keywords: low-density lipoprotein cholesterol (LDL-C), lipids, guidelines, cardiovascular disease, statins
Strengths and limitations of this study.
Potential implications of the 2014 National Institute for Health and Care Excellence (NICE) lipid-modification therapy guidelines on clinical practice in the UK have not been evaluated in prior reports.
We analysed a cohort of high-risk patients representing the UK general practice from a large representative data source and developed estimates of the extrapolated number of individuals across the UK, including subgroups of interest, whose treatment was already concordant with the new guidelines and those for whom uptitration or initiation of statin therapy would be needed to achieve full concordance.
Our study provides novel data on clinical practice in many high-risk subgroups such as those with ischaemic stroke, peripheral arterial disease, diabetes without vascular disease and chronic kidney disease.
A limitation of the study is that though the definition of medication usage was optimised to provide valid point-in-time estimates concurrent with lipid measurements, whether patients actually took their medications as prescribed cannot be ensured from the data source.
The aim of the study was to provide a comparison of 2014 clinical practice relative to guidelines released in 2014; these results cannot be interpreted in terms of the impact of the new guidelines on clinical practice.
Introduction
Despite a decade of continuing decline in cardiovascular (CV) disease mortality, CV deaths remain the leading cause of mortality in the UK, accounting for ∼31% of all deaths, with ischaemic heart disease and stroke representing the vast majority (17% and 10%, respectively).1 2 Reducing low-density lipoprotein cholesterol (LDL-C) with statin therapy has been shown to reduce all-cause and CV mortality, as well as CV outcomes such as non-fatal myocardial infarction (MI), coronary revascularisation procedures, and non-fatal ischaemic stroke in populations with prior atherosclerotic CV disease (ASCVD) and in certain primary-prevention populations.3 4 The high tolerability and safety of statins have also been established across these subgroups.3–5 Despite this, appropriate statin use and atherogenic lipid level reduction remain suboptimal in clinical practice.6
Statins are recommended by the National Institute for Health and Care Excellence (NICE) as first-line lipid-modifying therapy (LMT) for the reduction of CV event risk in patients with ASCVD as well as diabetes mellitus (DM), familial hypercholesterolaemia, chronic kidney disease (CKD) and other high-risk primary-prevention populations.7 In line with evidence from randomised trials and the recent availability of generic atorvastatin, the 2014 NICE guidelines recommend more intensive statin therapy compared with the 2008 guidelines. The recommended regimens include atorvastatin 80 mg for patients with ASCVD and atorvastatin 20 mg or higher for those with most other high-risk conditions; although lower doses of atorvastatin can be used in cases of potential drug to drug interactions, high risk of adverse effects or patient preference.
The present study assessed real-world clinical practice in 2014 relative to the updated NICE guidelines. In a large, representative UK population, we analysed LMT usage for each of the following indications: recent acute coronary syndrome (ACS), other coronary heart disease (CHD), ischaemic stroke/transient ischaemic attack (TIA), peripheral arterial disease (PAD), type 1 (T1) DM, type 2 (T2) DM and CKD. We provide an estimate of the extrapolated number of individuals in the UK within each subgroup whose treatment was already concordant with the guidelines as well as those for whom uptitration or initiation of statin therapy would be needed for full concordance. For the same patient subgroups, we also evaluated achievement of LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) goals as defined by the 2011 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) lipid management guidelines.8
Methods
This study was based on a retrospective, cross-sectional and observational cohort.
Database and cohort selection
We used the The Health Improvement Network (THIN) database, which represents anonymised patient records from general practitioners (GPs) in the UK. As of the end of 2014, the database represented 422 active GP practices and 3.5 million unique active patients. The THIN database has been found to be broadly representative of the UK population, and the validity of recorded information has been established in previous studies,9 10 including the validity of using Read codes to identify ASCVD, other high CV risk conditions and incident CV events.11 12 Owing to the extensive use of GP electronic prescribing in the UK, the recording of prescriptions is expected to be complete and accurate.13 Furthermore, the scope of THIN data to inform complex epidemiological observations was supported by the validation of the updated QRISK2 model for estimating the 10-year incident risk of CV disease in the UK general population.14
The following inclusion criteria were used: presence of a lipid profile measurement in 2014 (last LDL-C measurement in 2014 was considered the index date); ≥20 years of age and presence of ≥1 high CV risk condition for which statins would be recommended as per NICE guidelines (see below). In order to ensure complete capture of pertinent demographic and clinical characteristics, and to assess for prior statin use among those not currently treated with statins, we also required continuous representation in the database for ≥2 years prior to the index date.
Determination of ASCVD and non-ASCVD categories
NICE 2014 guidelines recommend statin treatment in following groups: (1) ASCVD, where atorvastatin 80 mg is recommended; (2) T2DM with QRISK2 10-year risk ≥10%, where atorvastatin 20 mg is recommended; (3) T1DM with age >40 years or DM duration of >10 years or presence of nephropathy or other CV risk factors, where atorvastatin 20 mg is recommended; (4) CKD, where atorvastatin 20 mg is recommended and (5) all other individuals with QRISK2 10-year risk ≥10%, where atorvastatin 20 mg is recommended. Our overall study cohort represents groups 1–4 (excluding 5), with group 1 representing the ASCVD population and groups 2–4 representing a high-risk non-ASCVD population.
