Abstract
To better understand the mechanisms that hydrological conditions control chemical weathering and carbon dynamics in the large rivers, we investigated hydrochemistry and carbon isotopic compositions of dissolved inorganic carbon (DIC) based on high-frequency sampling in the Wujiang River draining the carbonate area in southwestern China. Concentrations of major dissolved solute do not strictly follow the dilution process with increasing discharge, and biogeochemical processes lead to variability in the concentration-discharge relationships. Temporal variations of dissolved solutes are closely related to weathering characteristics and hydrological conditions in the rainy seasons. The concentrations of dissolved carbon and the carbon isotopic compositions vary with discharge changes, suggesting that hydrological conditions and biogeochemical processes control dissolved carbon dynamics. Biological CO2 discharge and intense carbonate weathering by soil CO2 should be responsible for the carbon variability under various hydrological conditions during the high-flow season. The concentration of DICbio (DIC from biological sources) derived from a mixing model increases with increasing discharge, indicating that DICbio influx is the main driver of the chemostatic behaviors of riverine DIC in this typical karst river. The study highlights the sensitivity of chemical weathering and carbon dynamics to hydrological conditions in the riverine system.
Rivers play a crucial role in biogeochemical processes involved in the global carbon cycle, which link major carbon reservoirs, including atmosphere, biosphere, terrestrial geosphere, and oceans1. Chemical weathering is a fundamental geochemical process regulating the atmosphere-land-ocean fluxes and the climate on earth2,3. Forward and inverse models have been used for studying chemical weathering and CO2 consumption in many rivers around the world4,5,6,7. Many studies have focused on the chemical weathering and CO2 consumption in carbonate-rich catchments8,9,10, however, few of them have investigated the temporal dynamics of stream chemistry based on long term and high frequency sampling campaigns11, especially in carbonate-rich areas12,13. Carbonate weathering is highly sensitive to human activities and climate change2,14,15,16,17,18, the latter of which may partly be controlled by local hydrology14. Understanding the dynamics of the major dissolved solutes is useful for characterizing chemical weathering in rivers11,16. The feedback between hydrological conditions and chemical weathering is hypothesized to play a crucial role in modulating CO2 concentrations in carbonate-rich areas. Thus, time series observations are critical to better constrain the chemical fluxes, chemical weathering rates and the processes controlling the fate of chemical species in rivers14,19,20.
The annual fluvial carbon flux to the oceans is estimated to be 1 Pg C/y (0.8–1.2Pg C/y), of which 38% is in the form of dissolved inorganic carbon (DIC) and 25% is in the form of dissolved organic carbon (DOC)21,22, the rest is in the form of particulate carbon. The recognition that the carbon in inland rivers could be a substantial component in regional or global carbon budgets, has led to increased momentum in riverine biogeochemistry studies23. However, the patchy global estimates have been poorly constrained with respect to hydrological conditions, anthropogenic activities, acid rain etc.22,24,25,26. Riverine DIC and DOC concentrations are often, closely, associated with variations in hydrology27,28, as well as variations in sources and fluxes of dissolved carbon26,29. Detailed information on dissolved carbon dynamics with respect to hydrological conditions is still scarce, and relevant controlling processes are largely unknown. To constrain the DIC sources and clarify the relative biogeochemical processes, carbon isotopes can be used to identify the carbon sources and carbon biogeochemical processes26,30,31,32.
The continuous exposure of carbonate rocks on the Yunnan-Guizhou plateau is the largest karst area in the world8,10,25,26. The Wujiang River as one of the largest rivers in the Yunnan-Guizhou plateau is an ideal river to study the chemical weathering and carbon dynamics in the carbonate-rich areas8,9,10. A series of field campaigns have been conducted to sample river water under different hydrological conditions. This study uses data collected through the field campaigns to investigate correlations among chemical weathering, dissolved carbon dynamics, biogeochemical processes and hydrology. Carbon isotopes of dissolved inorganic carbon denoted as δ13CDIC, are used to identify carbon sources and constrain their contributions, which can provide insights into the main factors controlling carbon dynamics over time.
Results
Hydrochemical characteristics
pH of the river water samples is mildly alkaline (7.93–8.30) within the range of the whole Wujiang basin (7.6 to 8.9)8. The electrical conductivity (EC) values range from 315 to 407 μS/cm for the whole hydrological year (Table S1). Mean discharge-weighted concentrations can be calculated as Σ(QiCi)/ΣQi, where the subscript i represents each sample during the hydrological year33. The total dissolved solids (TDS) range from 239 to 356 mg/L (Table S1), with an average of 265 mg/L, which is higher than the world average value (97 mg/L)22. In comparison to rivers draining carbonate terrain worldwide, the average TDS value of the Wujiang River is higher than that of the South Han River (174 mg/L) in South Korea34, the Ganges and Indus rivers (164 mg/L) draining the Himalayas35,36 and the Mackenzie River (160 mg/L) draining the Rocky Mountains37. However, the TDS values are in the same range with that of the upper Yellow River (274 mg/L)7, the upper Xijiang River (297 mg/L)25 but lower than that of the Houzhai River (441 mg/L)26 in China. The total cationic charge (TZ+ = Na++K++2Ca2++2Mg2, in μeq/L) and total dissolved anions (TZ− = Cl− + 2SO42− + HCO3− + NO3−, in μeq/L) are well balanced, indicating that the unanalyzed ions play a minor role in charge balance. The mean discharge-weighted concentrations of major cations are as follows: Ca2+(1.33 mmol/L) > Mg2+(0.40 mmol/L) > Na+(0.17 mmol/L) > K+(0.04 mmol/L) (Fig. S1). The mean discharge-weighted concentrations of major anions are as follows: HCO3− (2.37 mmol/L) > SO42− (0.44 mmol/L) > Cl− (0.13 mmol/L) (Fig. S1). The Wujiang River shows a dominance of Ca2+, Mg2+, HCO3− and SO42−, which is similar to the characteristics of rivers draining karst areas10,20,36,37.
