Abstract
The relationship between selective serotonin-reuptake inhibitors (SSRIs) use during first trimester and cardiovascular-related malformations of infants is still uncertain. Therefore, we conducted this systematic review and meta-analysis to assess the aforementioned association. A systematic literature review identified studies for cohort studies about SSRIs use and cardiovascular-related malformations in PubMed and Web of Science. We summarized relative risk (RRs) and 95% confidence intervals (CIs) of cardiovascular-related malformations using random-effects model, and heterogeneity and publication-bias analyses were conducted. Eighteen studies met the inclusion criteria. Pregnant women who were exposed to SSRIs at any point during the first trimester had a statistically significant increased risk of infant cardiovascular-related malformations (RR = 1.26, 95%CI = 1.13–1.39), with moderate heterogeneity (I2 = 53.6). The corresponding RR of atrial septal defects (ASD), ventricular septal defects (VSD), ASD and/or VSD was 2.06 (95%CI = 1.40–3.03, I2 = 57.8), 1.15 (95%CI = 0.97–1.36; I2 = 30.3), and 1.27 (95%CI = 1.14–1.42; I2 = 40.0), respectively. No evidence of publication bias and significant heterogeneity between subgroups was detected by meta-regression analyses. In conclusion, SSRIs use of pregnant women during first trimester is associated with an increased risk of cardiovascular-related malformations of infants including septal defects. The safety of SSRIs use during first trimester should be discussed to pregnant women with depression.
Depression in pregnancy is common1. Approximately 10% of pregnant women experience depression2 and up to 15% of pregnant women experience depression symptoms3,4. Since untreated depression may have potential risk to offspring, such as restricted fetal growth5,6, low birth weight7, and lower child body mass index (BMI)8, it is essential to manage antenatal depression9. After the introduction of SSRIs into the market, SSRIs have become the most commonly prescribed pharmacological treatment for depression during pregnancy10,11, which was considered relatively safe to take during pregnancy prior to 200512. The study carried out by Einarson et al. as well as a meta-analysis and two database failed to find SSRIs was associated with a risk of major malformations12,13,14,15. However, the studies16,17 published after 2005 reported that SSRIs might increase the incidence of cardiovascular malformations in infants. Since approximately 75% of pregnant women who experienced depression were treated with SSRIs in the first trimester of pregnancy18, the safety of SSRIs use has attracted more attention and has become a clinical issue as a result.
The association between SSRIs use during pregnancy and birth defects risk has still been controversial. There have been many studies conducted to determine the association between SSRIs use and cardiovascular malformations. Some studies reported maternal SSRIs use was related to an increased risk of congenital cardiac malformations19,20,21,22, while other studies suggested there was no association12,23,24. Three meta-analyses25,26,27 demonstrated that SSRIs use during pregnancy did not increase the risk of major or minor cardiovascular malformations. By contrast, other two meta-analyses28,29 reported that SSRIs were associated with an increased risk of cardiovascular malformations. However, several limitations were observed in these five meta-analyses: (1) case-control studies were included which might generate more bias (selection and information bias), (2) the exposure period of SSRIs of pregnant women was not consistent. For example, the meta-analysis carried out by Grigoriadis et al.29 included studies with different exposure periods of SSRIs (e.g., first, second, third, and term trimester), (3) the inclusion and exclusion criteria were not unify. For example, the meta-analysis conducted by Wang et al.27 only included four studies which were relatively small part of these studies should be included and (4) whether findings were robust in subgroup analyses stratified by adjustment for potential confounders were limited.
Additionally, cohort studies30,31,32,33,34,35,36 with larger number of populations have been conducted to explore the aforementioned association in Europe and North America recently. For example, in 2015, a prospective cohort study conducted by Berard et al.31 reported that there was no association between maternal use of SSRIs during pregnancy and cardiac malformations whose risk ratio of cardiac malformations was 1.10 (95%CI = 0.82–1.48). However, Furu et al.30 reported that, in a prospective cohort study which contained 2,303,647 singleton live births in Nordic countries including Denmark, Finland, Iceland, Norway, and Sweden, cardiac defects was associated with SSRIs use during pregnancy whose odds ratio of any cardiac defect was 1.15 (95%CI = 1.05–1.26). In order to better understand the relationship between SSRIs use in pregnant women during the first trimester and cardiovascular-related malformations of infants, we performed a systematic review and meta-analysis using data from cohort studies.
