Abstract
Background
The HCV RNA testing of potential cornea donors frequently relies on blood samples stored pre mortem. The recommended storage time of maximum 72 h frequently excludes a significant fraction of donors.
Methods
The influence of storage time of EDTA plasma samples at 4 °C on the viral load measured with the Roche HCV Quantitative Test vs. 2.0 was evaluated for 43 samples from HCV-positive individuals.
Results
The mean reduction of the viral load after 4 °C storage for 6-8 days was 0.46 log10 IU/ml (range +0.17 to −1.66 log10 IU/ml). After 1-3 days a mean loss of 0.19 log10 IU/ml (range +0.30 to −1.41 log10 IU/ml) and after 3-5 days of 0.32 log10 IU/ml (range +0.36 to −1.81 log10 IU/ml) was observed. In 23.3% of samples, a viral load reduction ≥ 1 log10 IU/ml (1.0-1.81 log10 IU/ml) was found after prolonged storage (5-8 days). In none of the samples did the HCV load fall below the detection limit.
Conclusion
Plasma storage for up to 8 days can quantitatively reduce the HCV RNA load, yet has no influence on the reliability of a qualitative HCV RNA detection by this ultrasensitive test to determine the HCV status of serologically negative cornea donors.
Keywords: Cornea donor testing, HCV-PCR, Prolonged sample storage at 4 °C
Introduction
Basic requirements for the safety of tissues and cells for human application (patient treatment) related to the risk of transmitting a viral infection are defined in the EU directives 2004/23/EC and 2006/17/EC [1,2] as well as in accompanying regulations. Standards were fully implemented into German law on July 20, 2007 via the Tissues and Cells Act also anchored in guidelines issued by the federal authority [3,4], including minimum requirements for in vitro testing for biological markers of donor blood.
Cadaveric blood samples of cornea donors which can be obtained at maximum 24 h post mortem are often of poor quality. Therefore, stored ante mortem blood samples frequently have to be considered for analysis [5,6,7]. Serologic assays usually allow testing of samples that are stored at 4 °C (2-8 °C) for up to 7 days. In contrast, for the detection of HCV RNA by PCR mostly shorter storage times have been defined by the manufactures – e.g., for the Roche Assay (COBAS Ampliprep/COBAS TaqMan HCV Quantitative Test Version 2.0 (CAP/CTM), Roche Diagnostics, Basel, Switzerland) a maximal storage time of 72 h for serum and plasma specimens after centrifugation has been defined. A common diagnostic problem that arises in the context of cornea donor testing is a considerable hemodilution in the acute phase pre mortem (up to one-third of the excluded cornea donors). This in turn may sometimes require the use of ‘older’, stored blood samples collected more than 72 h prior to testing. This storage is usually done at 4 °C in the refrigerator. The 72-hour storage restriction leads to a loss of potential cornea donors because the required HCV RNA test can no longer be performed.
Although previous studies showed that samples could be stored at 4 °C for several weeks without significant loss of HCV RNA [8,9], the suitability of the diagnostic test system has to be proven in the above described context.
In the current study the influence of the storage time of plasma samples at 4 °C was evaluated for 43 samples from HCV-positive individuals. Specifically, we quantified the HCV RNA load in these samples to assess whether or not a relevant viral load reduction could be seen.
Material and Methods
For HCV RNA detection and quantitation the COBAS AmpliPrep/COBAS TaqMan HCV Quantitative Test Version 2.0 (Roche Diagnostics, Basel, Switzerland) was used according to the manufacturer's recommendations. Patient samples were diluted 1:5 in negative plasma prior to use. This step was routinely performed in our laboratory with cadaveric samples obtained up to 24 h post mortem to minimize the role of interfering factors in plasma and, in the context of this particular study, to obtain a sufficient volume for repeat testing. The detection limit of the CAP/CTM assay is 15 IU/ml in undiluted samples according to the manufacturer's information.
A total of 43 different left-over samples from routinely tested patients with different HCV RNA loads were used in this study.
Samples were centrifuged for 20 min at 1,600 × g, the plasma was aliquoted and stored at 4 °C (which always refers to a temperature range of 2-8 °C). For the majority of samples (n = 27) one additional aliquot was frozen at ≤-18 °C and retested at the end of the study period (between day 7 and 90).
