Abstract
Purpose
The purpose of this work was to describe the design and implementation of a digital pathology laboratory, the Retinoblastoma Collaborative Laboratory (RbCoLab) in Kenya.
Method
The RbCoLab is a central lab in Nairobi that receives retinoblastoma specimens from all over Kenya. Specimens were processed using evidence-based standard operating procedures. Images were produced by a digital scanner, and pathology reports were disseminated online.
Results
The lab implemented standard operating procedures aimed at improving the accuracy, completeness, and timeliness of pathology reports, enhancing the care of Kenyan retinoblastoma patients. Integration of digital technology to support pathology services supported knowledge transfer and skills transfer. A bidirectional educational network of local pathologists and other clinicians in the circle of care of the patients emerged and served to emphasize the clinical importance of cancer pathology at multiple levels of care. A ‘Robin Hood’ business model of health care service delivery was developed to support sustainability and scale-up of cancer pathology services.
Discussion
The application of evidence-based protocols, comprehensive training, and collaboration were essential to bring improvements to the care of retinoblastoma patients in Kenya. When embraced as an integrated component of retinoblastoma care, digital pathology offers the opportunity for frequent connection and consultation for development of expertise over time.
Key Words: Cancer, Pathology, Retinoblastoma, Kenya, Digital pathology, Implementation, Global health, Translation
Introduction
Poor access to health care results in dismal outcomes for retinoblastoma, particularly in sub-Saharan Africa [1]. Efforts to combat this health inequity have emerged in East Africa in the form of organized national strategies, with the aim of raising awareness, coordinating care, and improving health services [2,3].
For one such group, the Kenyan National Retinoblastoma Strategy (KNRbS), cancer pathology emerged as an area of immediate focus [3]. Histopathology services are necessary for effective management and treatment of retinoblastoma patients, promoting informed decision-making for timely intervention, and increasing chance of cure. However, this relatively simple but essential part of cancer care is absent or delayed in sub-Saharan Africa [4].
Aiming to improve access to and accuracy of histopathology for retinoblastoma, the KNRbS developed the Retinoblastoma Collaborative Laboratory (RbCoLab): a centralized digital pathology laboratory that applied evidence-based protocols via newly trained and mentored staff. Here, we describe the design and execution of the project, and provide results for the first phase of the laboratory's operation.
Methods
Project Design
To describe the design of the project, we used Paul Farmer's ‘Staff, Stuff, Space, Systems’ approach to building capacity in global health care [5,6] as a framework. There were no animal or human subjects involved, and the evaluation was carried out in accordance with Article 2.5 of the Tri-Council Policy Statement for Ethical Conduct of Research [7].
Outcomes
Results of the project are reported as: service outcomes (beneficiaries: lives improved or touched); training outcomes (intermediaries), and sustainability outputs for phase I of the project (January 31, 2012 to January 31, 2014). These are consistent with the Grand Challenges Canada Results-Based Management and Accountability Framework (RMAF) (www.grandchallenges.ca).
Results
Project Design
The project was designed iteratively over time with experimentation, using the annual KNRbS meetings as a basis for defining and refining the problem (fig. 1), reviewing progress, and mapping next steps [3].
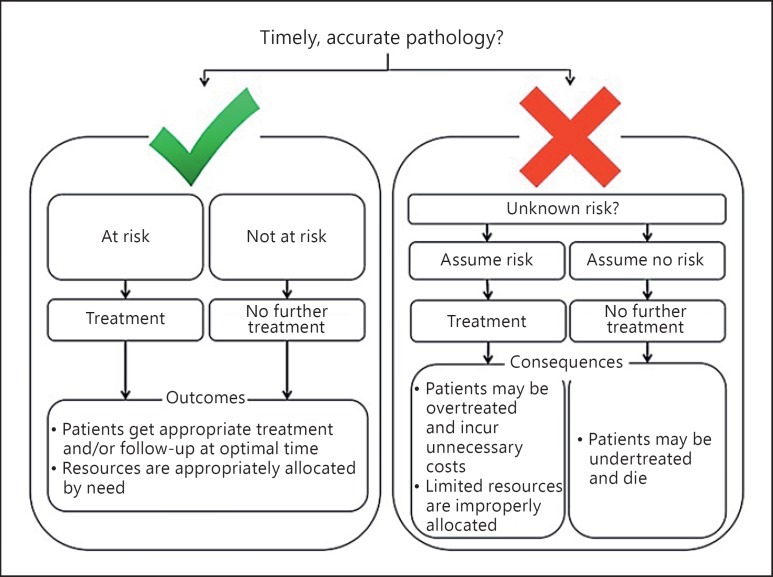
Fig. 1.