Results
Literature search
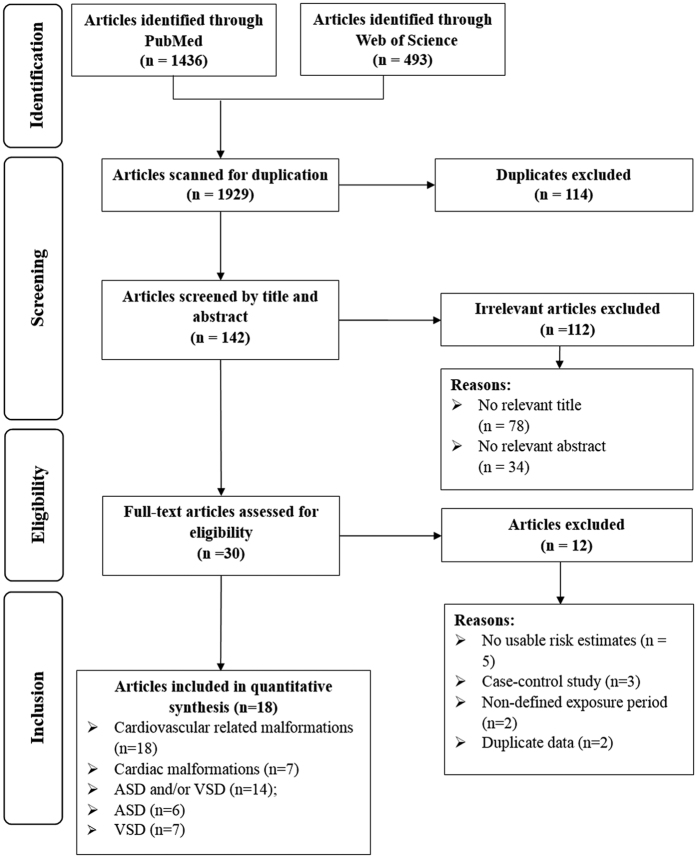
We identified 1436 studies from PubMed and 493 studies from Web of science via the search strategy. Of all studies, 1929 studies were excluded after we screened the titles and abstracts for the general criteria. Only 18 studies were eligible for inclusion into the final meta-analysis19,20,21,22,30,31,32,33,34,35,36,37,38,39,40,41,42,43 after the full text was reviewed for the 30 potential studies (Fig. 1).
Figure 1. Flow-chart of study selection.
Study characteristics
Characteristics of these 18 studies are shown in Supplementary Table S1. These studies were published between 1998 and 2015. Of all these included studies, eleven studies were conducted in Europe19,30,32,34,35,36,37,38,39,40,42, five studies were conducted in Northern America22,31,33,41,43, and one study each were conducted in Australia21 and Israel20. Sample sizes of these studies ranged from 534 to 2,303,647, and the number of cardiovascular-related malformations varied from 6 to 27,309.
Quality of included studies
Supplementary Table S2 presents the study-specific quality according to Newcastle-Ottawa quality scale44. In the ‘control for important factor or additional factor’ category, nine studies20,21,22,31,33,34,38,41,43 were not assigned two scores because they adjusted, less than two, important confounders in their primary analyses. In the ‘follow-up long enough for outcomes to occur’ and ‘adequacy of follow-up of cohorts’ categories, six equivalent studies22,32,37,38,42,43 were not assigned a score because they did not refer to follow-up in their studies. The maximum score is nine and the minimum score is five.
Cardiovascular-related malformations
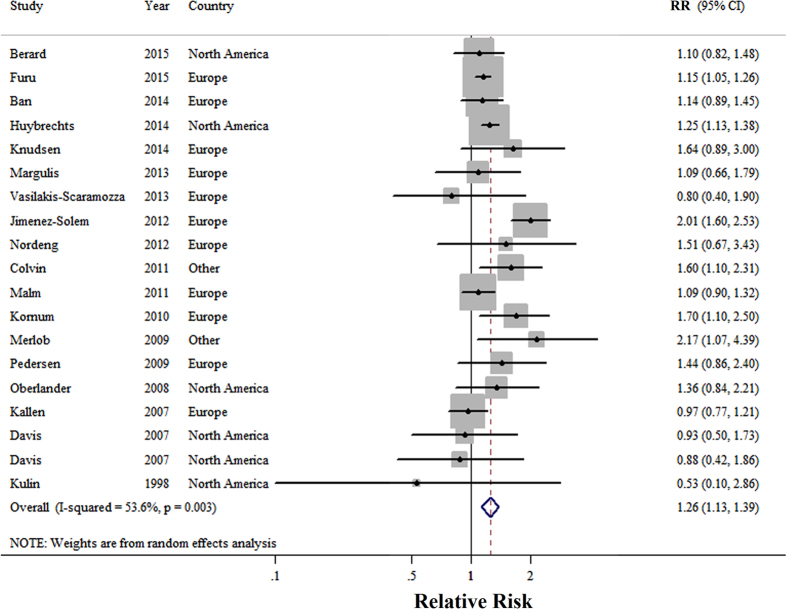
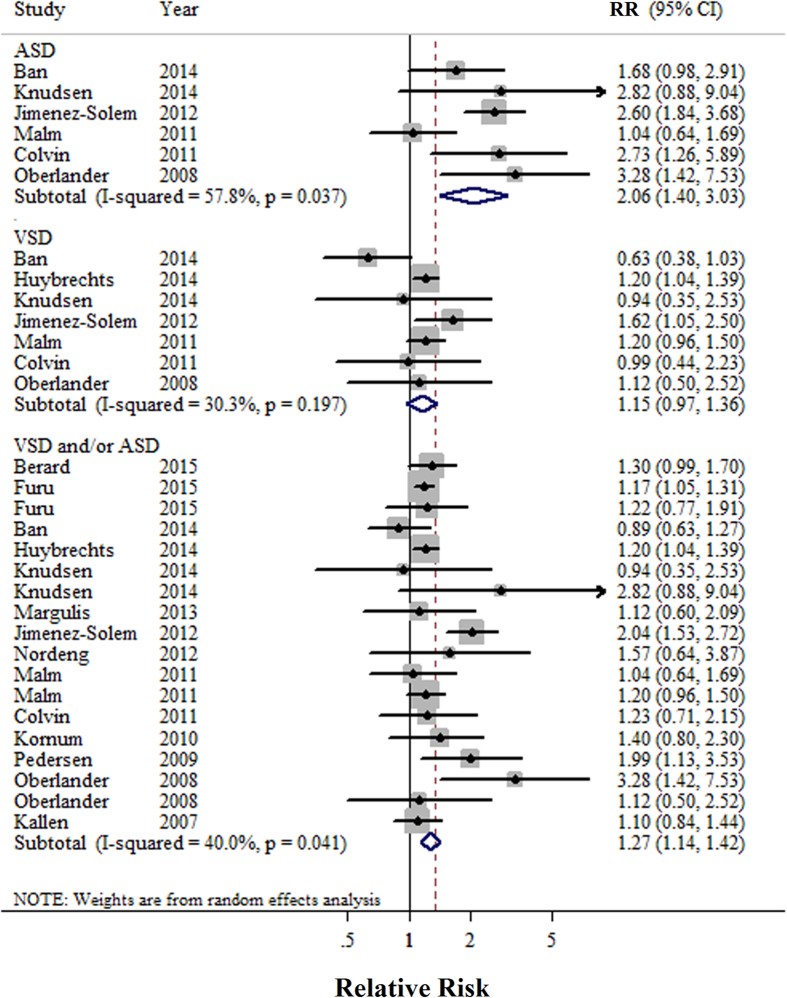
A total of 18 studies19,20,21,22,30,31,32,33,34,35,36,37,38,39,40,41,42,43 reported the association between SSRIs use of pregnant women during first trimester and cardiovascular-related malformations of infants. Pregnant women who were exposed to SSRIs at any point during the first trimester had a statistically significant increased risk of infant cardiovascular-related malformations (RR = 1.26, 95%CI = 1.13–1.39), with moderate heterogeneity (P = 0.003, I2 = 53.6) (Fig. 2). There was no indication of a publication bias according to Begg’s test (P-bias = 0.889) or Egger’s test (P-bias = 0.601), and there was no asymmetry in funnel plots when inspected visually. Additionally, analyses of the seven studies30,31,33,36,40,42,43 that reported cardiac malformations per our criteria showed that there was a statistically significant increased risk of cardiac malformations in infants born to mothers who used SSRIs in the trimester (RR = 1.17, 95%CI = 1.06–1.28; P = 0.19, I2 = 31.0). Furthermore, analyses of studies reported the cardiac malformations of interest were ASD21,22,30,32,34,37,39, VSD21,22,32,33,34,37,39 and ASD and/ or VSD19,21,22,30,31,32,33,34,36,37,38,39,40,42 with corresponding RR scores of 2.06 (95%CI = 1.40–3.03), 1.15 (95%CI = 0.97–1.36), and1.27 (95%CI = 1.14–1.42) respectively (Fig. 3).