Carbon characteristics
DIC is the sum of CO2 (aq), carbonic acid (H2CO3), HCO3−, and carbonate (CO32−) ions26. The DIC concentrations in the river water vary from 2303 to 2783 μmol/L (Table S2), which is triple times higher than the world average concentration (852 μmol/L)19. The dissolved organic carbon (DOC) has a narrow range from 0.89 mg/L to 1.32 mg/L (Table S2), with no significant temporal variations. The partial pressure of carbon dioxide (pCO2) is a function of respiration, which can lead to increases in both riverine pCO2 and the dissolution of carbonate26. The pCO2 values range from 711 μatm to 1619 μatm (Table S2), which is much higher than the local atmospheric pCO2 (349 μatm) (Eq. S1). The value of δ13CDIC ranges from −14.8‰ in the high-flow season to −9.4‰ in the low-flow season (Table S2), with the average value of −12.1‰.
Temporal variations in major elements
The concentrations of major elements show significant temporal variations in the Wujiang River (Fig. S2). The discharge (Q) is low and relatively stable from November 2013 to March 2014 and relatively high from April 2014 to October 2014, reaching a maximum in July 2014 (Fig. S2). Generally, all the major elements (except Si) exhibit slightly decreasing trends during the high-flow season due to dilution and reach the maxima in February and March during the low-discharge period (Fig. S2). However, Si shows an increasing trend in the high-flow season relative to the low-flow season (Fig. S2).
Relationship between elemental concentrations and discharge
Godsey et al.33 and Clow and Mast38 have demonstrated that the concentrations of weathering products are negatively correlated with discharge and can be approximated as power-law functions:
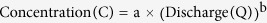 |
where a is a constant, and b represents the index that explains the deviation from chemostatic behavior39.
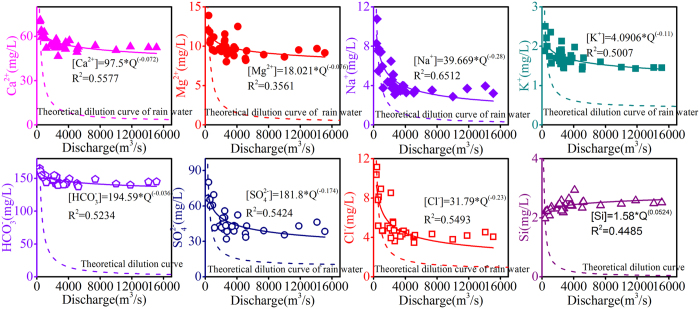
The regression coefficient b in the relationship between C and Q has a physical interpretation. If b = 0, the catchment behaves entire chemostatically; and if b = −1, Q is the only controller on C, constant solute fluxes being diluted by variable fluxes of water27. However, when b > 0, no dilution effect is present, because large amounts of inputs are induced by high discharge. Significant relationships (R2 values ranging from 0.35 to 0.65) between C and Q were found for the Wujiang River (Fig. 1). The slope values suggest that nearly all dissolved solutes (except Si) become diluted with increasing discharge and that the concentrations of Ca2+, Mg2+and HCO3− do not vary as much as those of Na+, K+, SO42− and Cl− (Fig. 1). Although all these dissolved solutes except Si decrease with increasing discharge, they do not strictly follow the theoretical dilution curve. Godsey et al.33 have postulated that concentrations are relatively constant in wide ranges of discharge, which maybe due to large amount of water stored in a catchment flows into the river induced by intense precipitation. Matrix porosity is widely distributed in the carbonate-rich areas, which stored large amount of “old” water. The near chemostatic behavior in the catchment may be ascribed to the carbonate-rich characteristics. The concentration of Si has a positive relationship with discharge (Fig. 1), indicating that Si is not affected by dilution, and that multiple biogeochemical processes counteract the dilution effect.
Figure 1.
Concentration-discharge relationships of major elements (Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl−, Si). The theoretical dilution curve means that these elements are diluted by deionized water (b = −1), and the theoretical dilution curve of rain water means that these elements are diluted by rain water, which is calculated by rainwater of Guiyang.
Discussion
Elemental ratio-discharge relationship
The dissolved loads of the river water are derived from atmosphere, rock weathering and anthropogenic pollutions40. The chemistry of river water in the Wujiang River is dominated by carbonate weathering (limestone and dolomite) (Fig. S3a). All of the samples have [Na+]/[Cl−] ratios exceeding 1 (Fig. S3b), indicating that silicate weathering is a clear source of major ions. There is no geological evidence for the exposure of evaporites in the river basin8, the contribution from evaporites is thus neglected. During carbonate weathering, the cations Ca2+and Mg2+are released into the dissolved phase. During silicate weathering, the cations Na+, K+, Ca2+ and Mg2+as well as Si, are released into the dissolved phase. Carbonate weathering is the primary sources of Ca2+, Mg2+ and HCO3−, and silicate weathering is the major source of Na+, K+ and Si in the basin8. Cl− has two major sources: atmospheric and anthropogenic inputs8. Changes in DOC concentrations can be ascribed to mixing of multiple sources and biogeochemical processes41.