Outcomes of timely, accurate pathology compared to consequences of untimely, inaccurate pathology. In the presence of timely and accurate pathology, the risk of tumor spread can be assessed, and if justified, subsequent treatment may be applied in a timely manner as the evidence-based guidelines indicate. When pathology is late, unreliable, or absent, the treating physician is left without any evidence to guide clinical decision-making. An assumption of risk leads to treating all patients, even those who are truly not at risk. These patients incur treatment costs that they would not otherwise have to incur, and hospital resources are strained unnecessarily. If the physician assumes no risk, then some patients will be undertreated and run the risk of recurrence, metastasis, and death.
The first major tasks were obvious: namely, to fill the need for a trained pathologist and technician in retinoblastoma (staff), and to provide the appropriate equipment (stuff) and laboratory (space) to begin working. The University of Nairobi (UoN) School of Dental Sciences, Nairobi, Kenya, offered the space and staff time for the retinoblastoma pathology project; as a gesture of good faith, partners from the University of Toronto, Toronto, Ont., Canada, acquired a used microtome to solidify the deal. This simple ‘handshake’ agreement set the tone for trust between partners, and a memorandum of understanding (MoU) was created to reflect this arrangement. The lack of rigid structures and design allowed the project design to develop organically, as described below.
(A) Staff: Training and Education
Pathologist Training. UoN pathologist (E.A.O.D.) completed a 3-month fellowship in Toronto in ocular and retinoblastoma pathology. She observed the multidisciplinary nature of retinoblastoma care following patients through diagnosis and eye removal, processing of the eye in the laboratory, histological evaluation of the resultant slides, discussion of the case at rounds, and subsequent management. She was trained on manual and digital systems for pathology. Research training included participation in a quality assurance audit of retinoblastoma pathology. Standard Operating Procedures (SOPs) were carefully studied and transcribed for adoption in Kenya. Additional training in ocular pathology was conducted with shadowing opportunities at a larger vision science campus in Toronto (St. Michael's Hospital).
Technician Training. Upon the fellow's return to UoN, she trained pathology technicians on retinoblastoma-specific protocols. Together, they led a workshop to train additional technicians from other labs. A technician from The Hospital for Sick Children, Toronto, Ont., Canada, travelled to Nairobi to assist with the workshop, and DECF-K provided administration support. Participants trained with bovine eyes, which are roughly analogous to human eyes. A video was developed and shared on processing of eye specimens (https://youtu.be/I35_Y5YCimY). Copies of pathology textbooks, guidelines, and SOPs were provided to all participants.
Follow-up visits to all pathology labs represented at the training workshop were performed 3-6 months after training. Using a standard form, RbCoLab-based technicians performed a retrospective audit of eye pathology (e.g., adherence to SOPs, volume and quality of eyes processed). Most labs tried to implement the SOPs, but only 4 had received eyes to process (see online suppl. table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000451000).
Scale-Up of Training: Clinician Network. Kenyan pathologists participated in retinoblastoma trainings combined with annual KNRbS meetings. Pathologists (15 in total) were recruited from hospitals where retinoblastoma cases were likely to be diagnosed (i.e., main referral hospitals, provincial district hospitals, private or mission hospitals). KNRbS ophthalmologists were invited to attend, to facilitate collaboration, and cross talk on the various components of the pathology process, from preparation of the eye after surgery, to the format, content, and interpretation of the resultant report.
In early annual KNRbS meetings, the pathology training focus was on development of proformas for service request and reporting, and gaining consensus on SOPs for laboratory operations. Later, KNRbS meetings hosted sessions on pathology TNM guidelines [8] for retinoblastoma, and applied them to real cases. Training also included instruction on the use of the digital pathology software. National e-pathology rounds were conducted in person during the KNRbS meetings with the use of digital images of retinoblastoma pathology.
Pathology involvement from outside Kenya (beyond the fellowship training in Toronto) included remote consultation for complex cases at the discretion of the Kenyan team.