Figure 2. Forest plots of the relationship between SSRIs use and risk of cardiovascular related malformations.
Squares indicate study-specific risk estimates (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; diamond indicates the summary relative risk with its 95% CI. RR: relative risk.
Figure 3. Forest plots of the relationship between SSRIs use and risk of septal defects.
Squares indicate study-specific risk estimates (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; diamond indicates the summary relative risk with its 95% CI. ASD: atrial septal defect; RR: relative risk; VSD: ventricular septal defect.
Subgroup and sensitivity analysis
We performed subgroup analyses in terms of geographic location and potential for confounded adjustment (Table 1). When stratified by geographic location, all strata showed significant results. Additionally, although the directions of the results of subgroup analyses stratified by potential confounders were consistent with the main findings, not all of them showed statistically significance.
Table 1. Summary risk estimates of the association between selective serotonin reuptake inhibitor use and cardiovascular-related malformations.
| No. of study | Summary RR (95%CI) | I2 (%) | P* | P** | |
|---|---|---|---|---|---|
| Overall | 18 | 1.26 (1.13–1.39) | 53.6 | 0.003 | |
| Cardiac malformation | 7 | 1.17 (1.06–1.28) | 31.0 | 0.19 | |
| ASD | 6 | 2.06 (1.40–3.03) | 57.8 | 0.04 | |
| VSD | 7 | 1.15 (0.97–1.36) | 30.3 | 0.20 | |
| ASD and/or VSD | 14 | 1.27 (1.14–1.42) | 40.0 | 0.04 | |
| Geographic Location | 0.52 | ||||
| Northern America | 5 | 1.22 (1.12–1.34) | 0.0 | 0.51 | |
| Europe | 11 | 1.27 (1.08–1.48) | 67.3 | 0.001 | |
| Others | 2 | 1.71 (1.23–2.37) | 0.0 | 0.45 | |
| Adjustment for confounders | |||||
| Maternal age | 0.39 | ||||
| Yes | 13 | 1.23 (1.07–1.41) | 62.7 | 0.001 | |
| No | 5 | 1.34 (1.13–1.59) | 18.0 | 0.30 | |
| Socioeconomic status | 0.23 | ||||
| Yes | 4 | 1.39 (0.97–2.00) | 75.1 | 0.007 | |
| No | 14 | 1.19 (1.09–1.30) | 29.5 | 0.14 | |
| Smoking or alcohol drinking | 0.72 | ||||
| Yes | 9 | 1.24 (1.05–1.47) | 72.7 | <0.001 | |
| No | 9 | 1.27 (1.14–1.41) | 6.0 | 0.39 | |
| Pregnancy BMI | 0.29 | ||||
| Yes | 3 | 1.10 (0.89–1.36) | 0.0 | 0.70 | |
| No | 15 | 1.29 (1.15–1.46) | 62.2 | 0.001 | |
| Pregnancy complications | 0.06 | ||||
| Yes | 6 | 1.13 (1.05–1.22) | 0.0 | 0.96 | |
| No | 12 | 1.40 (1.17–1.67) | 63.1 | 0.002 | |
| Parity | 0.73 | ||||
| Yes | 6 | 1.31 (1.05–1.63) | 82.0 | <0.001 | |
| No | 12 | 1.24 (1.14–1.34) | 0.0 | 0.47 | |
*P for heterogeneity within each subgroup.