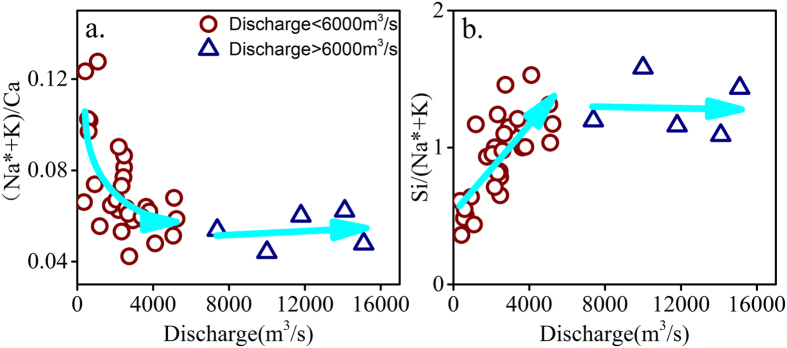
Elemental ratio-discharge relationships can be used to identify source changes and examine biogeochemical processes during various hydrological conditions16. Changes in elemental ratios are related to changes in the source or differential dissolution/precipitation rates between minerals, especially for carbonate and silicate minerals, with changing discharge14,16. As weathering progresses, saturation with respect to secondary silicate and the retention of silicate in the reservoir can buffer the concentration of dissolved Si, while the concentrations of dissolved cations that are not readily partitioned into secondary silicates continue to increase16,42. The variation in the ratio of (Na* + K)/Ca (Na* = [Na+] − [Cl−]) with changing discharge (Fig. 2a) suggests that the relative contribution of silicate mineral dissolution to the dissolved loads changes with discharge, which is similar to the findings of other studies38,43. Si, Na+ and K+ are from same lithologic source, and variations in Si/(Na* + K+) with discharge can be used to interpret the balance between secondary mineral precipitation and primary silicate weathering. The Si/(Na* + K) ratio can be regarded as a proxy commonly related to the “intensity” of silicate weathering44.
Figure 2.
(a) Correlation between (Na* + K)/Ca ratio and discharge for the Wujiang River. (b) Correlation between Si/(Na* + K) ratio and discharge for the Wujiang River.
Because multiple concentration-discharge relationships occur with increasing discharge (Fig. 1), reactive transport can generate various behaviors in the ratio-discharge relationships. The observed variation in (Na* + K)/Ca with discharge in Wujiang River doesn’t show a linear positive relationship or negative relationship. As the mean discharge of the Wujiang River in the monsoon season is approximately 3000 m3/s, we define that discharge is two times greater than the mean discharge (i.e., 6000 m3/s) as extremely high discharge. The (Na* + K)/Ca ratio decreases rapidly with increasing discharge at discharge below 6000 m3/s (Fig. 2a). Thus, the slope of the (Na* + K)/Ca ratio-discharge relationship suggests that the relative proportion of solutes derived from silicate weathering decreases with increasing discharge at discharges below 6000 m3/s. However, (Na* + K)/Ca has a relative stable value with increasing discharge when the discharge is higher than 6000 m3/s (Fig. 2a). Under high discharge conditions with short fluid transit times, water cannot reach equilibrium with rocks14,16. With increasing discharge, carbonate minerals dissolve rapidly and can drastically alter the water chemistry composition relative to the silicate minerals14,45; thus, the (Na* + K)/Ca ratio decreases with increasing discharge. When discharge is greater than 6000 m3/s, as most of dissolved solutes concentrations have relative constant values with increasing discharge (Fig. 1), the relative proportion of solutes derived from silicate weathering versus carbonate has a narrow range.
The power law exponent describing the relationship between Si and discharge shows a positive trend (Fig. 1). Dissolved Si concentration is maintained by equilibrium with respect to secondary silicate mineral and the retention of silicate in the reservoir38,42,43. The Si/(Na* + K) ratio increases with increasing discharge for discharge below 6000 m3/s, suggesting that the relative release of Si to Na* + K from primary silicates is greater than lower discharge and less dissolved Si is retained in the reservoir with increasing discharge for discharges below 6000 m3/s. Increasing discharge, decreases the transit time of fluids, leading to less time for the fluids and minerals to reach equilibrium with a secondary Si-bearing phase, and less time for retention in the reservoir in the upper reach. Thus, the Si/(Na* + K) ratios increase with increasing discharge for discharge below 6000 m3/s. Extreme discharge will shift deep flow paths to fast near-surface flow paths. Because of the less transit time of water, there is less time for biogeochemical processes such as ions exchange, biological uptake. So the Si/(Na* + K) ratio is near to the ratio from silicate weathering at extremly high discharge. Therefore, samples show relative stable Si/(Na* + K) ratios with increasing discharge when the discharge is higher than 6000 m3/s.
Chemical weathering fluxes affected by hydrological conditions
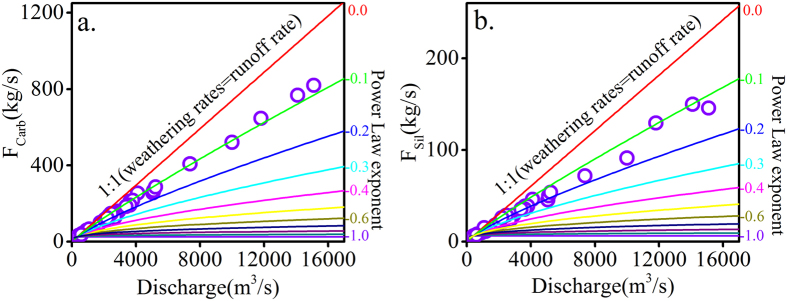
The temporal variability in discharge on the Yunnan-Guizhou Plateau in southwestern China mainly depends on rainfall associated with the monsoon climate. As discussed above, the major element dynamics are dominated by discharge, and hydrologic flushing further induces chemostatic behavior by increasing the reactive mineral surface area, which accelerates the mineral weathering38. In this study, a forward model is used to constrain the elemental sources (Eqs S1–9). Carbonate weathering fluxes (FCarb) and silicate weathering fluxes (FSil) are calculated using Eqs S11 and 12, respectively.