ASCVD and non-ASCVD conditions were identified using standardised Read codes (see online supplementary table S1) as follows: (1) recent ACS (MI or unstable angina within 12 months prior to index date); (2) other CHD (eg, ACS >12 months prior to index date, any coronary revascularisation, stable angina or ischaemic cardiomyopathy); (3) ischaemic stroke or TIA; (4) PAD (presence of revascularisation/surgery for significant peripheral arterial, aortic or carotid disease); (5) T2DM with QRISK2 10-year risk ≥10%; (6) T1DM with age >40 years (represents a slight simplification of guideline criteria with application of only the age limit) and (7) CKD stage III–V (estimated glomerular filtration rate <60 mL/min/1.73 m2 or dialysis, herewith referred to as ‘CKD’). A thorough process involving clinical cardiology and coding expert review was undertaken to optimise the specificity of Read codes for each condition. Read codes were also used to identify non-CV comorbidities. The QRISK2 10-year risk was estimated for individuals with T2DM in the non-ASCVD cohort via application of the algorithm to patient-level data (details available in online supplementary appendix).14
bmjopen-2016-013255supp_appendix.pdf (819.1KB, pdf)
Patients with conditions 1–4 were collectively referred to as ‘ASCVD’, while those with only conditions 5–7 were collectively referred to as ‘non-ASCVD’. Those with ASCVD were categorised hierarchically (as above) into four mutually exclusive groups: (1) recent ACS; (2) other CHD; (3) ischaemic stroke/TIA and (4) PAD. A sensitivity analysis was also conducted by categorising these same patients by each condition present, referred to as prevalent disease categorisation. As an example, an individual with a history of elective coronary revascularisation and PAD would be categorised hierarchically as ‘other CHD’, but as ‘other CHD’ and ‘PAD’ under the prevalent disease categorisation. Patients without ASCVD were categorised into five mutually exclusive categories to better evaluate the association of specific conditions with LMT usage and lipid goal achievement. These categories were: (1) T2DM and QRISK2 ≥10% with CKD; (2) T2DM and QRISK2 ≥10% without CKD; (3) T1DM and age >40 years with CKD; (4) T1DM and age >40 years without CKD and (5) other CKD not meeting the criteria of the other categories. For enhanced readability, we omitted the qualifier QRISK2 10-year risk ≥10% from the non-ASCVD T2DM population, and age >40 years from the non-ASCVD T1DM population, considering them implicit in the definitions. The fifth category was referred to as ‘CKD alone’.
Determination of medication treatment
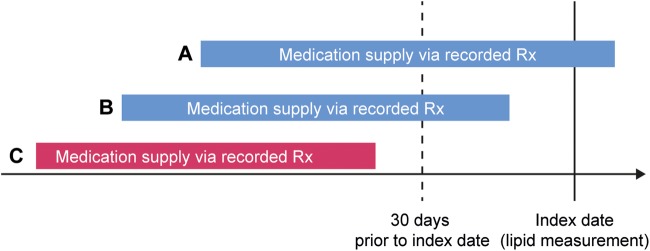
For any particular medication, patients were considered to have been treated on the index date if the medication supply via a recorded prescription was present on or within 30 days prior to the index date, regardless of the duration of the prescription (figure 1). This point-in-time assessment approach was used to better control for factors such as discontinuation over time. In addition, this also ensured that lipid levels best reflected the impact of the treatment regimen as the assessment of both measures was concurrent. Those not treated on the index date but with evidence of a prior recorded prescription were considered to have a history of being treated. Those without any evidence of a recorded prescription for a medication within the 2 years prior to the index date were considered to have no documented history of being treated. LMT was categorised as high-intensity, medium-intensity and low-intensity statin therapy, as well as non-statin therapy (see online supplementary table S2).7
Figure 1.
Determination of treatment status as of the index date. Blue bars representing scenarios A and B (medication supply via recorded prescription (Rx) on or within 30 days prior to the index date) define the patient as being treated as of the index date. The red bar representing scenario C (medication supply via recorded Rx more than 30 days prior to the index date) defines the patient as not being treated as of the index date.
We also summarised statin usage as per 2014 NICE guideline recommendations, which recommend treatment with atorvastatin 80 mg and 20 mg in patients with ASCVD and patients without ASCVD, respectively (for our study, we interpreted this as regimens of equivalent or higher potency: atorvastatin 80 mg equivalent or higher (atorvastatin 80 mg, rosuvastatin 40 mg); and atorvastatin 20 mg equivalent or higher (atorvastatin 20, 40 and 80 mg, rosuvastatin 10, 20 and 40 mg and simvastatin 80 mg)). It should be noted, however, that guidelines allow for a consideration of a lower dose of atorvastatin in presence of drug interactions, risk of adverse effects or patient preference. Statin categories included statins administered as either monotherapy or in combination with ≥1 non-statin medications.
Determination of LDL-C and non-HDL-C levels
We assessed the achievement of LDL-C and non-HDL-C levels relative to the ESC/EAS 2011 guidelines. They recommend goals of LDL-C <1.8 mmol/L (70 mg/dL) and non-HDL-C <2.6 mmol/L (100 mg/dL) for the ASCVD and non-ASCVD populations reported in the current study.8
Patient involvement
Our study was based on an analysis of patient-level data represented in an electronic medical record (EMR) database used by a set of GPs in the UK using the Vision EMR software. In order to protect patient privacy, the analyses reported in our study are based on a de-identified version of the database where patient identifiers are encrypted such that it is not possible to link an individual patient in the data set to a specific individual in the real world. As such, our study did not involve any direct patient-level interaction.
Statistical analyses
All statistical analyses were descriptive in nature. Cohort characteristics, LMT usage and achieved LDL-C and non-HDL-C levels were summarised via proportions and mean±SD as appropriate. Findings on number of patients treated with LMT were extrapolated to the UK population via an adjusted extrapolation methodology that accounted for differential weights by clinical and demographic profiles (details available in online supplementary appendix). All analyses were conducted with SAS software V.9.4.