(B) Stuff: Equipment and Consumables
Forms and Consensus Building. Developing and vetting the standard request and reporting proformas at the KNRbS meetings had the dual purpose of training pathologists in the network and building consensus. Proformas were created with the input of both ophthalmologists (requestors) and pathologists (reporters) and guided by the RbCoLab lead pathologist, ensuring that the end result met current guidelines and recommendations [8].
Equipping of Laboratory. Grant funding enabled purchase of a new microtome (replacing the MoU-initiating, donated microtome) and standard laboratory consumables (e.g., formalin, alcohols, stains, mounting medium, surgical blades, microscope slides, and coverslips, etc.). A perceived challenge was that some items would not be available locally (e.g., specimen containers or embedding cassettes suitable for eyes), given that similar items had been received only from international donors previously. However, this idea was quickly disproven, as local operators were identified who could provide most of the needed items. The team prioritized local purchases over international where possible, in order to support local biomedical procurement channels.
A Specimen Collection Kit was designed (fig. 2) and 100 initial kits were assembled. Each kit included an instruction sheet, request form, a 100-ml specimen bottle, and a sealable biohazard bag with separate pocket for documentation. The instruction sheet described how to package, document, and transport the surgical eye specimen using the kit.
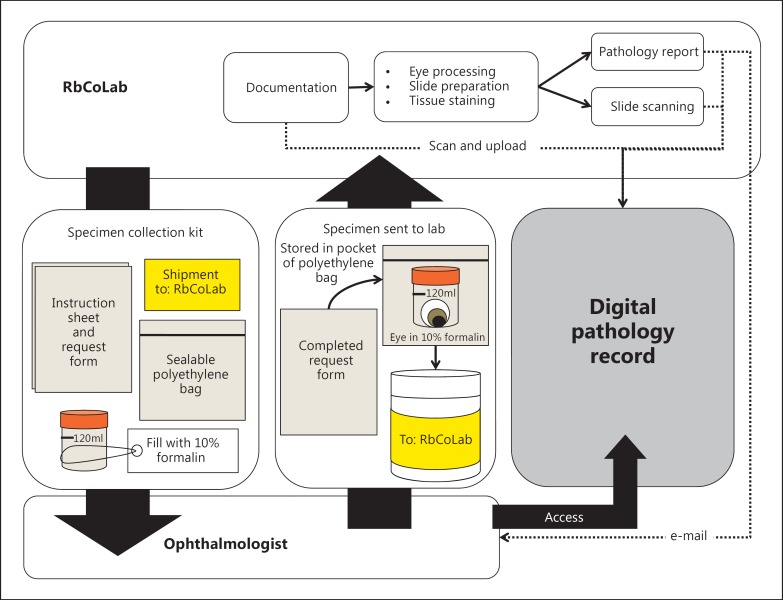
Fig. 2.
RbCoLab operations. Specimen collection kits were provided to all ophthalmologists who treat retinoblastoma patients. Kits were sent to the lab after surgery. The laboratory technicians documented the specimen in a logbook and scanned the pathology request form to be uploaded into the digital system. The technicians followed evidence-based SOPs to process the tissue and prepare stained slides. The pathologist read the slides and prepared a report. The report was uploaded onto the digital system. Select slides that complemented the report (i.e., clearly showed a reported feature) were marked by the pathologist and scanned by the technicians to be uploaded into the system. A copy of the report was also e-mailed to the ophthalmologists to alert them that the digital record was available for viewing. The ophthalmologists could access the digital record at any time using their unique login and password.
Since accurate cataloguing of slides and blocks rests on the ability to safely store them, rust and water resistant storage units were purchased. A digital pathology slide scanner (Ventana iScan Coreo; Roche) and imaging software (Aurora MScope 3.6.1) were donated to the project; while not part of the original program design, the donations were seen as an opportunity to (a) enhance communication and facilitate understanding of pathology results by clinicians, and (b) create a digital archive of specimens to facilitate future research. The company that provided the scanner also provided trouble shooting support and limited cost-free maintenance of the digital scanner during phase I. The imaging software (and subsequent images) was housed in a local computer server (2GHz CPU with 2GB RAM and Cent-OS 5.2 operating system).