The results show a broad range: FCarb ranges from 26.1 kg/s to 819.7 kg/s, FSil varies from 3.7 kg/s to 149.8 kg/s. FCarb and FSil have strong correlations with discharge (Fig. 3a and b). The strong correlations between chemical weathering fluxes and discharge indicate that chemical weathering is dominated by hydrological conditions. Contours of different power law exponents spanning from dilution (−1) to “chemostasis” (0) (Fig. 3a and b) illustrate the sensitivity of chemical weathering to discharge changes. The power law exponent between FCarb and discharge is approximately −0.1. FSil has a power law exponent of approximately −0.2 at relatively low discharge rates (e.g., discharge of <6000 m3/s) and approximately −0.1 at extremely high discharge rates (e.g., discharge of >6000 m3/s). Both FCarb and FSil shows strong chemostatic responses to varying discharge, and FCarb shows a slightly more obvious chemostatic response. The near chemostatic behavior of chemical weathering fluxes responding to changing discharge may be ascribed to the hypothesis that fluids will quickly approach chemical equilibrium in rapidly eroding environments16,43,46. Variations in chemical weathering fluxes versus discharge can be ascribed to the dissolution kinetics, because the dominance of physical erosion during high discharge allows the more easily weatherable carbonate to dissolve14. And Importantly, our results characterize the sensitivity of chemical weathering fluxes to changes in discharge.
Figure 3.
(a) Relationship between carbonate weathering fluxes (FCarb) and discharge. (b) Relationship between silicate weathering fluxes (FSil) and discharge.
Response of dissolved carbon dynamics to hydrological changes
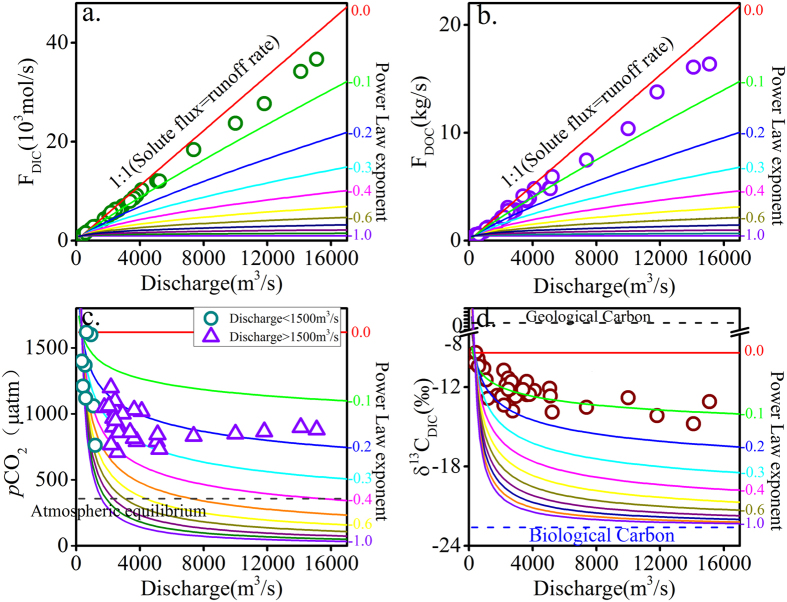
DIC is an important part of the total fluvial carbon to the ocean47. DOC is a significant constituent in aquatic ecosystems, and its concentrations in streams is influenced by both temperature and water flow pathway dynamics associated with changes in discharge48. The fluxes of DIC (FDIC) and DOC (FDOC) in rivers are strongly linked to climate condition (Fig. 4a and b). As discussed above, DIC and DOC show strong chemostic response with respect to varying discharge. Both FDIC and FDOC have strong positive relationship with discharge (Fig. 4a and b). The chemostic behaviors of DIC should be ascribed to primary production in the basin, as well as dissolution and precipitation. The power law exponents of both FDIC versus discharge and FDOC versus discharge are close to 0, indicating that the fluxes of dissolved carbon in the Wujiang River are dominated by hydrological conditions, and fluxes of DIC and DOC are sensitive to hydrological variability.
Figure 4.
(a) Relationship between flux of DIC (FDIC) with discharge. (b) Correlation between flux of DOC (FDOC) with discharge. (c) Links between pCO2 and discharge. (d) δ13CDIC values versus increasing discharge.
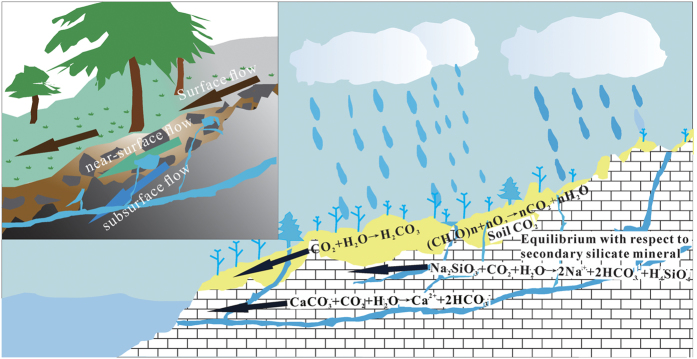
Biological CO2 discharge, in situ biodegradation and photosynthesis is the primary driver of the pCO2 in water26,30. Because of the relative low value of DOC and few amounts of aquatic plants in valley type of river channel, the effect of biodegradation and photosynthesis to pCO2 in the Wujiang river could be neglected. Therefore, biological CO2 discharge should be the main control on pCO2, especially during the flooding stage. The values of pCO2 show a negative correlation with discharge (Fig. 4c) and a power-law dilution effect with increasing discharge. The pCO2 values exhibit strong chemostatic behavior with respect to increasing discharge when the discharge is greater than 1500 m3/s. Biological CO2 produced by microbiologic activities and plant respiration would increase under high temperature conditions in summer at Southwest China26. So biological CO2 discharge is likely responsible for the chemostatic behavior. At extreme discharge rates, the fluid follows near-surface flow paths rather than deep flow paths, and biological CO2 does not have enough time to react with rocks. Therefore, biological CO2 can be transported to the river directly, which counteracts the dilution effect following periods of high discharge (Fig. 5). Li et al.26 showed that soil CO2 plays an important role in shifting δ13CDIC values in a small karst catchment. Epikarst aquifers water, with more negative δ13CDIC values than that in riverine water, have an important impact on karstic water carbon in carbonate-rich areas due to the active exchange between the surface water and subsurface flow water. Thus, the water stored in the matrix porosity and soil water with high contents of biological CO2 would flow into the river induced by high discharge, leading to decrease of δ13CDIC in the Wujiang River. As indicated in Fig. 4d, there is a generally negative correlation between δ13CDIC values and discharge. The values of δ13CDIC in Wujiang River changing with hydrological variability could not be interpreted in terms of simple dilution. Chemical weathering enhanced by increasing discharge produces large amounts of DIC, which is more positive than those of biological CO2. Thus, the δ13CDIC does not respond dramatically to the increasing discharge. As seen in Fig. 4d, the δ13CDIC values exhibit a power-law mixture effect with increasing discharge, indicating that there is a power-law relationship between the δ13CDIC values and discharge, as follows:
Figure 5. Schematic of chemical weathering and dissolved carbon dynamics in response to changing hydrological conditions in a typical karst catchment.