Results
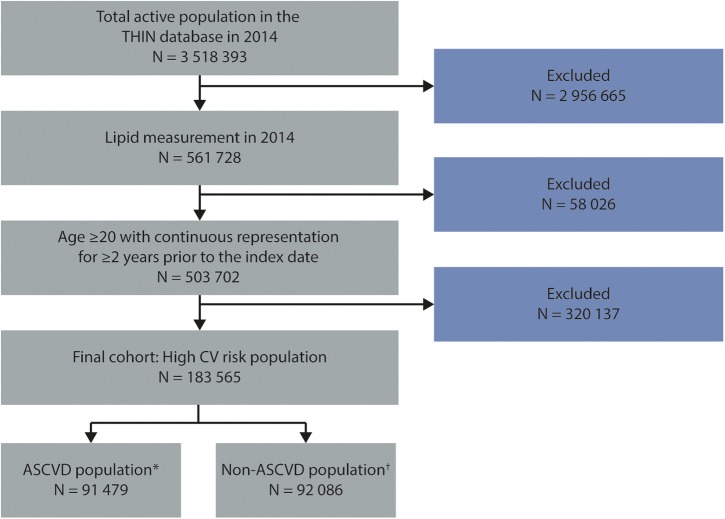
The inclusion criteria were met by 183 565 patients, of whom approximately one-half (n=91 479) had established ASCVD, with the remainder (n=92 086) having non-ASCVD high-risk conditions (figure 2). According to hierarchical disease categorisation for the ASCVD group, 3% had a recent ACS, 64% had another CHD diagnosis, 22% had a history of ischaemic stroke/TIA and 11% had PAD. Approximately one-third of the ASCVD cohort had concomitant DM; and close to one-fourth had concomitant CKD. Baseline characteristics of the ASCVD group by hierarchical categorisation are presented in table 1 and by prevalent categorisation in online supplementary table S3. Baseline characteristics for the non-ASCVD group are also presented in table 1. In the non-ASCVD group, 12% had T2DM with CKD; 57% had T2DM without CKD; 1% had T1DM with CKD; 5% had T1DM without CKD and 25% had CKD alone.
Figure 2.
Flow chart of the cohort selection for the study. *ASCVD includes acute coronary syndrome, other coronary heart disease, ischaemic stroke/transient ischaemic attack and peripheral arterial disease. †Includes type 2 diabetes mellitus with QRISK2 ≥10%, type 1 diabetes mellitus with age >40 years and chronic kidney disease not meeting the previous diabetes mellitus criteria. ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular disease; THIN, The Health Improvement Network.
Table 1.
Baseline characteristics for the overall ASCVD and non-ASCVD cohorts and subgroups
| ASCVD cohort (n=91 497) |
Non-ASCVD cohort (n=92 086) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recent ACS (3%) | Other CHD (64%) | Ischaemic stroke/TIA (22%) | PAD (11%) | Total ASCVD | T2DM with CKD (12%) | T2DM without CKD (57%) | T1DM with CKD (1%) | T1DM without CKD (5%) | CKD alone (25%) | Total non-ASCVD | |
| Demographic characteristics | |||||||||||
| Age, mean, years | 67.8 | 72.8 | 73.0 | 72.3 | 72.6 | 75.7 | 67.2 | 69.7 | 57.2 | 74.6 | 69.7 |
| Male, % | 66.5 | 63.5 | 50.3 | 62.8 | 60.7 | 41.3 | 58.6 | 40.7 | 57.6 | 35.0 | 50.3 |
| SDI*, mean | 2.7 | 2.7 | 2.6 | 2.8 | 2.7 | 2.7 | 2.8 | 2.7 | 2.6 | 2.6 | 2.7 |
| Current smoker, % | 15.2 | 12.1 | 13.5 | 26.6 | 14.1 | 7.0 | 14.9 | 10.8 | 16.3 | 7.1 | 11.9 |
| BMI, mean, kg/m2 | 28.4 | 28.6 | 27.8 | 27.6 | 28.3 | 30.4 | 30.9 | 29.8 | 28.3 | 28.3 | 30.1 |
| Systolic BP, mean | 128.9 | 131.5 | 133.1 | 134.6 | 132.1 | 134.5 | 134.7 | 134.7 | 131.1 | 134.1 | 134.3 |
| Baseline clinical characteristics | |||||||||||
| Recent ACS, % | 100.0 | 0.0 | 0.0 | 0.0 | 3.4 | N/A | N/A | N/A | N/A | N/A | N/A |
| Other CHD, % | 64.2 | 100.0 | 0.0 | 0.0 | 66.0 | N/A | N/A | N/A | N/A | N/A | N/A |
| Ischaemic stroke/TIA, % | 7.6 | 10.5 | 100.0 | 0.0 | 28.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| PAD, % | 10.1 | 11.2 | 14.0 | 100.0 | 21.7 | N/A | N/A | N/A | N/A | N/A | N/A |
| DM, % | 27.0 | 30.1 | 25.6 | 33.7 | 29.4 | 100.0 | 100.0 | 100.0 | 100.0 | 7.1 | 76.3 |
| Hypertension, % | 50.4 | 61.5 | 62.1 | 64.2 | 61.5 | 77.4 | 62.2 | 74.4 | 41.8 | 73.2 | 66.0 |
| History of CHF, % | 14.0 | 11.2 | 4.2 | 5.1 | 9.1 | 1.4 | 0.5 | 6.9 | 1.0 | 7.1 | 2.4 |
| CKD, stage III, % | 17.2 | 24.4 | 22.1 | 23.0 | 23.5 | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 | 38.2 |
| CKD, stage IV–V, %‡ | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 1.2 | 0.0 | 2.0 | 0.0 | 0.8 | 0.3 |
| Concomitant medication use§ | |||||||||||
| β-blockers, % | 81.4 | 60.1 | 23.5 | 22.4 | 48.7 | N/A | N/A | N/A | N/A | N/A | N/A |
| ACEI/ARBs, % | 85 | 65.3 | 52.1 | 52.4 | 61.7 | 74.6 | 59.3 | 77.8 | 52.7 | 59.9 | 61.1 |
| Antiplatelets, %¶ | 52.5 | 13.5 | 29.7 | 15.4 | 18.5 | N/A | N/A | N/A | N/A | N/A | N/A |
ASCVD subgroups represent hierarchical categorisation. Non-ASCVD categorisations have been simplified by consideration of the qualifiers QRISK2 ≥10% and age >40 years as implicit in the definitions of T2DM and T1DM, respectively, with and without CKD, and of the qualifiers without T2DM + QRISK2 ≥10% or T1DM + age >40 as implicit in the definition of CKD alone.
*Social deprivation index (SDI) as defined by the Townsend deprivation index score analysed in quintiles, 1=most affluent and 5=least affluent.
†Includes Indian, Pakistani, Bangladeshi and other South Asian individuals.
‡Stage V CKD includes end-stage renal disease and dialysis.
§Medication use on index date.
¶Clopidogrel/ticagrelor/prasugrel.