In undiluted patient samples with known HCV infection the HCV load was first assessed on the day of sampling (day 0) or 1 day later (day 1) (termed ‘original sample’). In a second run at the same day (day 0 or 1) or up to 3 days later a 1:5 dilution of the sample (stored at 4 °C) in HCV-negative plasma was re-tested to estimate the influence of sample dilution. The estimated viral loads of the undiluted original samples ranged between 640 und 6,400,000 IU/ml (mean 4.84 ± 0.84 log10 IU/ml). A second and third HCV RNA testing of samples stored at 4 °C was performed on day 2-5 and day 6-8 after sampling, respectively. All stored samples were diluted 1:5 in negative plasma prior to testing. These test values were multiplied by the dilution factor and transferred in log10 IU/ml. Time intervals of testing, storage conditions, and sample dilution are summarized in table 1.
Table 1.
Storage conditions and sample dilution
| Pre-analytical treatment | Duration of storage prior testing |
|---|---|
| Original sample (undiluted) | 0–1 days at 4 °C (2–8 °C) |
| 1:5 dilution* | 1–3 days at 4 °C (2–8 °C) |
| 1:5 dilution | 3–5 days at 4 °C (2–8 °C) |
| 1:5 dilution | 6–8 days at 4 °C (2–8 °C) |
| undiluted sample** | 7–90 days at ≤–18 °C |
Storage undiluted, 1:5 dilution immediately prior testing.
n = 27 samples.
Results
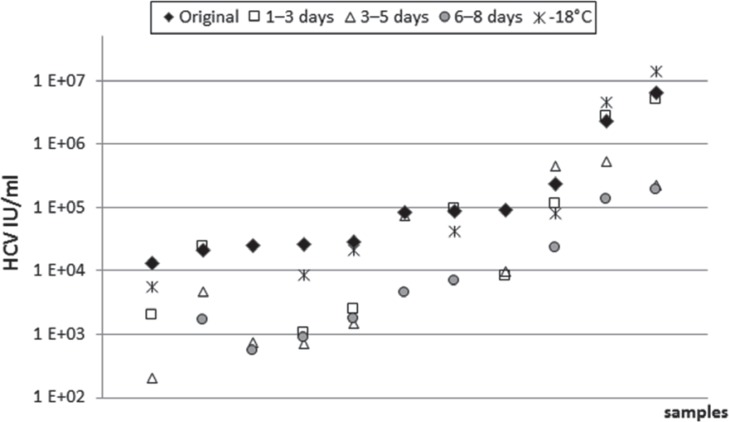
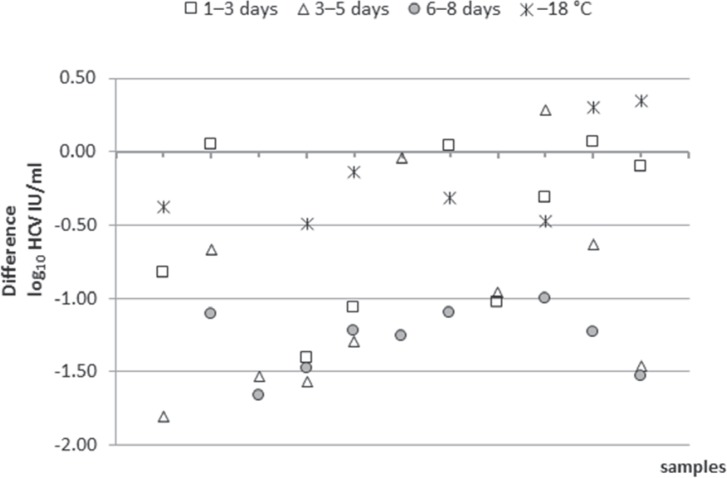
The quantification of the HCV RNA load in relation to storage time showed partially heterogenic results, especially after storage at 4 °C for longer periods of time (6-8 days, table 2). The mean reduction of the viral load after 4 °C storage for 6-8 days in comparison to the result determined for the original sample (days 0 or 1) was 0.46 log10 IU/ml (range +0.17 to −1.66 log10 IU/ml, fig. 1). In 9 of the investigated samples (20.9%) a viral load reduction ≥ 1 log10 IU/ml (1.0-1.66 log10 IU/ml) was found after prolonged storage (6-8 days). In two additional samples (No. 8 and 27, table 2) a reduction of the viral load of 1.81 and 1.03 log10 IU/ml was seen after storage of 5 and 1-3 days, respectively. Unfortunately, insufficient quantities of these plasma samples precluded testing of this plasma also at later time points. Six of these 9 samples, that had been stored at ≤-18 °C in parallel, could be re-tested. In three of them a viral load reduction of more than 1 log10 IU/ml (storage at 4 °C) compared to viral loads in the respective aliquots stored ≤-18 °C could be confirmed (samples No. 15, 41, 43, table 2). In the other three samples (samples No. 14, 26, 33) the viral load decrease ranged between 0.53 and 0.99 log10 IU/ml. Interestingly, storage at ≤-18 °C resulted in a high variance of the test results, too. On average the viral load was reduced by only 0.06 log10 IU/ml, but the range of differences between the viral load in the original sample compared to that stored at ≤-18 °C varied between −0.49 and +0.34 log10 IU/ml. In table 2 the differences between the viral loads of the stored samples (4 °C for 6-8 days or ≤-18 °C for ≥ 7 days) and the baseline value are shown. In figure 1 and 2, the 11 samples with ≥1 log10 IU/ml differences of viral load in comparison to the ‘original samples’ are presented. The samples were arranged in ascending order according to their viral load result in the original sample. In addition to the above mentioned 9 samples, 8 samples were found to have a viral load reduction by more than 1 log10 IU/ml already after a storage time of less than 6-8 days: in 3 cases after a storage of 1-3 days (samples No. 14, 15, 27) and in 5 cases after (or additionally after) 3-5 days (samples No. 8, 13, 14, 15, 43). Importantly, in none of the investigated samples in this study the HCV RNA test result became negative after prolonged storage.