This figure was drawn by software Coredraw X7 (http://www.corel.com/cn/).
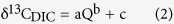 |
where a, b and c are constants. Usually, c is equal to the δ13C value of biological CO2. The exponent in the power-law relationship depends on the amount of biological CO2. A power law exponent of zero indicates that dilution will not change the values of δ13CDIC and that biological CO2 will not be directly discharged into the river under high discharge conditions. A power law exponent close to −1 means that a large amount of biological CO2 will be discharged into the river, shifting the δ13CDIC values.
The δ13CDIC values increase with increased (Na* + K)/Ca ratios, which represent the relative contribution of silicate weathering versus carbonate weathering (Fig. S4a). The results indicate that silicate weathering is not responsible for the lower values of δ13CDIC in the high-flow season due to relative low (Na* + K)/Ca ratios. Thus, more soil CO2 dissolution following rain water discharge would drop δ13C-DIC value in the river and counteract the riverine pCO2 at the same time in the high-flow season. There is a positive relationship between δ13CDIC values and SO4/DIC ratios (Fig. S4b), suggesting that the lower values of δ13CDIC are ascribed to the soil CO2 discharge and the reduced ratio of carbonate weathering by H2SO4. In this study, the power law exponent of δ13CDIC versus discharge is close to −0.1, indicating that relative high carbonate weathering rate and biological CO2 contribution are stimulated by high discharge likely occurs.
DIC sources
The sources of DIC can be constrained by δ13CDIC values due to the large difference between biological carbon and geological carbon1,31,41. As pCO2 in riverine water is much higher than pCO2 in the atmosphere (Fig. 4c), the contribution of atmospheric CO2 to DIC can be neglected5,25,30,32,49,50. Thus, the riverine DIC has two major sources: geological source and biological source. As discussed by Telmer and Veizer32, carbon isotopes in marine limestones and dolostones deposited since the end of the Proterozoic show typical marine values close to 0‰. C3 plants dominate the study area25 with the average δ13C value of −27‰. Cerling et al.51 have reported that there is carbon isotope fractionation of approximately 4.4‰ during the diffusion of CO2. Therefore, the carbon isotope of soil CO2 in the Wujiang river basin is likely −22.6‰. Given the different δ13CDIC of these two endmembers, the source contribution to riverine DIC can be calculated as follows:
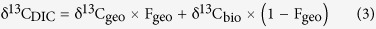 |
where δ13Cgeo and δ13Cbio is the δ13C values of geological carbon and biological carbon, respectively, and Fgeo is the proportion of carbon from the geological source. In the case that the DIC is affected by carbonate precipitation and CO2 degassing, the Fgeo is overestimated because of the isotope fractionation52.
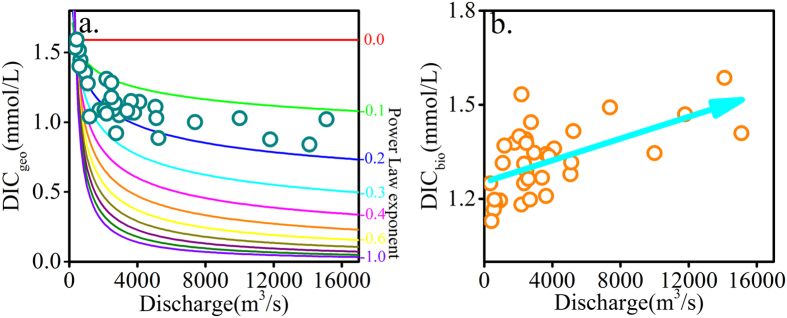
The contribution of DICbio (DIC from biological sources) to DIC in river water increases from 41.5% to 65.4% based on the calculation (Eq. 3). Furthermore, the concentrations of DICgeo (DIC from geological sources) and DICbio can be determined based on the relative proportions of DIC. The DICgeo concentrations show a power-dilution relationship with discharge (Fig. 6a), indicating that DICgeo exhibits strong chemostatic behavior with respect to discharge changes. Increasing carbonate weathering rates are likely responsible for this chemostatic situation. In contrast, DICbio values show a positive relationship with discharge (Fig. 6b), suggesting that biological DIC influx is the main driver of the chemostic behavior of total DIC with increasing discharge. Atmospheric precipitation infiltrates into the soil and flush soil CO2 into the river. Therefore, high discharge brings excessive biological DIC into the river, leading to increasing DICbio concentrations with increasing discharge.
Figure 6.
(a) Correlation between DICgeo and discharge. (b) Correlation between DICbio and discharge.