**P for heterogeneity between subgroups with meta-regression analysis.
Abbreviations: ASD, atrial septal defect; BMI, body mass index; CI, confidence interval; RR, relative risk; VSD, ventricular septal defect.
In a sensitivity analysis, we evaluated the effect of removing a single study from the total, for each of the 18 studies, in order determine its effect on the summarized estimate for heterogeneity and to assess whether one study had a significant influence on the meta-analytic RR. The 18 study-specific RRs of cardiovascular-related malformation ranged from a low of 1.19 (95%CI = 1.10–1.28; P = 0.25, I2 = 17.3%) after omission of the study by Jimenez-Solem et al.39 to a high of 1.29 (95%CI = 1.16–1.44; P = 0.005, I2 = 53.7) after omission of the study by Kallen et al.42.
Discussion
In this meta-analysis of 18 cohort studies, we found that SSRIs use during the first trimester increase the risk for cardiovascular and septal defects malformations by 26% and 27%, respectively. The same positive findings were also observed in most of the subgroup analyses. Considering the high prescription rate of SSRIs in pregnant women with depression, the safety of SSRI should be discussed with women in the first trimester.
The biological mechanisms of SSRIs use and cardiovascular-related malformations are still unclear thus far. SSRIs could cross the placenta and may, therefore, increase the incidence of fetal heart defect and alter placental and fetal heart serotonin signaling45. Experimental study indicates that myocardial staining in mice embryos exposed to serotonin restricts the development endocardial cushion forming regions and was became almost completely blocked with uptake inhibitors. This process appeared to be mediated by serotonin transporters46. The blockage of serotonin uptake by paroxetine could decrease the number of 5-bromo-deoxyuridine immunoreactive and MF20-im cells, and this data indicates that serotonin and serotonin transporters has a significant role in heart development47. Another study, conducted by Buskohl et al.48, reported elevated serotonin in avian induced atrioventricular valvuloseptal defect in vivo. Severe heart defects may be induced via a transforming growth factor-beta/serotonin signaling pathway. Since there is a relationship between signaling networks and cell/tissue level, and little information about other signal pathways, more research into the biological mechanism between SSRIs and cardiovascular-related defects should be pursued. Besides, a study conducted by Lage et al.49 showed that genetic and environmental risk factors modulate critical biological systems during heart development, especially influencing protein networks driving the development of specific anatomical structures. Therefore, further research should pay more attention to the influence of environmental and epigenetic factors between SSRIs and cardiovascular-related defects.
Although all results of subgroup analyses stratified by geographic locations showed significance, the point estimates were slightly different. This might be attributed to the fact that different populations may have different exposure rates of SSRIs. Berard et al.31 reported the exposure rate of SSRIs in pregnant women was 12.59% based on 18,493 participants between 1998 and 2010 from Quebec Pregnancy Cohort in Canada; whereas Margulis et al.36 reported the exposure rate was 2.40% on the basis of 149,464 participants between 1996 and 2010 from the Clinical Practice Research Datalink’s Mother Baby Link in UK. By comparison, the aforementioned rate was 3% and 0.35% for pregnant women from Australia21 and Israel20, respectively. Additionally, several studies reported SSRIs on the basis of several specific antidepressants, which might result in the different results of geographic locations. For example, Furu et al.30 noted that in the studies conducted by Huybrechs et al.33, seven kinds of SSRIs were contained in their study including fluoxetine, citalopram, paroxetine, sertraline, fluvoxamine, escitalopram and venlafaxine, whereas there were only three kinds of SSRIs including paroxetine, sertraline and fluoxetine.
The strengths of this meta-analysis include: the large sample size of 7,280,932 participants, and excluding cases that may bias the results. This sample size was chosen to provide sufficient statistical power to detect the association between SSRIs use and cardiovascular-related malformations In addition, because we only included cohort studies in the present study, recall and selection bias is not likely to affect the results. Moreover, compared with previous meta-analyses, numerous subgroup and sensitivity analyses were carried out to explore heterogeneity of the data.
Despite the clear strengths of this study, some limitations of our study should be acknowledged. First of all, in almost all studies, the data only consisted of live births and therefore lacked information about pregnancies that did not end with a live birth, such termination of pregnancy, stillbirth, or miscarriages. If pregnant women exposed to SSRIs had a higher incidence rate of abortions as a result of the severe heart malformations and defects, it could mask the teratogenic effect of SSRIs and introduce an unintentional selection and detection bias. Pregnant women exposed to SSRIs were reported to have an increased rate of taking ultrasound examinations compared with the women not exposed to SSRIs50. More frequent ultrasound examinations could also increase the risk of congenital heart defects detection and the detection of malformations could also lead to pregnancy termination.