(C) Space: Physical and Virtual
Annual KNRbS meetings provided the ‘space’ for planning and implementation of the project. The RbCoLab was physically housed in the Head and Neck Pathology Laboratory in the UoN School of Dental Sciences. The UoN provided additional infrastructure support through laboratory and office space; renovation of laboratory floors, lab-bench counters, and the new office space to enhance security of scanning equipment, and hosting of the CPU server in the Chiromo Information Technology campus.
(D) System: How It Works
Lab Operations. The RbCoLab was launched with an e-mail message to the existing database of KNRbS clinicians. A print follow-up announcement with Specimen Collection Kits was sent by courier to hospitals known to treat retinoblastoma patients.
As retinoblastoma specimens arrived in the lab, they were assigned a unique specimen number and catalogued into a digital spreadsheet that documented the information from the request form, and the date and time of arrival. The hard-copy request form was also labeled with the specimen number, and the date and time of arrival. The request form, report, and digital pathology images were scanned and uploaded into the digital system (fig. 2).
Evidence-based SOPs were followed for the tissue processing, sectioning, staining, and reporting (fig. 2). The pathologist made the diagnosis by microscope, and directed the technicians on which slides to scan at 20× (e.g., representative pupil-optic nerve section) and areas of interest to scan at 40× (e.g., high-risk features). End users received login/password details and training for the digital system to access the report and images; reports were also e-mailed to circumvent technical challenges (fig. 2).
A summer intern and volunteer pathology technician assisted with the additional workload (documentation, scanning, and uploading files). A temporary lab manager liaised between the technical team and the end users, and enforced protocols.
Research. Being incubated in an academic setting, the team relied on clinician-scientists and research-scientists to advance research goals. To expand local capacity in clinical retinoblastoma pathology, two UoN Master of Medicine (Pathology) students were mentored. Together with their supervisors (pathologists at the UoN), the students designed and performed a quality assurance audit and investigated correlation between the spectrum of retinoblastoma histological features and clinical outcome. With respect to the quality assurance project, it is known that laboratories face enormous challenges achieving quality control and assurance in low-resource settings [9]. This student project is an important first step in this direction, and a comprehensive quality control program will be implemented in phase II of the project.
Oversight. An executive committee was assembled by DECF-K to provide leadership and oversight. Members came from the KNRbS and had expertise in either pathology or ophthalmology. The committee met quarterly with the research and implementation team, advising on issues such as ethics, research, and relevant university policies.
Partnerships and Support. Multidisciplinary partnerships were essential to garnering funding and in-kind support for implementation and execution of the RbCoLab. Incubating the RbCoLab within the activities of the KNRbS leveraged support from DECF-K in the form of administration, access to networks, and linking training to the annual KNRbS meetings.
Funding support for the initial pathology fellowship was acquired via the now defunct ‘Healthy Kids International’ (HKI) fellowship program of The Hospital for Sick Children, which included follow-up funding for the execution of the project upon return to Kenya. The fellow worked with Toronto-based researcher and KNRbS member (H.D.) to develop the initial plan for the RbCoLab. However, after funding was approved and the fellow returned home, HKI ceased operations, and only 25% of the original promised funding was awarded. Funding from other avenues was sought to make up the difference, and the investigators (E.A.O.D. and H.D.) were later awarded a grant from Grand Challenges Canada to fully execute the project.
The support from Grand Challenges Canada enabled the team to leverage support from existing and new partners. In-kind support was instrumental in outfitting the laboratory (e.g., scanner, software). Funding from the TUYF Charitable Trust supported broader KNRbS efforts, and a portion was allocated to the pathology program (table 1). Funding from the University of Toronto Center for International Experience supported the student intern who assisted in the RbCoLab for 3 months.
Table 1.
