Abstract
Anticoagulant-related nephropathy is a type of acute kidney injury caused by overcoagulation. We describe a case of an 84-year-old man with arterial hypertension, coronary heart disease and atrial fibrillation treated with acenocoumarol, who presented with haematoproteinuria and acute kidney injury during a phase of excessive anticoagulation. In addition to IgA nephropathy, renal biopsy also revealed acute tubular necrosis, red blood cell casts and positive iron staining in tubular cells. After this acute episode, renal function improved and proteinuria decreased below the nephrotic range.
Background
Anticoagulant therapy has a central role in diseases with thromboembolic events, such as atrial fibrillation and deep vein thrombosis.1 Acenocoumarol is a vitamin K antagonist with similar efficacy as warfarin in the treatment of venous and arterial thromboembolism.2 Closely monitoring of international normalised ratio (INR) is essential to avoid overcoagulation, because there is a large interindividual variability of dose requirements. In the past years, there has been recognised an association between overcoagulation and acute kidney injury (AKI), named as anticoagulant-related nephropathy (ARN), first described with warfarin and, because of this, is also called warfarin-related nephropathy (WRN). We report a case of a patient with underdiagnosed IgA nephropathy, presenting with AKI due to overcoagulation with acenocoumarol.
Case presentation
An 84-year-old patient presented with gross haematuria, fatigue and shortness of breath. He had a medical history of arterial hypertension for 10 years and coronary heart disease (the patient underwent triple coronary artery bypass grafting 10 years ago). He also had atrial fibrillation, with a pacemaker implanted 3 years ago, and is under oral anticoagulation with acenocoumarol (CHADS2-VASc score was 4). His medical history included prostate adenocarcinoma diagnosed in the year before (stage 1; T1cN0M0, according to prostate cancer staging of American Joint Committee on Cancer). The patient also had well-controlled hypothyroidism. His medical history was significant for bone tuberculosis at the age of 18, resolved without major sequelae. Medications included ramipril plus hydrochlorothiazide, levothyroxine, acenocoumarol, esomeprazole, amlodipine, tamsulosin, bicalutamide and goserelin.
On admission his blood pressure was 123/72 mm Hg, pulse rate was 92 bpm, temperature 36.8°C and oxygen saturation 98% on room air. Physical examination revealed crackles in the lung bases and 2+ pitting oedema in both lower extremities.
Investigations
Laboratory studies showed haemoglobin of 10.8 g/dL with normal leucocyte and platelet counts. Serum creatinine (SCr) was 4.68 mg/dL, glomerular filtration rate was 12 mL/min/1.73 m2, estimated by Modification of Diet in Renal Disease (MDRD) formula, and blood urea nitrogen was 82 mg/dL, with normal electrolytes. Serum albumin was low (32 g/L). The prothrombin time was 23.3 s and INR 2.03, but 1 week before this evaluation his INR was 6.96. Urinalysis revealed proteinuria (3.6 g/24 hour) and erythrocyturia (3394/μL). No abnormalities were noted on renal ultrasound scan. Analysing the blood tests and the urinalysis from the year before, we found that the patient had normal renal function in the previous month (SCr 1.0 mg/dL) and erythrocyturia was present in the past 2 years, but proteinuria was only noticed in this episode. Autoimmune markers including antineutrophil cytoplasmic antibody, antiglomerular basement membrane antibody and antinuclear antibody were negative. C3 and C4 levels were normal. Serum and urine immunofixation were negative for monoclonal gammopathy.
A renal biopsy was performed. The biopsy specimen contained 16 glomeruli with mild mesangial hypercellularity. The interstitium was oedematous with the presence of diffuse acute tubular necrosis and occlusive red blood cell (RBC) casts (figure 1). The arteries had severe intima thickness (figure 1). The immunofluorescence revealed mesangial deposits of IgA (+++), C3 (+++) and light chains κ and λ (++; figure 2). The histology findings allowed to diagnose two conditions: (1) ARN; (2) IgA nephropathy (M1E0S0T0, according with Oxford Classification of IgA nephropathy).
Figure 1.