ACEI, ACE inhibitors; ACS, acute coronary syndrome; ARB, angiotensin II receptor blocker; ASCVD, atherosclerotic cardiovascular disease; BMI, Body Mass Index; BP, blood pressure; CHD, coronary heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; N/A, not applicable; PAD, peripheral arterial disease; SDI, Social Deprivation Index; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TIA, transient ischaemic attack.
LMT usage in 2014
ASCVD population
Approximately 79% of the ASCVD population received treatment with a statin on the index date (table 2). By hierarchical categories, statin use was 87% in the recent ACS group, 82% in the other CHD group and 73% in the ischaemic stroke/TIA and PAD groups. Of patients currently treated with statins, 40% were receiving treatment with high-intensity statins (defined as per online supplementary table S2): 72% for recent ACS, 42% for other CHD, 32% for PAD and 29% for ischaemic stroke/TIA. Statins were predominantly used as monotherapy: 92% for high-intensity statins, 98% for medium-intensity statins and 96% for low-intensity statins. Of patients not currently treated with LMT, 34% had no evidence of any LMT treatment in the 2 years prior to the index date. LMT usage by prevalent categorisation in the ASCVD group is presented in online supplementary table S4.
Table 2.
Use of LMT in the overall ASCVD and non-ASCVD cohorts and subgroups
| ASCVD cohort (n=91 497) |
Non-ASCVD cohort (n=92 086) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recent ACS | Other CHD | Ischaemic stroke/TIA | PAD | Total ASCVD | T2DM with CKD | T2DM without CKD | T1DM with CKD | T1DM without CKD | CKD alone | Total non-ASCVD | |
| High-intensity statin, %* | 62.4 | 34.6 | 21.5 | 23.2 | 31.4 | 20.0 | 18.6 | 29.0 | 21.1 | 11.1 | 17.0 |
| Monotherapy, % | 93.3 | 91.8 | 93.2 | 92.3 | 92.2 | 91.1 | 93.5 | 92.6 | 90.2 | 93.3 | 93.0 |
| Plus ezetimibe, % | 1.0 | 4.8 | 2.9 | 3.4 | 4.1 | 3.2 | 2.4 | 4.1 | 5.1 | 2.7 | 2.8 |
| Plus other non-statin LMT, % | 5.8 | 3.4 | 3.9 | 4.3 | 3.7 | 5.7 | 4.0 | 3.2 | 4.7 | 4.0 | 4.3 |
| Medium-intensity statin, %* | 22.6 | 41.4 | 46.2 | 44.3 | 42.1 | 44.7 | 43.0 | 39.5 | 36.5 | 33.1 | 40.4 |
| Monotherapy, % | 98.6 | 97.9 | 98.7 | 98.6 | 98.2 | 97.9 | 98.4 | 96.3 | 97.7 | 98.9 | 98.4 |
| Plus ezetimibe, % | 0.6 | 1.2 | 0.7 | 0.6 | 1.0 | 0.6 | 0.6 | 1.7 | 1.4 | 0.5 | 0.6 |
| Plus other non-statin LMT, % | 0.9 | 0.9 | 0.5 | 0.8 | 0.8 | 1.5 | 1.0 | 2.0 | 1.0 | 0.7 | 1.0 |
| Low-intensity statin, %* | 2.1 | 5.9 | 5.5 | 5.1 | 5.6 | 6.4 | 4.9 | 4.9 | 4.4 | 4.8 | 5.0 |
| Monotherapy, % | 93.8 | 95.9 | 97.4 | 97.1 | 96.3 | 96.2 | 98.1 | 89.2 | 95.6 | 98.3 | 97.7 |
| Plus ezetimibe, % | 6.3 | 3.2 | 1.7 | 2.3 | 2.8 | 2.3 | 1.1 | 8.1 | 2.7 | 1.0 | 1.4 |
| Plus other non-statin LMT, % | 0.0 | 0.9 | 0.8 | 0.6 | 0.8 | 1.6 | 0.7 | 2.7 | 1.6 | 0.7 | 0.9 |
| Non-statins only, %* | 0.9 | 2.0 | 1.7 | 1.9 | 1.9 | 2.5 | 1.6 | 3.9 | 2.0 | 1.7 | 1.8 |
| Ezetimibe, % | 70.4 | 61.3 | 65.5 | 55.6 | 61.6 | 47.9 | 54.2 | 58.6 | 61.6 | 45.5 | 51.5 |
| Other non-statin LMT, % | 29.6 | 38.7 | 34.5 | 44.4 | 38.4 | 52.1 | 45.8 | 41.4 | 38.4 | 54.5 | 48.5 |
| Not currently treated by LMT, %* | 12.1 | 16.2 | 25.1 | 25.4 | 19.0 | 26.4 | 31.9 | 22.7 | 35.9 | 49.4 | 35.8 |
| Prev high-intensity statin, % | 20.7 | 16.4 | 8.6 | 9.3 | 13.2 | 7.7 | 6.1 | 18.2 | 9.1 | 3.3 | 5.5 |
| Prev medium-intensity statin, % | 22.8 | 40.9 | 37.9 | 33.0 | 38.4 | 33.2 | 27.5 | 30.6 | 29.9 | 17.7 | 24.7 |
| Prev low-intensity statin, % | 5.6 | 10.0 | 6.9 | 6.8 | 8.5 | 7.3 | 4.9 | 10.0 | 4.4 | 3.9 | 4.7 |
| Prev non-statin LMT, % | 4.8 | 7.2 | 4.8 | 3.6 | 5.9 | 6.1 | 3.5 | 5.3 | 3.8 | 2.4 | 3.4 |
| No prev LMT, % | 46.1 | 25.5 | 41.8 | 47.3 | 34.0 | 45.7 | 58.0 | 35.9 | 52.8 | 72.7 | 61.7 |
*Numbers in this row denote absolute percentages, and add up to 100% vertically. All other numbers are relative percentages of the absolute percentages. ASCVD subgroups represent hierarchical categorisation. Non-ASCVD categorisations have been simplified by consideration of the qualifiers QRISK2 ≥10% and age >40 years as implicit in the definitions of T2DM and T1DM, respectively, with and without CKD, and of the qualifiers without T2DM + QRISK2 ≥10% or T1DM + age >40 as implicit in the definition of CKD alone.