Partnerships and support
| Staff |
Stuff |
Space |
||||||
|---|---|---|---|---|---|---|---|---|
| item | financial support | in-kind support | item | financial support | in-kind support | item | financial support | in-kind support |
| Pathologist | HKI (training) | HSC, SMH (staff time, training) | Microtome | GCC (operations) | TGH (equipment) | Laboratory and offices | − | UoN (renovation, outfitting) |
| Technicians | HKI, GCC (training) | HSC (staff time, training) | Supplies | HKI, GCC (operations) | − | Digital storage space | − | UoN (hosting, admin) |
| Pathology network | GCC (training) | DECF-K (admin) | Scanner | − | Roche/Biolmagene (equipment, training, maintenance) | KNRbS meeting | TUYF charitable trust (meeting costs) | DECF-K (admin) |
| Volunteers | UoT (travel support) | individual volunteers (time, effort) | Digital software | − | Aurora mScope (equipment, training) | |||
| Research students | GCC (operations, research support) | UoN, DECF-K (admin) | ||||||
| Implementation team | − | UoN, UoT, DECF-K (staff time) | ||||||
GCC = Grand Challenges Canada; HKI = Healthy Kids International; UoT = University of Toronto; UoN = University of Nairobi; DECF-K = Daisy's Eye Cancer Fund-Kenya; TGH = Toronto General Hospital; HSC = Hospital for Sick Children; SMH = St. Michael's Hospital.
Outcomes
RbCoLab Service Outcomes
During phase I of the project, the laboratory received specimens from 103 patients (110 eyes) from 7 nationwide retinoblastoma treatment centers (table 2a). Thus, the total lives improved by this project are considered to be 103 (table 3). Considering the average Kenyan household size of 4.4 persons [10], the total lives touched by the RbCoLab in phase I were estimated to be 453 (tables 2a, 3).
Table 2.
RbCoLab service outcomes
a.
Lives improved, touched1
| Hospital | City | Patients, n | Eyes, n |
|---|---|---|---|
| Coast provincial | Mombasa | 1 | 1 |
| Sabatia Eye Hospital | Batere | 2 | 2 |
| Garissa | Garissa | 2 | 2 |
| Homabay | Homa Bay | 2 | 2 |
| Meru | Meru | 2 | 2 |
| Kikuyu Eye Unit | Kikuyu | 28 | 31 |
| Kenyatta National Hospital | Nairobi | 66 | 70 |
| Total | 103 | 110 | |
| Lives saved and/or improved (= total patients) | 103 | ||
| Lives touched (= total patients × 4.4 persons, average Kenyan household size) | 453 | ||
b.
Report turn-around time
| Report turn-around time | Average | Range |
|---|---|---|
| Overall | 13 days | 4 – 31 days |
| Year 1 | 15 days | 7 – 31 days |
| Year 2 | 12 days | 4 – 25 days |
Data are summarized from confidential RbCoLab monthly specimen logs.
Metrics from the Grand Challenges Canada Results-Based Management and Accountability Framework.
Table 3.
Results-based management and accountability framework (RMAF)
| Description of outcome/output | Description of indicator | Project results achieved |
Data source | |
|---|---|---|---|---|
| n | indicator | |||
| Ultimate outcomes | ||||
| (A) Lives saved in LMICs | Number of lives saved | 103 | Number of children served by RbCoLab still alive at the end of the granting period | table 2 |
| (B) Lives improved in LMICs | Number of individuals with improved health outcomes | 103 | Number of children served by RbCoLab | table 2 |
| Intermediate outcomes | ||||
| (A) Lives touched – BENEFICIARIES who accessed a product and/or service | Number of BENEFICIARIES who accessed products and/or services | 453 | Number of individuals (patients, parents, siblings) served by RbCoLab (based on the average Kenyan household size of 4.4 persons) | table 2 |
| (B) Lives touched – INTERMEDIARIES who accessed a product and/or service and improved their knowledge/attitudes/behaviors | Number of INTERMEDIARIES who accessed products and/or services | 145 | Number in attendance at technician training, pathology training digital rounds, laboratory site visits | table 3 |
| (C) Jobs created | Number of jobs created in Canada as a result of the project |
0 |
n.a. |
n.a. |
| Number of jobs created in LMIC as a result of the project | 5 | Researchers (n = 2); technicians (n = 2); management roles (n = 1) | RbCoLab job descriptions, research proposals and DECF-K records | |
| (D) Changes in policy, legislation and/or regulation | Number of policies developed and/or adopted through the project | 1 | Clinical practice guidelines recommending use of RbCoLab dervice, n | KNRbS guidelines endorsed by the Ministry of Medical Services |
| Outputs | ||||
| (A) Building tools and capacity to execute | Number of innovative prototypes and/or service delivery models developed |
2 |
Development of business plan; RbCoLab operating model |
e-pathology East Africa business plan produced by Afribusiness Development; RbCoLab operations (fig. 2) |
| Funds leveraged | many In-kind or financial contributions from new or existing partners | table 1 | ||
LMICs = Low- and middle-income countries; n.a. = not available.