Fluvial DIC concentrations and δ13CDIC values primarily reflect the mixing of compositionally distinct endmembers (Fig. 6a and b). Physical and biological processed take turns to alter the composition of the DIC pool during changing hydrological conditions (Fig. 5). δ13CDIC may be more sensitive than DIC concentrations to hydrological changes (Fig. 4a and d), which is in agreement with Waldran et al.53. Clearly, physical and biological processes affect DIC concentrations during changing hydrological conditions. Therefore, continuous high-frequency monitoring during field programs should be conducted53. The results suggest that δ13CDIC values can be useful for revealing the response of biogeochemical processes to riverine hydrological conditions.
Methods
The study area
The Wujiang River basin (Fig. S5) is located in the center of the Southeast Asian Karst Region, the largest karst area in the world8,25. The Wujiang River is the largest tributary on the south bank of the upper Changjiang River, which is the 3rd longest river in the world8,9. The drainage area is 87 920 km2, and the region features a warm subtropical climate9. The mean annual precipitation for the last several years has ranged from 850 to 1600 mm8, and the occurrence of a seasonal monsoon results in high precipitation during summer and low precipitation during winter10. Carbonate rocks are widely exposed in this area, with no significant outcrops of evaporites8.
Sampling and analyses
The sampling site is located at the outlet of the Wujiang River (Fig. S5), where is 45 km away from the mainstream of the Changjiang River. The water samples for chemical and isotopic analyses were collected, monthly, from November 2013 to October 2014, i.e., throughout one entire hydrological year. Additional samples were collected during high-discharge periods. Samples were collected in the middle of the river by boat. pH, teeasured in the field. Alkalinity was determined with 0.02 μM hydrochloric acid mperature(T) and Ec were mwithin 24 hours. The samples were filtered through 0.45 μM cellulose-acetate filter paper. Major cations (K+, Na+, Ca2+ and Mg2+) and Si were acidified to pH = 2 with ultrapurified HNO3 and measured via Inductively Couples Plasma-optical Emission Spectrometry (ICP-OES) (with an error of 3%). Anions (SO42−, Cl− and NO3−) were analyzed using a Diones ICS90 (with an error of 5%). The DOC was measured using an OI Analytical Aurora 1030 TOC analyzer. For the δ13CDIC analysis, the method of Li et al.25 was used, with a precision of 0.2%. All these analyses were conducted at the State Key Laboratory of Environmental Geochemistry (Institute of Geochemistry, Chinese Academy of Sciences). Daily water discharge data were obtained online from the Ministry of Water Resources (http://www.hydroinfo.gov.cn/). Finally, pCO2, SIc and all DIC species were calculated based on mass action relationships and the relative equilibrium constants.
Additional Information
How to cite this article: Zhong, J. et al. Sensitivity of chemical weathering and dissolved carbon dynamics to hydrological conditions in a typical karst river. Sci. Rep. 7, 42944; doi: 10.1038/srep42944 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work is financially supported by National Natural Science Foundation of China (Grant Nos. 41422303, 41571130072 and 41130536) and the Ministry of Science and Technology of China through Grant Nos. 2016YFA0601000 and 2013CB956700.
Footnotes
The authors declare no competing financial interests.
Author Contributions Si-Liang Li designed the research, Jun Zhong conducted the field sampling, analyzed the samples, Si-Liang Li and Jun Zhong drafted the manuscript, Faxiang Tao, Fujun Yue and Cong-Qiang Liu contributed to the results discussion and the manuscript modification.
References
- Khadka M. B., Martin J. B. & Jin J. Transport of dissolved carbon and CO2 degassing from a river system in a mixed silicate and carbonate catchment. Journal of Hydrology 513, 391–402, doi: 10.1016/j.jhydrol.2014.03.070 (2014). [DOI] [Google Scholar]
- Guo J., Wang F., Vogt R. D., Zhang Y. & Liu C. Q. Anthropogenically enhanced chemical weathering and carbon evasion in the Yangtze Basin. Scientific reports 5, 11941, doi: 10.1038/srep11941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquet J.-S. et al. Chemical weathering and atmospheric/soil CO2 uptake in the Andean and Foreland Amazon basins. Chemical Geology 287, 1–26, doi: 10.1016/j.chemgeo.2011.01.005 (2011). [DOI] [Google Scholar]
- Gaillardet J., Dupre B., Louvat P. & Allegre C. J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical Geology 159, 3–30 (1999). [Google Scholar]
- Galy A. & France-Lanord C. Weathering processes in the Ganges–Brahmaputra basin and the riverine alkalinity budget. Chemical Geology 159 (1999). [Google Scholar]
- Wu W., Xu S., Yang J. & Yin H. Silicate weathering and CO2 consumption deduced from the seven Chinese rivers originating in the Qinghai-Tibet Plateau. Chemical Geology 249, 307–320, doi: 10.1016/j.chemgeo.2008.01.025 (2008). [DOI] [Google Scholar]
- Wu L., Huh Y., Qin J., Du G. & van Der Lee S. Chemical weathering in the Upper Huang He (Yellow River) draining the eastern Qinghai-Tibet Plateau. Geochimica et Cosmochimica Acta 69, 5279–5294, doi: 10.1016/j.gca.2005.07.001 (2005). [DOI] [Google Scholar]