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; CKD, chronic kidney disease; LMT, lipid-modifying therapy; PAD, peripheral arterial disease; Prev, previously on; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TIA, transient ischaemic attack.
Non-ASCVD population
In the non-ASCVD population, 62% received treatment with a statin on the index date (table 2). In the subgroups, statin use was 71% in T2DM with CKD, 66% in T2DM without CKD, 73% in the T1DM with CKD, 62% in T1DM without CKD and 49% in CKD alone. Of the patients receiving statins, at least a medium-intensity statin was used in 92%. As with patients with ASCVD, statins were most commonly used as monotherapy: 93% for high-intensity statins and 98% for medium-intensity and low-intensity statins. Of patients not currently treated with LMT, 62% had no evidence of any LMT treatment in the 2 years prior to the index date.
Comparison of LMT usage in 2014 with 2014 NICE recommendations
ASCVD population
Statin treatment already concordant with the NICE 2014 guidelines (atorvastatin 80 mg equivalent or higher; which is a subset of statins regarded as high-intensity) was observed in 6% of the ASCVD cohort, with 73% treated with a less intensive statin regimen than recommended and 21% not treated with any statin (table 3). Extrapolated to the UK ASCVD population, this identified ∼202 000 individuals whose treatment was already concordant with the updated guidelines, 2.4 million who would require statin uptitration, and 680 000 in whom statin initiation would be recommended to achieve full concordance. For those requiring statin initiation, 31% represented a need for de novo initiation, 60% reinitiation due to past discontinuation and 9% statin addition to non-statin LMT. NICE 2014 guidelines recommended statin therapy was most commonly used in patients with a recent ACS (33%) and the least used in patients with history of an ischaemic stroke/TIA or PAD (2% each).
Table 3.
Statin treatment according to NICE guidelines in the overall ASCVD and non-ASCVD cohorts and subgroups
| ASCVD cohort (n=91 497) |
Non-ASCVD cohort (n=92 086) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recent ACS | Other CHD | Ischaemic stroke/TIA | PAD | Total ASCVD | T2DM with CKD | T2DM without CKD | T1DM with CKD | T1DM without CKD | CKD alone | Total non-ASCVD | |
| Study cohort, % | |||||||||||
| Treated as per NICE guidelines* | 33.1 | 6.7 | 2.0 | 2.2 | 6.1 | 18.6 | 17.2 | 26.2 | 19.3 | 10.3 | 15.8 |
| Treated with statins of lower potency* | 54.0 | 75.1 | 71.2 | 70.5 | 73.0 | 52.5 | 49.3 | 47.3 | 42.7 | 38.6 | 46.6 |
| Treated with only non-statin LMT | 0.9 | 2.0 | 1.7 | 1.9 | 1.9 | 2.5 | 1.6 | 3.9 | 2.0 | 1.7 | 1.8 |
| Not treated by LMT | 12.1 | 16.2 | 25.1 | 25.4 | 19.0 | 26.4 | 31.9 | 22.7 | 35.9 | 49.4 | 35.8 |
| Adjusted extrapolation to the UK population, N | |||||||||||
| Treated as per NICE guidelines* | 34 085 | 147 215 | 15 653 | 4869 | 201 822 | 112 119 | 209 589 | 10 642 | 18 830 | 156 621 | 507 801 |
| Treated with statins of lower potency* | 55 615 | 1 639 122 | 550 930 | 158 966 | 2 404 633 | 316 269 | 600 916 | 19 220 | 41 649 | 586 243 | 1 564 297 |
| Treated with only non-statin LMT | 894 | 44 456 | 13 266 | 4318 | 62 934 | 15 311 | 19 897 | 1575 | 1994 | 26 006 | 64 783 |
| Not treated by LMT | 12 488 | 352 657 | 194 365 | 57 299 | 616 809 | 159 085 | 388 359 | 9230 | 35 017 | 749 786 | 1 341 477 |
ASCVD subgroups represent hierarchical categorisation. Non-ASCVD categorisations have been simplified by consideration of the qualifiers QRISK2 ≥10% and age >40 years as implicit in the definitions of T2DM and T1DM, respectively, with and without CKD, and of the qualifiers without T2DM + QRISK2 ≥10% or T1DM + age >40 as implicit in the definition of CKD alone.
*NICE 2014 guidelines recommend atorvastatin 80 mg and 20 mg, respectively, for the ASCVD and non-ASCVD populations in the table. We have included statins of equivalent or higher potency with ASCVD definition based on atorvastatin 80 mg, rosuvastatin 40 mg and non-ASCVD definition based on atorvastatin 20, 40 and 80 mg, rosuvastatin 10, 20 and 40 mg and simvastatin 80 mg. It should be noted that NICE guidelines allow for consideration of a lower dose based on clinical considerations and patient preference.
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; CKD, chronic kidney disease; LMT, lipid-modifying therapy; NICE, National Institute for Health and Care Excellence; PAD, peripheral arterial disease; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TIA, transient ischaemic attack.
Non-ASCVD population
Statin treatment already concordant with the NICE 2014 guidelines (atorvastatin 20 mg equivalent or higher) was observed in 15% of the non-ASCVD cohort, with 47% treated with a less intensive statin regimen than recommended and 37% not treated with any statin (table 3). Extrapolation identified ∼508 000 UK patients without ASCVD (ie, fulfilling the DM and/or CKD indications) whose treatment was already concordant with the updated guidelines, 1.6 million who would require uptitration, and 1.4 million in whom statin initiation would be recommended to achieve full concordance. For those requiring statin initiation, 59% represented a need for de novo initiation, 36% reinitiation due to past discontinuation and 5% statin addition to non-statin LMT. Of the subgroups, T1DM with CKD was most likely to already be receiving NICE 2014 guidelines recommended statin therapy (26%) and CKD alone was least likely (10%).