Table 2.
HCV PCR test results (log10 IU/ml) of original samples (n = 43) in comparison to the test results after storage for 6–8 days at 4 ° C (2–8 ° C) and the retested samples after storage at ≤–18 ° C
| No. | A original sample | B* storage at ≤–18 °C | Viral load reduction B – A | E* storage at 4 °C, 6–8 days | Viral load reduction E – A | Viral load reduction E – B |
|---|---|---|---|---|---|---|
| 1 | 2.81 | n.d. | − | 2.13 | −0.68 | − |
| 2 | 3.22 | 3.07 | −0.15 | 3.08 | −0.14 | 0.01 |
| 3 | 3.57 | n.d. | − | 3.14 | −0.43 | |
| 4 | 3.76 | 3.98 | 0.22 | 3.63 | −0.13 | −0.34 |
| 5 | 3.77 | 3.87 | 0.10 | 3.35 | −0.43 | −0.52 |
| 6 | 4.10 | n.d. | − | 3.76 | −0.33 | − |
| 7 | 4.11 | n.d. | − | 3.58 | −0.54 | − |
| 8 | 4.12 | 3.74 | −0.38 | n.d. | − | − |
| 9 | 4.13 | n.d. | − | 4.02 | −0.11 | − |
| 10 | 4.14 | n.d. | − | 3.44 | −0.70 | − |
| 11 | 4.26 | 3.80 | −0.45 | 3.51 | −0.74 | −0.29 |
| 12 | 4.33 | n.d. | − | 3.23 | −1.11 | − |
| 13 | 4.40 | n.d. | − | 2.74 | −1.66 | − |
| 14 | 4.42 | 3.93 | −0.49 | 2.94 | −1.48 | −0.99 |
| 15 | 4.46 | 4.32 | −0.14 | 3.23 | −1.22 | −1.08 |
| 16 | 4.49 | 4.42 | −0.07 | 3.91 | −0.57 | −0.50 |
| 17 | 4.60 | n.d. | − | 4.73 | 0.13 | − |
| 18 | 4.65 | 4.61 | −0.03 | 4.68 | 0.03 | 0.07 |
| 19 | 4.72 | 4.85 | 0.13 | 4.89 | 0.17 | 0.04 |
| 20 | 4.74 | 5.03 | 0.29 | 4.66 | −0.08 | −0.37 |
| 21 | 4.84 | n.d. | − | 4.30 | −0.54 | − |
| 22 | 4.86 | 5.18 | 0.32 | 4.46 | −0.39 | −0.71 |
| 23 | 4.87 | 4.63 | −0.24 | 4.38 | −0.49 | −0.25 |
| 24 | 4.91 | n.d. | − | 3.65 | −1.26 | − |
| 25 | 4.93 | n.d. | − | 4.47 | −0.46 | − |
| 26 | 4.94 | 4.62 | −0.32 | 3.84 | −1.10 | −0.78 |
| 27 | 4.95 | n.d. | − | n.d. | − | − |
| 28 | 4.97 | 5.05 | 0.08 | 4.90 | −0.07 | −0.15 |
| 29 | 5.06 | 4.89 | −0.18 | 4.91 | −0.16 | 0.02 |
| 30 | 5.24 | 5.14 | −0.10 | 5.25 | 0.01 | 0.11 |
| 31 | 5.30 | 5.23 | −0.07 | 5.23 | −0.07 | 0.00 |
| 32 | 5.36 | 5.49 | 0.14 | 5.20 | −0.16 | −0.30 |
| 33 | 5.36 | 4.89 | −0.47 | 4.36 | −1,00 | −0.53 |
| 34 | 5.38 | 5.41 | 0.03 | 5.22 | −0.16 | −0.19 |
| 35 | 5.66 | 5.17 | −0.49 | 5.43 | −0.23 | 0.26 |
| 36 | 5.69 | 5.43 | −0.25 | 5.32 | −0.37 | −0.12 |
| 37 | 5.76 | 5.69 | −0.07 | 5.84 | 0.08 | 0.15 |
| 38 | 5.87 | 5.92 | 0.05 | 5.94 | 0.07 | 0.03 |
| 39 | 5.90 | 5.90 | 0.00 | 5.97 | 0.08 | 0.07 |
| 40 | 6.00 | 6.13 | 0.13 | 6.06 | 0.06 | −0.07 |
| 41 | 6.37 | 6.66 | 0.30 | 5.13 | −1.23 | −1.53 |
| 42 | 6.49 | 6.62 | 0.13 | 6.61 | 0.13 | 0.00 |
| 43 | 6.81 | 7.15 | 0.34 | 5.28 | −1.53 | −1.87 |
| Mean | −0.06 | −0.46 | −0.34 | |||
| Maximal viral load reduction | −0.49 | −1.66 | −1.87 | |||
n.d. = Not done; – = not calculable, since no sufficient sample volume was available for testing. However, these samples were tested at other times points (e.g. 1–3 days and/or 3–5 days).