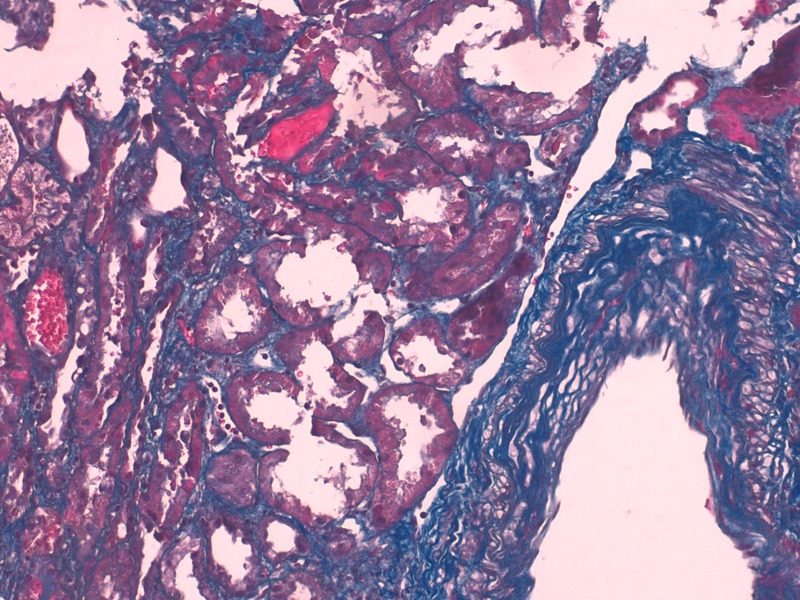
Red blood cells casts and acute tubular necrosis. It also showed an artery with marked intima thickness. Masson's trichrome staining (×400).
Figure 2.
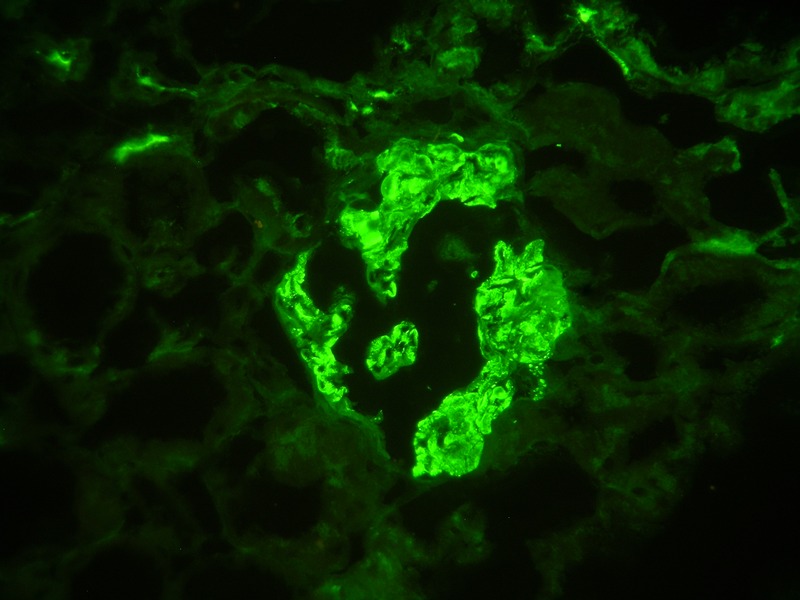
Immunofluorescence microscopy demonstrating granular mesangial deposits of IgA (×400).
Treatment, outcome and follow-up
Ramipril plus hydrochlorothiazide and acenocoumarol were withdrawn. INR was normalised and, at this time, enoxaparin 60 mg once daily was started. His renal function slowly improved and the patient was discharged with SCr 3.45 mg/dL. Two months later his SCr decreased to 2.7 mg/dL, but proteinuria remained in the nephrotic range (4.6 g/24 hour). Thus, enalapril 5 mg once daily was prescribed and enoxaparin was replaced by a new oral anticoagulant: apixaban 2.5 mg two times a day. Eleven months later, his SCr continued to decrease to 1.7 mg/dL and proteinuria markedly improved (400 mg/24 hour). However, significant erythrocyturia (840/μL) was still present, although without more episodes of gross haematuria.
Discussion
ARN is defined as AKI without obvious aetiology in the setting of an INR of >3.0,3 accompanied by microscopic or gross haematuria.3
Previous reports on ARN focused on anticoagulation with warfarin and acenocoumarol;4 however, recent case reports suggest that it can also occur with new oral anticoagulants.5–7 ARN is an underdiagnosed condition for the reason that nephrologists are reluctant to perform renal biopsy in patients under anticoagulation therapy, because of concerns related to the risk of thrombosis/thromboembolism while anticoagulation is withheld and the risk of bleeding when the anticoagulation is restarted. Thus, most of cases have a presumptive diagnosis, being difficult to determine the real incidence of ARN. Morphologically, ARN is characterised by glomerular haemorrhage and tubular obstruction by RBC casts, causing acute and chronic tubular damage, predominantly in distal nephron segments. The glomeruli show little or no abnormalities by light, immunofluorescence or electron microscopy.8
To best of our knowledge, three cases of ARN in patients with IgA nephropathy have been reported: two cases occurred in patients treated with dabigatran6 7 and one in a case of anticoagulation with warfarin.9 Patients with chronic kidney disease are particularly susceptible, but there are other underlying risk factors, such as age, diabetes and diabetic nephropathy, arterial hypertension, glomerulonephritis and heart failure.10 In this case, the presence of proteinuria in nephrotic range raised the suspicion that an underlying glomerulopathy might also be present, which gave an indispensable role to the renal biopsy to ensure a correct diagnosis. In IgA nephropathy, glomerular haemorrhage leads to a tubular injury but, in our patient, we assumed that haematuria as a consequence of IgA nephropathy as a minor role in the development of AKI. One study showed that only few patients (<1.5%) with IgA nephropathy and gross haematuria had evidence of AKI, which leads to think that other mechanisms may be involved.11 Thrombin, a vitamin K-dependent coagulation factor stimulates signalling cascades, binds and activates a family of proteinase-activated receptors (PARs) expressed in endothelial cells.12 It is suggested that activation of PARs is important to maintain endothelial integrity (including in glomeruli), and the reduction in thrombin levels caused by anticoagulants affects the endothelial permeability, allowing glomerular bleeding. Recently, one study showed that activated protein C signalling has trophic and antiapoptotic effects in cultured podocytes. It is demonstrated that in patients treated with vitamin K antagonists the levels of protein C activated are decreased, and thus, it is another possible pathway of glomerular injury by these drugs.13
In this case, the patient had prostate cancer, which is a reported cause of haematuria. Nevertheless, we assumed that haematuria had, mainly, a glomerular origin, since RBC casts were found on renal biopsy.