ESC/EAS-recommended lipid goal achievement
ASCVD population
The mean LDL-C was 2.3 mmol/L with ∼31% achieving a LDL-C <1.8 mmol/L. Mean non-HDL-C was 2.9 mmol/L with ∼42% achieving a non-HDL-C <2.6 mmol/L. Online supplementary tables S5 and S6 provide a detailed summary of the mean LDL-C and non-HDL-C levels, achievement of LDL-C <1.8 mmol/L and non-HDL-C <2.6 mmol/L, by LMT treatment for prevalent and hierarchical subgroups. Achievement of LDL-C and non-HDL-C goals was associated with the hierarchy of ASCVD subgroups, with recent ACS being the highest and PAD being the lowest. Patients with recent ACS receiving high-intensity statins had the highest achievement of both lipid goals: LDL-C <1.8 mmol/L was 53% and non-HDL-C <2.6 mmol/L was 65%. LDL-C and non-HDL-C goal achievement by LMT regimen was somewhat difficult to interpret due to the unadjusted nature of analyses and potential self-selection bias, resulting from the choice of LMT regimen in clinical practice being influenced by baseline lipid levels and other patient-level factors.
Non-ASCVD population
Mean LDL-C was 2.4 mmol/L with ∼26% achieving LDL-C <1.8 mmol/L. Mean non-HDL-C was 3.2 mmol/L with ∼33% achieving non-HDL-C <2.6 mmol/L. A summary of mean LDL-C and non-HDL-C levels and lipid goal achievement are presented in online supplementary table S7. Achievement of LDL-C and non-HDL-C goals was highest for the T1DM with CKD subgroup and lowest for the CKD alone subgroup. The T1DM with CKD subgroup receiving medium-intensity statins had the highest lipid goal achievement: 52% for LDL-C <1.8 mmol/L and 69% for non-HDL-C <2.6 mmol/L.
Discussion
The use of statins for reducing atherogenic lipid levels is recommended across all global guidelines for the prevention of incident ASCVD events. Recent guidelines have shifted towards an approach of consistent and immediate initiation of the appropriate statin intensity regimen for a particular indication regardless of baseline lipid levels. In contrast, the standard approach has been to lower LDL-C (or non-HDL-C) below defined thresholds. Our study, representing a UK general practice population at high risk for ASCVD events, provides several insights regarding the state of concordance of 2014 clinical practice with the updated NICE 2014 guidelines,7 which focus on the intensity of the statin regimen. As a comparison, we also report on concordance with the ESC/EAS 2011 guidelines,8 which focus on treatment to defined LDL-C goals.
We observed that 79% of individuals with ASCVD were receiving statin treatment, with 6% being prescribed a regimen already concordant with the NICE 2014 guidelines (atorvastatin 80 mg or equivalent). For the non-ASCVD group, we observed that 62% were receiving statin treatment, with 15% being prescribed a regimen already concordant with the NICE 2014 guidelines (at least atorvastatin 20 mg or equivalent). Extrapolation of these findings to the UK population indicated that, out of 3.3 million individuals with ASCVD, 2.4 million would require uptitration of statin therapy and 680 000 would require statin initiation (31% de novo initiation, 60% reinitiation and 9% addition to non-statin LMT) to have full population-level concordance with these recommendations. Of the 3.5 million individuals with high-risk non-ASCVD (ie, fulfilling DM-based and/or CKD-based guideline criteria), 1.6 million would require uptitration of statin therapy and 1.4 million would require statin initiation (59% de novo initiation, 36% reinitiation and 5% addition to non-statin LMT).
Our findings highlight a considerable gap between the 2014 UK clinical practice and achievement of full concordance with the NICE 2014 guideline recommendations. Optimising the implementation of the guidelines represents best practice and would result in a reduction in ASCVD event risk. The practical impact on patients, and in particular GPs, however, merits consideration. First, for patients who are well established on a treatment regimen, changes in therapy can lead to reduced compliance, greater reporting of adverse events and additional time within the healthcare system to monitor the changes in therapy. Second, in an environment where primary care is already stretched, the impact of counselling 90% of eligible patients for statin regimen changes is likely to be considerable on time and resources available in primary care without, for instance, an increase in the number of GPs. Finally, as cholesterol targets no longer appear in the updated 2014/2015 Quality Outcomes Framework (QOF) in the UK for rewarding the provision of quality of care by GPs,15 and with QOF lacking mention of statin intensity, it is unclear how narrowing of the gap between guidelines and clinical practice might be effectively incentivised.
As mentioned earlier, an alternative approach to atherogenic lipid management is to aim for lipid goal achievement as recommended in the ESC/EAS guidelines. For the ASCVD population, we observed that achievement of LDL-C <1.8 mmol/L and non-HDL-C <2.6 mmol/L8 was only 31% and 42% overall; 38% and 52% for those on medium-intensity statins; and 37% and 48% for those on high-intensity statins. Comparatively, lower goal achievement for those on high-intensity statin likely reflected the unadjusted nature of analysis, with patients on high-intensity statins likely starting with higher baseline lipid levels. For the non-ASCVD population considered in the analysis (ie, fulfilling DM-based and/or CKD-based guideline criteria), the ESC/EAS guidelines recommend the same thresholds of <1.8 mmol/L and <2.6 mmol/L for LDL-C and non-HDL-C, respectively.8 We observed achievement of these goals in only 26% and 33% of the patients without ASCVD; 40% and 49% for those on medium-intensity statin and 36% and 42% for those on high-intensity statin.