Secondly, several studies failed to control for potential confounders, which might introduce bias in an unpredictable direction. In fact, there are some known or suspected risk factors for cardiac defects such as age of delivery, state of residence, age, race, and parity, etc33. However, these potential confounders were not consistent in each study. Some studies did not adjust for any confounders while others adjusted for non-consistent confounders. For example, Colvin et al.21 did not note the adjustment for any potential confounders in their results while Berard et al.31 and Ban et al.32 adjusted for six and nine kinds of potential confounders, respectively. In addition, the study30 conducted by Furu et al. use the sibling analysis adjusting more potential confounders (e.g. family related factors), but these attenuated results were generated from only 2,288 participants which might be attributed to limited statistical power when comparing to primary cohort (n = 2,303,647). Besides, some specific confounding factors such as maternal BMI or obesity, which seems to itself increase the risk of congenital heart defects including the septal ones51,52,53. Any further studies should fully adjust these potential confounders or report analyses stratified by these risk factors to better be able to rule out residual confounding.
Thirdly, because the majority of included studies (15/18)19,20,21,22,30,31,32,33,34,35,36,38,40,42,43 were based on register data, we could not get information on diagnostic tests for all cardiovascular-related malformations. Although echocardiography has been the most useful diagnostic test to confirm the presence of congenital heart defects54 in utero, it greatly dependent on the clinical skills and knowledge of operators. Therefore, the comparison of detection rates varies according to an operators’ ability. Additionally, although most articles referenced the International Classification of Diseases (ICD), different revisions were used, for example Ninth Revision (ICD-9) or Tenth Revision (ICD-10), to identify the malformations. We failed to get a uniform consensus in cardiovascular-related malformations containing all of the conditions for birth defects of the circulatory system. For example, 4 studies21,22,35,38 used “cardiovascular anomalies” to describe any circulatory defect, whereas 7 studies30,31,33,36,40,42,43 used “cardiac defect” or “cardiac malformation.” Further studies should establish consistent definitions of cardiovascular-related diseases by explicitly defining every kind of disease included in overall outcomes to reduce bias among different studies.
Finally, the estimates based on blank control group were included in our meta-analysis when the studies presented different measures of association. For example, the study conducted by Huybrechts et al.33 presented three kinds of estimates with increasing levels of confounding adjustments, but the adjusted estimates were based on pregnant women with depression. Therefore, we chose crude estimates instead of adjusted estimates.
Conclusion
Our meta-analysis suggests that SSRIs use in pregnant women during first trimester is associated with an increased risk of cardiovascular-related malformations of infants including septal defects. Additional studies are needed to provide more detailed results, including research into every possible SSRI that is used by pregnant with results stratified by the different kinds of cardiovascular-related congenital defect after better adjustment for any potential confounders.
Methods
Literature search
We followed the guideline of the Preferred Reporting Items for Systematic reviews and Meta-Analyses55 to perform and report this meta-analysis. We conducted computerized literature searches of the databases including PubMed and Web of Science and reviewed the data from the database index date through December 31, 2015. The following search key words and Medical Subject Heading (MeSH) terms were used: (serotonin reuptake inhibitors OR SSRI OR fluoxetine OR paroxetine OR citalopram OR escitalopram OR sertraline OR fluvoxamine) AND (malformations OR birth outcome OR obstetrical outcome OR congenital abnormalities). Additionally, the references cited in the retrieved articles were scrutinized by manual search.
Study selection
The studies that were included were considered if they met the following criteria: (1) used cohort study design, (2) defined the exposure period of SSRI as the first trimester of pregnancy, (3) defined the non-exposure group as normal pregnant women who did not use any antidepressant drug during first trimester, (4) reported any cardiovascular-related malformations at birth, (5) reported any usable risk estimates (e.g., odds ratio, risk ratio or relative risk with 95% CIs or necessary data for calculation) of the association between SSRI use and cardiovascular malformations.
The studies were excluded if they: (1) were review articles, systemic review and meta-analysis, commentaries, editorials or meeting abstracts, (2) used non-cohort study designs (e.g., case-control study, descriptive study, randomized controlled trial, etc.), (3) did not define the exposure period of SSRI as the first trimester, (4) included pregnant women who were exposed to two kinds of anti-depressants or more at the same time, (5) used pregnant women who took any antidepressant drug as reference group, (6) were not human studies or published in English.
When duplicate articles from the same study were identified, we included the most recent report that contained the largest number of the cohorts or cases that matched our interest. The selection and exclusion were carried out by 2 independent researchers (T-NZ and Z-QS). Disagreements were discussed and agreed-upon prior to selection.
Data extraction
Data was independently extracted according to a standardized format by 2 researchers (T-NZ and Z-QS) for each eligible study. Disagreements were resolved by a third researcher (Q-JW) through discussion. From each study, we extracted the information as follows: first author, year of publication, geographic location, sample size (cases and cohort size), study period, outcome with their risk estimates and 95%CIs. Since there were only a limited number of studies with the specific outcomes of interest (e.g., atrioventricular septal defects, transposition of great arteries, situs anomalies and looping defect, etc), we summarized and presented the outcomes that were generally cardiovascular anomalies, cardiac malformations, and septal defects (ASD and/or VSD). We also extracted the adjusted confounders information of each study. If there were multiple estimates of the association, we extracted the estimate that was adjusted for the largest number of potential confounders. If there was no adjusted estimate in the study, we used a crude estimate.