The glomerular lesions concerning IgA nephropathy were mild (M1E0S0T0, according with Oxford Classification of IgA nephropathy), but the tubulointerstitial involvement was more evident with acute tubular necrosis, extensive RBC casts and positive iron staining in the cytoplasm of proximal tubular cells (figure 3). The mechanism of iron tubular toxicity seems to be related with oxidative stress.14 In high concentrations, free haemoglobin released by RBCs in the tubular lumen is uptaken via the megalin-cubulin receptors and the haeme molecule causes oxygen-radical formation, increasing lipid peroxidation and denaturation of proteins, which leads to apoptosis.14 Haeme also induces mitochondrial injury through oxidative damage and augments ischaemia-induced peroxidation.15
Figure 3.
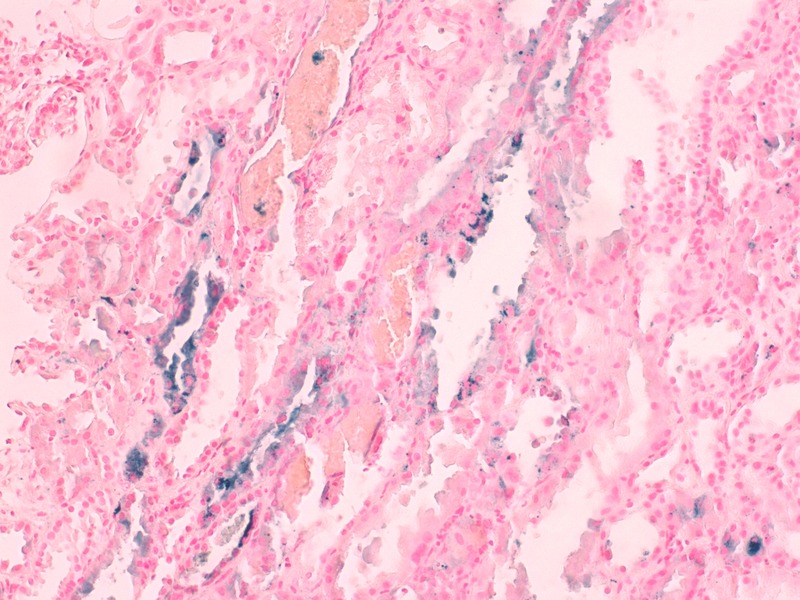
Iron in cytoplasm of tubular epithelial cells. Perls Prussian blue staining. Exuberant red blood cells casts are also noted (×400).
In our case, in addition to hypertension, cardiovascular disease and age, the presence of IgA nephropathy appears to be a predisposing factor to ARN. Renal function was normal prior to this episode of AKI, which is according with histological findings. Beyond severe intima thickness of arteries due to hypertension, there were no signs of chronic damage on renal biopsy, such as glomerulosclerosis, tubular atrophy and interstitial fibrosis. In glomeruli, the presence of mild mesangial hypercellularity was the only change noticed. Thus, in this case, we assume that AKI is explained mostly by ARN and its acute tubulointerstitial involvement, in a background of IgA nephropathy. At previous evaluation, the proteinuria significantly decreased (400 mg/24 hour) as a consequence of enalapril, and renal function markedly improved (SCr 1.7 mg/dL). Owing to good response to therapy—proteinuria below 1 g/24 hour—the patient was not submitted to any additional treatment (like steroids) for IgA nephropathy.