- Han G. & Liu C.-Q. Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chemical Geology 204, 1–21, doi: 10.1016/j.chemgeo.2003.09.009 (2004). [DOI] [Google Scholar]
- Li C. & Ji H. Chemical weathering and the role of sulfuric and nitric acids in carbonate weathering: Isotopes (13C, 15N, 34S, and 18O) and chemical constraints. Journal of Geophysical Research: Biogeosciences 121, 1288–1305, doi: 10.1002/2015jg003121 (2016). [DOI] [Google Scholar]
- Han G., Tang Y. & Xu Z. Fluvial geochemistry of rivers draining karst terrain in Southwest China. Journal of Asian Earth Sciences 38, 65–75, doi: 10.1016/j.jseaes.2009.12.016 (2010). [DOI] [Google Scholar]
- Kirchner J. W. & Neal C. Universal fractal scaling in stream chemistry and its implications for solute transport and water quality trend detection. Proceedings of the National Academy of Sciences of the United States of America 110, 12213–12218, doi: 10.1073/pnas.1304328110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercod D. J., Brady P. V. & Gregory R. T. Catchment-scale coupling between pyrite oxidation and calcite weathering. Chemical Geology 151(1–4), 259–276 (1998). [Google Scholar]
- Szramek K., McIntosh J. C., Williams E. L., Kanduc T., Ogrinc N. & Walter L. M. Relative weathering intensity of calcite versus dolomite in carbonate-bearing temperate zone watersheds: Carbonate geochemistry and fluxes from catchments within the St. Lawrence and Danube river basins. Geochemistry, Geophysics, Geosystems, 8(4), doi: 10.1029/2006GC001337 (2007). [DOI] [Google Scholar]
- Tipper E. et al. The short term climatic sensitivity of carbonate and silicate weathering fluxes: Insight from seasonal variations in river chemistry. Geochimica et Cosmochimica Acta 70, 2737–2754, doi: 10.1016/j.gca.2006.03.005 (2006). [DOI] [Google Scholar]
- Ibarra D. E. et al. Differential weathering of basaltic and granitic catchments from concentration–discharge relationships. Geochimica et Cosmochimica Acta 190, 265–293, doi: 10.1016/j.gca.2016.07.006 (2016). [DOI] [Google Scholar]
- Torres M. A., West A. J. & Clark K. E. Geomorphic regime modulates hydrologic control of chemical weathering in the Andes–Amazon. Geochimica et Cosmochimica Acta 166, 105–128, doi: 10.1016/j.gca.2015.06.007 (2015). [DOI] [Google Scholar]
- Raymond P. A. & Oh N.-H. Long term changes of chemical weathering products in rivers heavily impacted from acid mine drainage: Insights on the impact of coal mining on regional and global carbon and sulfur budgets. Earth and Planetary Science Letters, doi: 10.1016/j.epsl.2009.04.006 (2009). [DOI] [Google Scholar]
- Jiang Y. J., Wu Y. X. & Yuan D. X. Human Impacts on Karst Groundwater Contamination Deduced by Coupled Nitrogen with Strontium Isotopes in the Nandong Underground River System in Yunan, China. Environmental science & technology 43, 7676–7683, doi: 10.1021/es901602t (2009). [DOI] [PubMed] [Google Scholar]
- Voss B. M. et al. Tracing river chemistry in space and time: Dissolved inorganic constituents of the Fraser River, Canada. Geochimica et Cosmochimica Acta 124, 283–308, doi: 10.1016/j.gca.2013.09.006 (2014). [DOI] [Google Scholar]
- Gao Q. et al. Chemical weathering and CO2 consumption in the Xijiang River basin, South China. Geomorphology 106, 324–332, doi: 10.1016/j.geomorph.2008.11.010 (2009). [DOI] [Google Scholar]
- Amiotte Suchet P., Probst J.-L. & Ludwig W. Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Global Biogeochemical Cycles 17, 1038, doi: 10.1029/2002gb001891 (2003). [DOI] [Google Scholar]
- Li S. & Bush R. T. Changing fluxes of carbon and other solutes from the Mekong River. Scientific reports 5, 16005, doi: 10.1038/srep16005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon S. et al. Contrasting biogeochemical characteristics of the Oubangui River and tributaries (Congo River basin). Scientific reports 4, 5402, doi: 10.1038/srep05402 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond P. A. & Cole Jonathan J. Increase in the export of alkalinity from north america’s largest river Science (2003). [DOI] [PubMed]
- Li S.-L., Calmels D., Han G., Gaillardet J. & Liu C.-Q. Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: Examples from Southwest China. Earth and Planetary Science Letters 270, 189–199, doi: 10.1016/j.epsl.2008.02.039 (2008). [DOI] [Google Scholar]
- Li S.-L. et al. Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: Isotopic and chemical constraints. Chemical Geology 277, 301–309, doi: 10.1016/j.chemgeo.2010.08.013 (2010). [DOI] [Google Scholar]
- León J. G. & Pedrozo F. L. Lithological and hydrological controls on water composition: evaporite dissolution and glacial weathering in the south central Andes of Argentina (33°–34°S). Hydrological Processes 29, 1156–1172, doi: 10.1002/hyp.10226 (2015). [DOI] [Google Scholar]
- Voss B. M. et al. Seasonal hydrology drives rapid shifts in the flux and composition of dissolved and particulate organic carbon and major and trace ions in the Fraser River, Canada. Biogeosciences 12, 5597–5618, doi: 10.5194/bg-12-5597-2015 (2015). [DOI] [Google Scholar]
- Hélie J.-F., Hillaire-Marcel C. & Rondeau B. Seasonal changes in the sources and fluxes of dissolved inorganic carbon through the St. Lawrence River — isotopic and chemical constraint. Chemical Geology 186, 117–138 (2002). [Google Scholar]