Quality evaluation
Two independent researchers (T-NZ and S-YG) conducted the quality assessment of these included studies according to the Newcastle-Ottawa Scale (NOS) for cohort studies56,57,58,59. All 8 items in the scale were applicable to our study question. The items can be divided into 3 domains (i.e., selection, comparability, and exposure/outcome). We used these NOS parameters to evaluate the studies instead of scoring them and categorizing them into high- or low quality on the basis of the scores.
Statistical analysis
The studies19,20,21,22,30,31,32,33,34,35,36,37,38,39,40,41,42,43, reported the outcomes of specific heart anomalies e.g. any cardiac defect, cardiac malformations, congenital heart defects, cardiovascular anomalies, all major cardiovascular anomalies, bulbus cordis anomalies and anomalies of cardiac septal closure, and other congenital anomolies of heart. We extracted this data in order to calculate the summarized overall RR. The studies19,21,22,30,31,32,33,34,36,37,38,39,40,42, reported the outcomes of ASD (including ostium secundum type atrial septal defect), VSD, septal defect, atrioventricular septal defect, and ASD and/or VSD. We extracted this data in order to calculate the summarized RR of ASD and/or VSD events. For the study31, that separately reported the risk estimates of SSRIs but did not combine them, we used the effective count method proposed by Hamling et al.60 to recalculate the total risk estimate61,62,63,64. We reported all results in terms of the RR for simplicity since absolute risk of cardiovascular malformations are low. If there was no estimate specified in a study, we calculated it by using the original data from the study22,43. We calculated summarized RRs and 95%CIs by using the random effects model described by DerSimonian and Laird65. The I2 statistic was used to evaluate the heterogeneity of RRs across studies and we considered the values 50% or less, 51–75% and 76% or more as low-, moderate-, and high-heterogeneity, respectively66,67,68. Subgroup analysis was carried out on the basis of the geographic location (Europe, Northern America, and other regions). Additionally, we also stratified the meta-analysis by potential confounders including age, socioeconomic status, pregnancy body mass index, pregnancy complications and parity. Heterogeneity between subgroups was evaluated by meta-regression analysis. We also performed sensitivity analyses by excluding one study at a time to explore whether results were strongly influenced by a specific study. Finally, publication bias was evaluated through Egger’s linear regression69, Begg’s rank-correlation methods70, and funnel plots. We assumed that there was a significant statistical publication bias if P is less than 0.05 for Egger’s or Begg’s test. All statistical analyses were performed with Stata 12.1 (StataCorp).
Additional Information
How to cite this article: Zhang, T.-N. et al. Use of selective serotonin-reuptake inhibitors in the first trimester and risk of cardiovascular-related malformations: a meta-analysis of cohort studies. Sci. Rep. 7, 43085; doi: 10.1038/srep43085 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by grants from the China National Health and Family Planning Commission (No. 201402006 to C-XL), the funding of the Obstetric Diseases Translational Medicine Research Center Project of Liaoning Province (No. 2014225007 to C-XL), the Natural Science Foundation of China (No. 81602918 for Qi-Jun Wu and No. 81402130 for Da Li) and the Doctoral Start-up Foundation of Liaoning Province (No. 201501007 for Qi-Jun Wu and No. 20141045 for Da Li), and the Fok Ying Tung Education Foundation (No. 151039 for Da Li). Qi-Jun Wu was supported by the Fogarty International Clinical Research Scholars and Fellows Support Center at the Vanderbilt Institute for Global Health, funded by the Fogarty International Center, NIH, through an R24 Training Grant (D43 TW008313 to Xiao-Ou Shu).
Footnotes
The authors declare no competing financial interests.
Author Contributions T.-N.Z., Z.-Q.S., and Q.-J.W. designed research; T.-N.Z., Z.-Q.S., and Q.-J.W. conducted research; T.-N.Z., S.-Y.G., Z.-Q.S., and Q.-J.W. analyzed data; T.-N.Z., D.L., C.-X.L., H.-C.L., Y.Z., T.-T.G., X.X., C.J., and Q.-J.W. wrote the draft; All authors read, reviewed and approved the final manuscript. Q.-J.W. had primary responsibility for final content.