Treatment of this condition is mainly supportive and includes the returning of INR to a therapeutic range. N-acetylcysteine, by its antioxidant effect, has been shown to prevent AKI in animal models of WRN.16 Steroids are not recommended and were used with success only in one case.7 The most important therapeutic approach is the prevention and early detection of ARN, maintaining INR in the therapeutic range and closely monitoring renal function, even in patients treated with new oral anticoagulants.
In most patients with ARN, renal function stabilises or improves slightly within the first few weeks.8 In our case, renal function, which was normal before this episode of AKI, was consistently improving and proteinuria decreased. Histologically, the absence of glomerulosclerosis and tubulointerstitial fibrosis are markers of good renal prognosis. The only histological marker of poor renal prognosis is the severe intima thickness of arteries according to the medical history of hypertension. In addition to renal prognosis, which appears to be favourable, the vital prognosis will be affected by patient's multiple comorbidities and, inclusively, by ARN. In one study of 4006 patients, presumptive ARN has been associated with an increase in mortality independent of age, sex, race, haemorrhage, atrial fibrillation, heart failure and diabetes mellitus.10
Learning points.
Anticoagulant-related nephropathy is a type of acute kidney injury caused by overcoagulation with vitamin K antagonists of other anticoagulants.
The risk of occurrence of this condition is higher in patients with diabetes, hypertension, heart failure, chronic kidney disease and glomerulonephritis.
Since the renal biopsy is not performed in most cases, the diagnosis is frequently presumptive.
Morphologically, it is characterised by glomerular haemorrhage and acute tubular necrosis due to obstruction by red blood cell casts and, ultimately, by haeme toxicity.
Treatment is mainly supportive and the central approach is prevention, closely monitoring the patients under anticoagulation.
Footnotes
Contributors: MG contributed in writing and intellectual input. AA treated the patient. FC contributed in analysis, data interpretation and reviewed the article. FN reviewed the article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Harter K, Levine M, Henderson SO. Anticoagulation drug therapy: a review. West J Emerg Med 2015;16:11–17. 10.5811/westjem.2014.12.22933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcellona D, Vannini ML, Fenu L et al. . Warfarin or acenocoumarol: which is better in the management of oral anticoagulants? Thromb Haemost 1998;80:899–902. [PubMed] [Google Scholar]
- 3.Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost 2016;14:461–7. 10.1111/jth.13229 [DOI] [PubMed] [Google Scholar]
- 4.Martín Cleary C, Moreno JA, Fernández B et al. . Glomerular haematuria, renal interstitial haemorrhage and acute kidney injury. Nephrol Dial Transplant 2010;25:4103–6. 10.1093/ndt/gfq493 [DOI] [PubMed] [Google Scholar]
- 5.Ryan M, Ware K, Qamri Z et al. . Warfarin-related nephropathy is the tip of the iceberg: direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant 2014;29:2228–34. 10.1093/ndt/gft380 [DOI] [PubMed] [Google Scholar]
- 6.Escoli R, Santos P, Andrade S et al. . Dabigatran-related nephropathy in a patient with undiagnosed IgA Nephropathy. Case Rep Nephrol 2015;2015:298261 10.1155/2015/298261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moeckel GW, Luciano RL, Brewster UC. Warfarin-related nephropathy in a patient with mild IgA nephropathy on dabigatran and aspirin. Clin Kidney J 2013;6:507–9. 10.1093/ckj/sft076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodsky SV, Satoskar A, Chen J et al. . Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis 2009;54:1121 10.1053/j.ajkd.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 9.Ng CY, Tan CS, Chin CT et al. . Warfarin related nephropathy: a case report and review of literature. BMC Nephrol 2016;17:15 10.1186/s12882-016-0228-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodsky SV, Nadasdy T, Rovin BH et al. . Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 2011;80:181–9. 10.1038/ki.2011.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kveder R, Lindic J, Ales J et al. . Acute kidney injury in immunoglobulin a nephropathy: potential role of macroscopic hematuria and acute tubulointerstitial injury. Ther Apher Dial 2009;13:273–7. 10.1111/j.1744-9987.2009.00723.x [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000;407:258–64. [DOI] [PubMed] [Google Scholar]
- 13.Madhusudhan T, Wang H, Straub BK et al. . Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood 2012;119:874–83. 10.1182/blood-2011-07-365973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zager RA, Gamelin LM. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol 1989;256:446–55. [DOI] [PubMed] [Google Scholar]
- 15.Moussavian MR, Slotta JE, Kollmar O et al. . Hemoglobin induces cytotoxic damage of glycine-preserved renal tubules. Transpl Int 2007;20:884–94. 10.1111/j.1432-2277.2007.00538.x [DOI] [PubMed] [Google Scholar]
- 16.Ware K, Qamri Z, Ozcan A et al. . N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am J Physiol Renal Physiol 2013;304:F1421–7. 10.1152/ajprenal.00689.2012 [DOI] [PubMed] [Google Scholar]