- Barth J. A. C., Cronin A. A., Dunlop J. & Kalin R. M. Influence of carbonates on the riverine carbon cycle in an anthropogenically dominated catchment basin: evidence from major elements and stable carbon isotopes in the Lagan River (N. Ireland). Chemical Geology 200, 203–216, doi: 10.1016/s0009-2541(03)00193-1 (2003). [DOI] [Google Scholar]
- Rivé K., Gaillardet J., Agrinier P. & Rad S. Carbon isotopes in the rivers from the Lesser Antilles: origin of the carbonic acid consumed by weathering reactions in the Lesser Antilles. Earth Surface Processes and Landforms 38, 1020–1035, doi: 10.1002/esp.3385 (2013). [DOI] [Google Scholar]
- Telmer K. & Veizer J. Carbon fluxes, pCO2 and substrate weathering in a large northern river basin, Canada carbon isotope perspectives. Chemical Geology 159 (1999). [Google Scholar]
- Godsey S. E., Kirchner J. W. & Clow D. W. Concentration-discharge relationships reflect chemostatic characteristics of US catchments. Hydrological Processes 23, 1844–1864, doi: 10.1002/hyp.7315 (2009). [DOI] [Google Scholar]
- Ryu J., Lee K., Chang H. & Shin H. Chemical weathering of carbonates and silicates in the Han River basin, South Korea. Chemical Geology 247, 66–80, doi: 10.1016/j.chemgeo.2007.09.011 (2008). [DOI] [Google Scholar]
- Karim A. & V. J. Weathering processes in the Indus River Basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chemical Geology 170 (2000). [Google Scholar]
- Dalai T. K. et al. Major ion chemistry in the headwaters of the Yamuna river system Chemical weathering, its temperature dependence and CO2 consumption in the Himalaya. Geochimica et Cosmochimica Acta 66, 3397–3416 (2002). [Google Scholar]
- Millot R. Northern latitude chemical weathering rates: clues from the Mackenzie River Basin, Canada. Geochimica et Cosmochimica Acta 67, 1305–1329, doi: 10.1016/s0016-7037(02)01207-3 (2003). [DOI] [Google Scholar]
- Clow D. W. & Mast M. A. Mechanisms for chemostatic behavior in catchments: Implications for CO2 consumption by mineral weathering. Chemical Geology 269, 40–51, doi: 10.1016/j.chemgeo.2009.09.014 (2010). [DOI] [Google Scholar]
- Basu N. B. et al. Nutrient loads exported from managed catchments reveal emergent biogeochemical stationarity. Geophysical Research Letters 37, L23404, doi: 10.1029/2010gl045168 (2010). [DOI] [Google Scholar]
- Xu Z. & Liu C. Chemical weathering in the upper reaches of Xijiang River draining the Yunnan–Guizhou Plateau, Southwest China. Chemical Geology 239, 83–95, doi: 10.1016/j.chemgeo.2006.12.008 (2007). [DOI] [Google Scholar]
- Jin J., Zimmerman A. R., Moore P. J. & Martin J. B. Organic and inorganic carbon dynamics in a karst aquifer: Santa Fe River Sink-Rise system, north Florida, USA Journal of Geophysical Research: Biogeosciences 119, 340–357, doi: 10.1002/2013jg002350 (2014). [DOI] [Google Scholar]
- Humborg C., Ittekkot V., Cociasu A. & Bodungen B., V I., A C. & von B. B. Effect of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature 386, 385–388 (1997). [Google Scholar]
- Maher K. The role of fluid residence time and topographic scales in determining chemical fluxes from landscapes. Earth and Planetary Science Letters 312, 48–58, doi: 10.1016/j.epsl.2011.09.040 (2011). [DOI] [Google Scholar]
- Moon S., Huh Y., Qin J. & Vanpho N. Chemical weathering in the Hong (Red) River basin: Rates of silicate weathering and their controlling factors. Geochimica et Cosmochimica Acta 71, 1411–1430, doi: 10.1016/j.gca.2006.12.004 (2007). [DOI] [Google Scholar]
- Wei G. et al. Seasonal changes in the radiogenic and stable strontium isotopic composition of Xijiang River water: Implications for chemical weathering. Chemical Geology 343, 67–75, doi: 10.1016/j.chemgeo.2013.02.004 (2013). [DOI] [Google Scholar]
- Maher K. & Chamberlain C. P. Hydrologic regulation of chemical weathering and the geologic carbon cycle. Science 343(1502), doi: 10.1126/science.1250770 (2014). [DOI] [PubMed] [Google Scholar]
- Meybeck M. Riverine transport of atmospheric carbon: sources, global typology and budget. Water Air Soil Pollut 70, 443–463 (1993). [Google Scholar]
- Winterdahl M., Laudon H., Lyon S. W., Pers C. & Bishop K. Sensitivity of stream dissolved organic carbon to temperature and discharge: Implications of future climates. Journal of Geophysical Research: Biogeosciences 121, 126–144, doi: 10.1002/2015jg002922 (2016). [DOI] [Google Scholar]
- Aucour A.-M., Sheppard S. M. F., Guyomar O. & Wattelet J. Use of 13C to trace origin and cycling of inorganic carbon in the Rhône river system. Chemical Geology 159, 87–105, doi: 10.1016/S0009-2541(99)00035-2 (1999). [DOI] [Google Scholar]
- Mayorga E. et al. Young organic matter as a source of carbon dioxide outgassing from Amazonian rivers. Nature 436, 538–541, doi: 10.1038/nature03880 (2005). [DOI] [PubMed] [Google Scholar]
- Cerling T. E., Solomon D. K., Quade J. & Bowman J. R. The Macalpine Hills Lunar Meteorite ConsortiumOn the isotopic composition of carbon in soil carbon dioxide. Geochimica et Cosmochimica Acta 55, 3403–3405, doi: 10.1016/0016-7037(91)90498-T (1991). [DOI] [Google Scholar]
- Frondini F. et al. Carbon dioxide degassing from Tuscany and Northern Latium (Italy). Global and Planetary Change 61, 89–102, doi: 10.1016/j.gloplacha.2007.08.009 (2008). [DOI] [Google Scholar]
- Waldran S., Scott E. M. & Soulsby C. Stable Isotope Analysis Reveals Lower-Order River Dissolved Inorganic Carbon Pools Are Highly Dynamic. Environ. Sci. Technol. 41, 6156–6162 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.