The average report turnaround time was 13 days (range 4-31; table 2b). This decreased from 15 (range 7-31) to 12 days (range 4-25) in year 1 versus year 2, respectively (table 2b).
Training Outcomes
The technician workshop, pathologist workshop, national e-pathology rounds, and 10 technical site visits trained 145 individuals (tables 3, 4).
Table 4.
RbCoLab training outcomes
| Events | Events, n | Intermediaries/event, n | Total intermediaries, n |
|---|---|---|---|
| Technician training workshop1 | 1 | 20 | 20 |
| Pathologist workshop2 | 1 | 28 | 28 |
| National e-pathology rounds3 | 1 | 40 | 40 |
| Laboratory site visits4 | 10 | 5 – 6 | 57 |
| Total | 145 | ||
Data from Daisy's Eye Cancer Fund – Kenya Attendance Records for Technician Training March 2012.
Data from Daisy's Eye Cancer Fund – Kenya Attendance Records Pathology Workshop September 2012.
Data from Daisy's Eye Cancer Fund – Kenya Attendance Records for Kenyan National Retinoblastoma Strategy Meeting #5, September 2013.
Data from RbCoLab Technicians’ Report on Laboratory Site Visits, October 2012.
The majority of technicians implemented eye pathology SOPs following initial training (see online suppl. table 1). However, many labs lacked or had inadequate equipment to appropriately implement the SOPs. Also, since most labs rarely received eyes, most technicians felt that they saw too few retinoblastoma cases at their respective institutions to justify conducting their own pathology. Overall, the training promoted sharing of knowledge and expertise, generated buy-in and fostered confidence in the RbCoLab. Only one major referral center continued processing their own retinoblastoma specimens, but remained an active part of the educational network by agreeing to send slides for scanning at the RbCoLab.
Despite training on the digital pathology system, few used it outside the RbCoLab staff, many citing technical difficulties. The software was used at national e-pathology rounds at a KNRbS meeting, but further efforts are needed to achieve full adoption and use.
Jobs Created and Policy Outputs
Five jobs were created: 2 research positions, 2 technician positions, and 1 management role (table 3). The consensus achieved at annual KNRbS meetings on pathology SOPs led to the development of pathology guidelines within the Ministry of Health endorsed ‘Kenyan Retinoblastoma Best Practice Guidelines’ [11].
Sustainability Outputs
The RbCoLab was designed using Grand Challenges Canada integrated innovation model [12], which combines science and technology, social, and business innovation. The science and technology component was the digital pathology and implementation of evidence-based best practices. The social innovation was the embedding of the RbCoLab within the multidisciplinary KNRbS structure, facilitating widespread adoption and use of the service. Finally, the business innovation was the business plan that was developed to guide the expansion of the pathology lab to other cancers. The business design aims to provide free or low-cost service for the poor, and at-cost where affordable, such that RbCoLab could potentially self-sustain its operations in future. This business model will be refined and tested in phase II of project implementation.
Discussion
We describe the first attempt to implement a digitized cancer pathology laboratory in Kenya. The idea for the RbCoLab first emerged from discussions and work within the KNRbS group, but its implementation relied on buy-in and support from laboratories outside the KNRbS network. Ownership and acceptance was built among nation-wide stakeholders by providing training workshops and site visits to build cancer pathology capacity, which also increased awareness, communication, and collaboration (i.e., pathology referrals to the RbCoLab). Our stakeholders support the lab and are supported by it rather than feeling competition. Their consultation in decision-making ensured their continued involvement in the RbCoLab [13]; these teams are now primed to support scale-up. Arguably, one of the most original contributions of the RbCoLab project was to create a culture shift in the retinoblastoma community that brought the importance of pathology for patient management to the forefront, and the consensus that application of evidence-based protocols was needed to improve care [3].
The path to scale encompasses four major areas: range of services offered, coverage, effect, and sustainability. In phase I, only retinoblastoma pathology services (range) were offered. Next, the implementation team will work closely with UoN academic units to determine the most logical cancer(s) to expand into and liaise with labs in the existing pathology network to expand cancer services in the chosen area of study.