For those not on any statin, treatment with a medium-intensity statin is expected to lower LDL-C by 35%, while for those on medium-intensity statin, increasing the dose to high-intensity statin is expected to lower LDL-C by an incremental 15%.7 Given that average LDL-C levels for those not on a statin in ASCVD and non-ASCVD cohorts was ∼3.0 mmol/L, and for those on medium-intensity statins in ASCVD and non-ASCVD cohorts was ∼2.0 mmol/L, it is unlikely that an average LDL-C of <1.8 mmol/L can be achieved in these populations even by maximising statin treatment. From the perspective of a treat to LDL-C goal approach, our results suggest an expanded role for evidence-based add-on therapies for LDL-C lowering, primarily ezetimibe based on available data from the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) in 2014.16
Prior reports from a generalisable UK population describing LMT usage and lipid attainment in ASCVD and non-ASCVD cohorts are limited. Our study provides novel data on how these populations are treated and how effective the treatment has been. The recent EUROpean Action on Secondary and Primary prevention by Intervention to Reduce Events (EUROASPIRE) IV Study, representing the 2012–2013 time period, was conducted in patients with a history of hospitalisation due to a coronary event with an assessment date of 6 months to 3 years post event.17 The study reported LDL-C <1.8 mmol/L achievement as only 19%. To the best of our knowledge, the most recent study that reported statin use in a generalisable UK population at high CV risk found statin use in 2007 as 72% for patients with a history of MI or coronary revascularisation and 36% for those with a history of angina but not MI.18 Statin use in the UK in those with ASCVD without coronary conditions (eg, ischaemic stroke or PAD) appears to be even further under-reported. From 1995–2005, in individuals aged ≥50 years with a history of stroke (not limited to ischaemic stroke), 32% of men and 26% of women were receiving treatment with LMT.19 Data on statin use in individuals with DM or CKD without ASCVD also do not appear to be well reported. During 2006–2008, a diabetes registry representing GPs in Scotland found 68% individuals with DM without ASCVD were prescribed a statin.20 When compared with these data, our study suggests statin use has increased in the UK. This increase is likely due to a multitude of influences, including the NICE 2008 guidelines,21 landmark trials such as the Stroke Prevention by Aggressive Reduction in Cholesterol Level (SPARCL) study which demonstrated the benefits of statins in the postischaemic stroke population,22 and incorporation of statins and lipid monitoring as quality measures in major pay-for-performance initiatives in the UK.23
The continued recommendation for statin therapy for the ASCVD population from the 2008 to the 2014 NICE guidelines7 21 suggest that the 21% rate of statin non-use in this population is largely due to discontinuation, a hypothesis that is supported by our findings which indicate that ∼60% of statin non-use in the ASCVD population can be attributed to discontinuation (hence overall 13% (=21%×60%) of statin non-use in ASCVD population is estimated to be due to discontinuation). High rates of statin discontinuation have been documented in the literature. For example, in a UK primary care database analysis representing the 1997–2006 time period, 20% of patients initiated on a statin after an MI discontinued therapy at 1 year.24 For the non-ASCVD population, the higher observed rate of statin non-use (38%) likely reflects statin discontinuation and many patients who have never been prescribed LMT. Our study suggests that ∼59% of statin non-use in the non-ASCVD population can be attributed to non-initiation of LMT therapy. Prior to the 2014 NICE guidelines, some of these patients may not have qualified for LMT due to higher risk thresholds in the NICE 2008 guidelines and the absence of certain conditions such as CKD as a stand-alone criterion for statin therapy.7 21
Limitations
Availability of demographic and clinical characteristics was limited to information available in the GP database. Certain data such as Body Mass Index, ethnicity, blood pressure and smoking status were not available for all patients. Lipid measurements were not prospectively specified and the study cohort represents individuals with an available lipid profile measurement, introducing the possibility of bias in findings. The definition of LMT usage at the time of lipid measurements was optimised to provide valid ‘on-treatment’ measures, but ensuring whether patients fully took their prescribed medications was not feasible from the database.
For consistency, we based LMT categorisation on the statin type and dose, regardless of concomitant non-statin medications. This meant, for example, atorvastatin 10 mg + ezetimibe was categorised under medium-potency statin, even though its overall lipid-lowering efficacy is close to atorvastatin 80 mg. NICE 2014 guidelines specifically recommend atorvastatin 80 mg and atorvastatin 20 mg, respectively, for the ASCVD and non-ASCVD populations considered in our study. During LMT categorisation, we included equivalent or higher potency statin therapy in order to include situations where quality of care with lipid-lowering treatment was similar or better. Of those treated with NICE 2014 guidelines according to our definition, 92% received atorvastatin 80 mg in the ASCVD population and 44% received atorvastatin 20 mg in the non-ASCVD population. Lower doses of atorvastatin are allowed by the guidelines in case of potential drug interactions, high risk of adverse effects and patient preference; but estimation of how frequently these cases occur was not feasible. In individuals ≥85 years of age, NICE 2014 guidelines recommend a careful consideration of patient-level factors informing the risk–benefit of statin treatment and intensity. Among those not treated according to NICE 2014 guidelines in our study, ∼14% and 10% individuals within the ASCVD and non-ASCVD populations, respectively, were ≥85 years of age.
Our study was focused on the secondary prevention and high-risk primary-prevention populations; it did not include those for whom treatment would be recommended for treatment based solely on a QRISK2 10-year risk of ≥10%. Thus, the analysis does not assess the full impact of the guidelines across all indications.
Conclusions
In the UK, although 79% and 62% of patients with ASCVD and high-risk patients without ASCVD, respectively, received treatment with statin therapy in 2014, there existed a large gap in terms of treatment concordance with the NICE 2014 guidelines recommendations released during the same year, with up to 90% of both populations requiring modification to their existing therapy. Extrapolating these results to the UK population, up to 3 million ASCVD and 3 million high-risk non-ASCVD individuals (ie, fulfilling DM-based and/or CKD-based guideline criteria) might require either statin uptitration or initiation in order to attain full concordance with NICE 2014 guidelines. Achievement of ESC/EAS 2011 guideline criteria would also require statin uptitration, as approximately two-thirds of individuals receiving moderate-intensity or high-intensity statins were not at the recommended LDL-C goal.
Acknowledgments
Medical writing support was provided by Jeff Alexander from SNELL Medical Communication, supported by Sanofi and Regeneron Pharmaceuticals, Inc. Editorial assistance for later drafts was provided by Neil Venn from Prime, supported by Sanofi and Regeneron Pharmaceuticals, Inc.
Footnotes
Contributors: DS was involved with the design of the study, interpretation of data and critical review of drafts. IK was involved with the design of the study, interpretation of data and critical review of drafts. DA was involved with the design of the study, acquisition, analysis and interpretation of data, and critical review of drafts. RJS was involved with the design of the study, interpretation of data and critical review of drafts. KKR was involved with the design of the study, interpretation of data and critical review of drafts. All authors provided final approval of the submitted manuscript.