References
- O’Keane V. & Marsh M. S. Depression during pregnancy. BMJ 334, 1003–5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellfolk M. & Malm H. Risks associated with in utero and lactation exposure to selective serotonin reuptake inhibitors (SSRIs). Reprod Toxicol 30, 249–60 (2010). [DOI] [PubMed] [Google Scholar]
- Bennett H. A., Einarson A., Taddio A., Koren G. & Einarson T. R. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol 103, 698–709 (2004). [DOI] [PubMed] [Google Scholar]
- Chatillon O. & Even C. Antepartum depression: prevalence, diagnosis and treatment. Encephale. 36, 443–51 (2010). [DOI] [PubMed] [Google Scholar]
- Field T. et al. Chronic prenatal depression and neonatal outcome. Int J Neurosci 118, 95–103 (2008). [DOI] [PubMed] [Google Scholar]
- Field T., Diego M. & Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant Behav Dev 29, 445–55 (2006). [DOI] [PubMed] [Google Scholar]
- Steer R. A., Scholl T. O., Hediger M. L. & Fischer R. L. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol 45, 1093–9 (1992). [DOI] [PubMed] [Google Scholar]
- Ertel K. A., Koenen K. C., Rich-Edwards J. W. & Gillman M. W. Antenatal and postpartum depressive symptoms are differentially associated with early childhood weight and adiposity. Paediatr Perinat Epidemiol 24, 179–89 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D. B., Yadon C. A. & Tregellas H. C. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Womens Ment Health 15, 1–14 (2012). [DOI] [PubMed] [Google Scholar]
- Ververs T. et al. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol 62, 863–70 (2006). [DOI] [PubMed] [Google Scholar]
- Andrade S. E., et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol 198, 191–4 (2008). [DOI] [PubMed] [Google Scholar]
- Einarson A. et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry 165, 749–52 (2008). [DOI] [PubMed] [Google Scholar]
- Einarson T. R. & Einarson A. Newer antidepressants in pregnancy and rates of major malformations: a meta-analysis of prospective comparative studies. Pharmacoepidemiol Drug Saf 14, 823–7 (2005). [DOI] [PubMed] [Google Scholar]
- Malm H., Klaukka T. & Neuvonen P. J. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol 106, 1289–96 (2005). [DOI] [PubMed] [Google Scholar]
- Wen S. W. et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol 194, 961–6 (2006). [DOI] [PubMed] [Google Scholar]
- Berard A. et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol 80, 18–27 (2007). [DOI] [PubMed] [Google Scholar]
- Cole J. A., Ephross S. A., Cosmatos I. S. & Walker A. M. Paroxetine in the first trimester and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf 16, 1075–85 (2007). [DOI] [PubMed] [Google Scholar]
- Cooper W. O., Willy M. E., Pont S. J. & Ray W. A. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol 196, 541–4 (2007). [DOI] [PubMed] [Google Scholar]
- Pedersen L. H., Henriksen T. B., Vestergaard M., Olsen J. & Bech B. H. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 339, b3569 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlob P. et al. Are selective serotonin reuptake inhibitors cardiac teratogens? Echocardiographic screening of newborns with persistent heart murmur. Birth Defects Res A Clin Mol Teratol 85, 837–41 (2009). [DOI] [PubMed] [Google Scholar]
- Colvin L., Slack-Smith L., Stanley F. J. & Bower C. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Res A Clin Mol Teratol 91, 142–52 (2011). [DOI] [PubMed] [Google Scholar]
- Oberlander T. F. et al. Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population-based health data. Birth Defects Res B Dev Reprod Toxicol 83, 68–76 (2008). [DOI] [PubMed] [Google Scholar]
- Louik C., Lin A. E., Werler M. M., Hernandez-Diaz S. & Mitchell A. A. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med 356, 2675–83 (2007). [DOI] [PubMed] [Google Scholar]
- Davis R. L. et al. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf 16, 1086–94 (2007). [DOI] [PubMed] [Google Scholar]
- Rahimi R., Nikfar S. & Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol 22, 571–5 (2006). [DOI] [PubMed] [Google Scholar]
- Nikfar S., Rahimi R., Hendoiee N. & Abdollahi M. Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: A systematic review and updated meta-analysis. DARU 20, 75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. et al. Selective Serotonin Reuptake Inhibitors (SSRIs) and the Risk of Congenital Heart Defects: A Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc 4, e001681 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reefhuis J., Devine O., Friedman J. M., Louik C. & Honein M. A. Specific SSRIs and birth defects: Bayesian analysis to interpret new data in the context of previous reports. BMJ 351, h3190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S. et al. Antidepressant exposure during pregnancy and congenital malformations: is there an association? A systematic review and meta-analysis of the best evidence. J Clin Psychiatry 74, e293–308 (2013). [DOI] [PubMed] [Google Scholar]
- Furu K. et al. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 350, h2235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard A., Zhao J. P. & Sheehy O. Sertraline use during pregnancy and the risk of major malformations. Am J Obstet Gynecol 212, 791–5 (2015). [DOI] [PubMed] [Google Scholar]
- Ban L. et al. Maternal depression, antidepressant prescriptions, and congenital anomaly risk in offspring: a population-based cohort study. BJOG 121, 1471–81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts K. F., Hernandez-Diaz S. & Avorn J. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 371, 1168–9 (2014). [DOI] [PubMed] [Google Scholar]
- Knudsen T. M., Hansen A. V., Garne E. & Andersen A. M. Increased risk of severe congenital heart defects in offspring exposed to selective serotonin-reuptake inhibitors in early pregnancy–an epidemiological study using validated EUROCAT data. BMC Pregnancy Childbirth 14, 333 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis-Scaramozza C., Aschengrau A., Cabral H. & Jick S. S. Antidepressant use during early pregnancy and the risk of congenital anomalies. Pharmacotherapy 33, 693–700 (2013). [DOI] [PubMed] [Google Scholar]
- Margulis A. V. et al. Use of selective serotonin reuptake inhibitors in pregnancy and cardiac malformations: a propensity-score matched cohort in CPRD. Pharmacoepidemiol Drug Saf 22, 942–51 (2013). [DOI] [PubMed] [Google Scholar]
- Malm H., Artama M. & Gissler M. & Ritvanen. Selective serotonin reuptake inhibitors and risk for major congenital anormalies. Obstet Gynecol 118, 111–20 (2011). [DOI] [PubMed] [Google Scholar]
- Nordeng H. et al. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol 32, 186–94 (2012). [DOI] [PubMed] [Google Scholar]
- Jimenez-Solem E. et al. Exposure to selective serotonin reuptake inhibitors and the risk of congenital malformations: a nationwide cohort study. BMJ Open 2, e001148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornum J. B., Nielsen R. B., Pedersen L., Mortensen P. B. & Norgaard M. Use of selective serotonin-reuptake inhibitors during early pregnancy and risk of congenital malformations: updated analysis. Clin Epidemiol 2, 29–36 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L. et al. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidem DR S 16, 1086–94 (2007). [DOI] [PubMed] [Google Scholar]
- Kallen B. A. & Otterblad O. P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol 79, 301–8 (2007). [DOI] [PubMed] [Google Scholar]
- Kulin N. A. et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA 279, 609–10 (1998). [DOI] [PubMed] [Google Scholar]
- Wells G. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa Hospital Research Institute. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Data of access: 15/January/2017) (2013).