In phase I, coverage of retinoblastoma reached 60% of the estimated new Kenyan patients per year [14]. In phase II, the group aims to expand coverage to reach 100% of retinoblastoma patients in Kenya. The ‘missing’ 40% are likely inclusive of children lost to follow-up (estimated at 20% [15]), eyes processed at the institution that declined to send specimens to the RbCoLab, and possibly commercial labs not captured in our network. The KNRbS network will be instrumental in reducing children lost to follow-up; enhanced outreach from the RbCoLab will generate new ways of collaborating with independent and commercial labs so all retinoblastoma patients get access to quality pathology.
The team will consider increasing retinoblastoma pathology coverage to other East African regions, including Uganda and Ethiopia, where national retinoblastoma strategy groups have sprouted. Such South-South partnerships have greater potential to develop regional pathology infrastructure in East Africa, rather than relying on analysis from Europe or North America, often the case for African pathology [16].
The effect of RbCoLab was to achieve an acceptable report turnaround time, improve report completeness, accuracy, and adherence to the evidence base, such that clinicians are better able to care for their patients. However, the lack of reliable data prior to this project limited accurate comparison to the standard of pathology previously; instead, the group relied on anecdotal evidence alone to assess improvements [3,17]. In phase II, strict monitoring and evaluation will continue, along with quality assurance activities as recommended from the pending study of phase I laboratory operations. Quality assurance analysis will determine the overall effect of the RbCoLab on patient care and identify how to decrease costs, increase productivity, and improve services [18].
A business plan to ensure sustainability was created, but requires evaluation to determine if it is indeed viable in the real-world setting. Linking scientific research and business is difficult, particularly when working from an academic setting, and on projects focused on patient populations, which may not represent a ‘viable market’ in the business sense [19]. While we developed a model for income generation as part of funding agency requirements, it is not yet clear if this is the most appropriate or feasible next step. A public-private partnership between the university and private sector could provide a different opportunity to commercialize research results and enhance sustainability of the service [20].
The scale-up process for health services can produce a different set of challenges than what has been observed thus far. Implementation on a large scale can increase the interventional complexity or the quality and quantity of nonfinancial resources required for an intervention. The new services offered might differ from the original intervention in their requirements of ‘staff, stuff, space, systems,’ making it more difficult to manage [21]. Simply replicating the same arrangements as those used in the pilot case may not reflect the dynamic context of the new setting [22]. These challenges will be met by catering to the local demand of cancer pathology services and mobilizing specialized task forces while maintaining our primary focus on retinoblastoma. Long-term partnership and strong local leadership [23], as was demonstrated in phase I, will be crucial in achieving sustainability in phase II.
Statement of Ethics
There were no animal or human subjects involved in this study. The evaluation was carried out in accordance with Article 2.5 of the Tri-Council Policy Statement for Ethical Conduct of Research.
Disclosure Statement
The authors confirm that they have no competing interests.
Acknowledgements
We acknowledge funding from Healthy Kids International (SickKids Foundation), Grand Challenges Canada, TUYF Charitable Trust, and the University of Toronto Center for International Experience. We acknowledge in-kind support from Roche (and former BioImagene) and Aurora mScope. We acknowledge the many contributions from staff at Daisy's Eye Cancer Fund, University of Nairobi, University of Toronto, and the Kenyan Ministry of Health. Finally, we acknowledge the intellectual contributions and support from the membership of the Kenyan National Retinoblastoma Strategy group.