Funding: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Competing interests: DS receives modest consulting fees from Sanofi and Regeneron. IK is a stockholder and employee of Sanofi. DA is an employee of IMS Health. RJS is a stockholder and employee of Regeneron Pharmaceuticals, Inc. KKR has received honoraria for advisory boards, lectures or for serving on the steering committee for clinical trials from Amgen, Sanofi, Regeneron, Pfizer, AstraZeneca, Aegerion, Kowa, IONIS Pharma, MedCo, Cerenis and Resverlogix, and has received research support by grants to his institution from Pfizer, MSD, Amgen, Sanofi and Regeneron Pharmaceuticals, Inc.
Ethics approval: This study was approved by the Scientific Review Committee, an independent UK-based ethics committee established to review studies from The Health Improvement Network (THIN).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The original analytic data set is available on request by emailing the corresponding author: SteenDL@ucmail.uc.edu.
Transparency: DS, as the lead author (the manuscript’s guarantor), affirms that the manuscript is an honest, accurate and transparent account of the study being reported; no important aspects of the study have been omitted and any discrepancies from the study as planned have been explained.
References
- 1.World Health Organization. Health statistics and information systems: estimated deaths (‘000) by cause, sex and WHO member state, 2012. http://www.who.int/entity/healthinfo/global_burden_disease/GHE_Deaths_2012_country.xls?ua=1 (accessed 14 Jan 2016).
- 2.Institution for health metrics and evaluation. Global burden of disease calculator: United Kingdom, 2013. http://vizhub.healthdata.org/gbd-compare. (accessed 14 Jan 2016).
- 3.Mihaylova B, Emberson J, Blackwell L et al. . Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–90. 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baigent C, Blackwell L, Emberson J et al. , Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010;376:1670–81. 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashani A, Phillips CO, Foody JM et al. . Risks associated with statin therapy: a systematic overview of randomised clinical trials. Circulation 2006;114:2788–97. 10.1161/CIRCULATIONAHA.106.624890 [DOI] [PubMed] [Google Scholar]
- 6.Halcox JP, Tubach F, Lopez-Garcia E et al. . Low rates of both lipid-lowering therapy use and achievement of low-density lipoprotein cholesterol targets in individuals at high-risk for cardiovascular disease across Europe. PLoS One 2015;10:e0115270 10.1371/journal.pone.0115270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Lipid modification: Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. Clinical guideline CG181. July 2014. http://www.nice.org.uk/guidance/cg181 (accessed 7 Feb 2015).
- 8.Reiner Z, Catapano AL, De Backer G et al. , European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–818. 10.1093/eurheartj/ehr158 [DOI] [PubMed] [Google Scholar]
- 9.Blak BT, Thompson M, Dattani H et al. . Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251–5. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JD, Schinnar R, Bilker WB et al. . Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393–401. 10.1002/pds.1335 [DOI] [PubMed] [Google Scholar]
- 11.Hammad TA, McAdams MA, Feight A et al. . Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the General Practice Research Database. Pharmacoepidemiol Drug Saf 2008;17:1197–201. 10.1002/pds.1672 [DOI] [PubMed] [Google Scholar]
- 12.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 2010;60:e128–136. 10.3399/bjgp10X483562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shephard E, Stapley S, Hamilton W. The use of electronic databases in primary care research. Fam Pract 2011;28:352–4. 10.1093/fampra/cmr039 [DOI] [PubMed] [Google Scholar]
- 14.Collins GS, Altman DG. Predicting the 10 year risk of cardiovascular disease in the United Kingdom: independent and external validation of an updated version of QRISK2. BMJ 2012;344:e4181 10.1136/bmj.e4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health Services (NHS) Employers, NHS England, and British Medical Association General Practitioners Committee. 2014/15 General medical services (GMS) contract quality and outcomes framework (QOF): Guidance for GMS contract 2014/15. March 2014. NHS England Gateway reference 01264. http://www.hscic.gov.uk/media/14019/QOF-Guidance-GMS-Contract-2014-15/pdf/QOF_guidance_GMS_contract_2014_15.pdf (accessed 22 Mar 2016).
- 16.Cannon CP, Blazing MA, Giugliano RP et al. , IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 17.Kotseva K, Wood D, De Bacquer D et al. , EUROASPIRE Investigators. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016;23:636–48. 10.1177/2047487315569401 [DOI] [PubMed] [Google Scholar]
- 18.Hawkins NM, Scholes S, Bajekal M et al. . The UK National Health Service: delivering equitable treatment across the spectrum of coronary disease. Circ Cardiovasc Qual Outcomes 2013;6:208–16. 10.1161/CIRCOUTCOMES.111.000058 [DOI] [PubMed] [Google Scholar]
- 19.Raine R, Wong W, Ambler G et al. . Sociodemographic variations in the contribution of secondary drug prevention to stroke survival at middle and older ages: cohort study. BMJ 2009;338:b1279 10.1136/bmj.b1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones NR, Fischbacher CM, Guthrie B et al. , Scottish Diabetes Research Network Epidemiology Group. Factors associated with statin treatment for the primary prevention of cardiovascular disease in people within 2 years following diagnosis of diabetes in Scotland, 2006-2008. Diabet Med 2014;31:640–6. 10.1111/dme.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute for Health and Clinical Excellence. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. NICE Clinical Guideline 67. May 2008. https://www.nice.org.uk/guidance/cg67 (accessed 22 Mar 2016). [Google Scholar]
- 22.Amarenco P, Bogousslavsky J, Callahan A III et al. . Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59. 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 23.Millett C, Gray J, Wall M et al. . Ethnic disparities in coronary heart disease management and pay for performance in the UK. J Gen Intern Med 2009;24:8–13. 10.1007/s11606-008-0832-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey IM, DeWilde S, Shah SM et al. . Statin use after first myocardial infarction in UK men and women from 1997 to 2006: Who started and who continued treatment? Nutr Metab Cardiovasc Dis 2012;22:400–8. 10.1016/j.numecd.2010.09.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013255supp_appendix.pdf (819.1KB, pdf)