- Laurent L. et al. In utero exposure to venlafaxine, a serotonin-norepinephrine reuptake inhibitor, increases cardiac anomalies and alters placental and heart serotonin signaling in the rat. Birth Defects Res A Clin Mol Teratol 106, 1044–55 (2016). [DOI] [PubMed] [Google Scholar]
- Yavarone M. S., Shuey D. L., Tamir H., Sadler T. W. & Lauder J. M. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology 47, 573–84 (1993). [DOI] [PubMed] [Google Scholar]
- Sari Y. & Zhou F. C. Serotonin and its transporter on proliferation of fetal heart cells. Int J Dev Neurosci 21, 417–24 (2003). [DOI] [PubMed] [Google Scholar]
- Buskohl P. R., Sun M. L., Thompson R. P. & Butcher J. T. Serotonin Potentiates Transforming Growth Factor-beta3 Induced Biomechanical Remodeling in Avian Embryonic Atrioventricular Valves. PLoS One 7, e42527 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage K. et al. Genetic and environmental risk factors in congenital heart disease functionally converge in protein networks driving heart development. Proc Natl Acad Sci USA 109, 14035–40 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Oz B. et al. Paroxetine and congenital malformations: Meta-analysis and consideration of potential confounding factors. Clin Ther 29, 918–26 (2007). [DOI] [PubMed] [Google Scholar]
- Brite J., Laughon S. K., Troendle J. & Mills J. Maternal overweight and obesity and risk of congenital heart defects in offspring. Int J Obes (Lond) 38, 878–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Carmichael S. L., Canfield M., Song J. & Shaw G. M. Socioeconomic status in relation to selected birth defects in a large multicentered US case-control study. Am J Epidemiol 167, 145–54 (2008). [DOI] [PubMed] [Google Scholar]
- Rivera H. M., Christiansen K. J. & Sullivan E. L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci 9, 194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K. J. & Schwarzwald C. C. Echocardiography for the Assessment of Congenital Heart Defects in Calves. Vet Clin North Am Food Anim Pract 32, 37–54 (2016). [DOI] [PubMed] [Google Scholar]
- Moher D. et al. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM Statement. Rev Esp Salud Publica 74, 107–18 (2000). [PubMed] [Google Scholar]
- Gong T. T., Wu Q. J., Vogtmann E., Lin B. & Wang Y. L. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int J Cancer 132, 2894–900 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. J. et al. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur J Cancer Prev 25, 196–205 (2016). [DOI] [PubMed] [Google Scholar]
- Wu Q. J. et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol 24, 1079–87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z. Q. et al. Sertraline use in the first trimester and risk of congenital anomalies: a systemic review and meta-analysis of cohort studies. Br J Clin Pharmacol, doi: 10.1111/bcp.13161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamling J., Lee P., Weitkunat R. & Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27, 954–70 (2008). [DOI] [PubMed] [Google Scholar]
- Cui H., Gong T. T., Liu C. X. & Wu Q. J. Associations between Passive Maternal Smoking during Pregnancy and Preterm Birth: Evidence from a Meta-Analysis of Observational Studies. PLoS One 11, e147848 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan N. N. et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr 98, 1020–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T. T., Wu Q. J., Wang Y. L. & Ma X. X. Circulating adiponectin, leptin and adiponectin-leptin ratio and endometrial cancer risk: Evidence from a meta-analysis of epidemiologic studies. Int J Cancer 137, 1967–78 (2015). [DOI] [PubMed] [Google Scholar]
- Chen J., Gong T. T. & Wu Q. J. Parity and gastric cancer risk: a systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep 6, 18766 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–88 (1986). [DOI] [PubMed] [Google Scholar]
- Wu Q. J., Yang Y., Wang J., Han L. H. & Xiang Y. B. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci 104, 1067–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Hou R., Gong T. T. & Wu Q. J. Dietary fat intake and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. Sci Rep 5, 16693 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. J., Gong T. T. & Wang Y. Z. Dietary fatty acids intake and endometrial cancer risk: a dose-response meta-analysis of epidemiological studies. Oncotarget 6, 36081–97 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M., Davey S. G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–101 (1994). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.