References
- 1.Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–1131. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 2.Kimani K, Ouma B, Gallie BL, White A, Dimaras H, Livingstone M.Rati's Challenge: A Vision for Africa. Report from the First Kenyan National Retinoblastoma Strategy Meeting. 2008. https://www.sickkids.ca/pdfs/SickKids%20international/19474-DECF-Kenya%20Report%202008.pdf
- 3.Hill JA, Kimani K, White A, et al. Achieving optimal cancer outcomes in East Africa through multidisciplinary partnership: a case study of the Kenyan National Retinoblastoma Strategy group. Global Health. 2016;12:23. doi: 10.1186/s12992-016-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14:e152–e157. doi: 10.1016/S1470-2045(12)70598-3. [DOI] [PubMed] [Google Scholar]
- 5.Farmer P. Who Lives and Who Dies. London Review of Books. 2015;37:17–20. [Google Scholar]
- 6.Farmer P, Mukherjee J.Ebola: countries need ‘staff, stuff, and systems’. Partners In Health, September 24, 2014. http://www.pih.org/blog/for-ebola-countries-need-tools-to-treat-patients-in-their-communities
- 7.Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada and Social Sciences and Humanities Research Council of Canada . Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. Ottawa: Canadian Institutes of Health Research; 2014. http://www.pre.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/Default/ [Google Scholar]
- 8.Finger PT, 7th Edition AJCC-UICC Ophthalmic Oncology Task Force The 7th edition AJCC staging system for eye cancer: an international language for ophthalmic oncology. Arch Pathol Lab Med. 2009;133:1197–1198. doi: 10.5858/133.8.1197. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HL, Omondi MW, Musyoka AM, et al. Challenges of maintaining good clinical laboratory practices in low-resource settings: a health program evaluation framework case study from East Africa. Am J Clin Pathol. 2016;146:199–206. doi: 10.1093/ajcp/aqw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenya Open Data Constituency Household Size, Constituency Population and Density – 2009, 2009 Census Volume 1 b Table 1 Constituency Population by Gender and Density. 2014. https://www.opendata.go.ke/Population/Constituency-Household-Size/uxuz-svgx
- 11.Kenyan National Retinoblastoma Strategy Group Retinoblastoma Best Practice Guidelines. 2014. http://guidelines.health.go.ke/#/category/6,7/4/meta
- 12.Grand Challenges Canada Integrated Innovation: An Update and Early Lessons. 2012. http://www.grandchallenges.ca/wp-content/uploads/An-Update-on-Integrated-Innovation-2012Dec06-EN.pdf
- 13.Marjanovic S, Hanlin R, Diepeveen S, Chataway J. Research capacity building in Africa: networks, institutions and jocal ownership. J Int Dev. 2013;25:936–946. [Google Scholar]
- 14.Nyamori JM, Kimani K, Njuguna MW, Dimaras H. The incidence and distribution of retinoblastoma in Kenya. Br J Ophthalmol. 2012;96:141–143. doi: 10.1136/bjophthalmol-2011-300739. [DOI] [PubMed] [Google Scholar]
- 15.Nyamori JM, Kimani K, Njuguna MW, Dimaras H. Retinoblastoma referral pattern in Kenya. Middle East Afr J Ophthalmol. 2014;21:321–327. doi: 10.4103/0974-9233.142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruger M, Hendricks M, Davidson A, et al. Childhood cancer in Africa. Pediatr Blood Cancer. 2014;61:587–592. doi: 10.1002/pbc.24845. [DOI] [PubMed] [Google Scholar]
- 17.Dimaras H, Dimba EA, Waweru W, Githanga J, Kimani K. Digital cancer pathology in Africa. Lancet Oncol. 2013;14:e289–e290. doi: 10.1016/S1470-2045(13)70246-8. [DOI] [PubMed] [Google Scholar]
- 18.Mosadeghrad AM. Healthcare service quality: towards a broad definition. Int J Health Care Qual Assur. 2013;26:203–219. doi: 10.1108/09526861311311409. [DOI] [PubMed] [Google Scholar]
- 19.Logie C, Dimaras H, Fortin A, Ramon-Garcia S. Challenges faced by multidisciplinary new investigators on addressing grand challenges in global health. Global Health. 2014;10:27. doi: 10.1186/1744-8603-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Easterbrook PJ. Institutional partnerships in global health. Clin Med. 2011;11:112–113. doi: 10.7861/clinmedicine.11-2-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangham LJ, Hanson K. Scaling up in international health: what are the key issues? Health Policy Plan. 2010;25:85–96. doi: 10.1093/heapol/czp066. [DOI] [PubMed] [Google Scholar]
- 22.Paina L, Peters DH. Understanding pathways for scaling up health services through the lens of complex adaptive systems. Health Policy Plan. 2012;27:365–373. doi: 10.1093/heapol/czr054. [DOI] [PubMed] [Google Scholar]
- 23.Curry L, Taylor L, Chen PG, Bradley E. Experiences of leadership in health care in sub-Saharan Africa. Hum Resour Health. 2012;10:33. doi: 10.1186/1478-4491-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]