Test values were multiplied by the dilution factor
Fig. 1.
HCV viral load (IU/ml) in 11 individual patient samples, which showed viral load differences ≥1 log10 IU/ml after different storage times at 4 ° C or at ≤-18 ° C (points plotted vertically originate from the same sample that was tested at different time points. The abscissa shows the samples blotted according to their viral load in the ‘original sample’ in ascending order).
Fig. 2.
Differences of viral load (≥1 log10 IU/ml) in comparison to the ‘original samples’ after different storage times at 4 ° C and after storage at ≤-18 ° C (n = 11 samples). Points plotted vertically originate from the same sample that was tested at different time points. The abscissa shows the samples blotted according to their viral load in the ‘original sample’ in ascending order.
Discussion
Several studies have investigated the influence of storage on the quantifiable HCV viral load in patient blood samples [8,9]. Most of these studies were conducted with small patient numbers with the aim of verifying routine sampling conditions. As a consistent finding, the HCV RNA load was in most instances stable for 72 h at 4 °C [8,9,10,11,12]. However, storage at other conditions (e.g. room temperature) or for longer time periods at 4 °C resulted in more discrepant outcomes. In some studies HCV RNA seemed to be relatively stable at 4 °C for several weeks [8,9,11], but other studies found significantly decreasing viral loads in some samples stored for more than 72 h [10,13]. As a confounding issue, in all of these studies only a small number (2-19) of individual samples were analyzed, and different material (serum or plasma), storage conditions, and also variation of diagnostic assaysmade an inter-study comparison even more difficult. Our HCV RNA load quantifications of 43 individual patient samples demonstrate that prolonged storage times at 4 °C can in some cases significantly reduce the viral load. This phenomenon (cut-off ‘reduction’ ≥ 1 log10 IU/ml) could be observed in 10 out of 43 samples (23.3% for a storage time of 5-8 days). The maximal reduction observed was 1.81 log10 IU/ml HCV in 1 sample after a 5-day storage at 4 °C corresponding to a 64-fold reduction of the original viral load. Interestingly, in 3 cases (7%) a reduction of the viral load of more than 1 log10 IU/ml was observed already after storage for 1-3 days. Of note, this time frame falls into the presumably uncritical storage time stated in the product sheet of the manufacturer (at 4 °C up to 72 h). Considering a loss of 0.5 log10 IU/ml or less ‘acceptable’, still 6 cases (14%) displayed a more strongly reduced HCV RNA load after 1-3 days, 11 samples (25.6%) after 3-5 days, and 15 samples (34.9%) after 6-8 days.
From a different perspective, the majority of HCV RNA-positive plasma samples showed only marginal changes of the viral loads after storage at 4 °C. After 1-3 days and 3-5 days, a mean loss of 0.19 log10 IU/ml (+0.30 to −1.41 log10 IU/ml), and 0.32 log10 IU/ml (+0.36 to −1.81 log10 IU/ml) was observed, respectively. At the endpoint of the study (day 6-8), the viral load had decreased on average by 0.46 log10 IU/ml (+0.17 to −1.66 log10 IU/ml). In none of the samples investigated, the storage resulted in a complete loss of detectable HCV RNA. In other words, none of these patient samples would have been classified as HCV RNA-negative.
Sample storage at ≤-18 °C for ≥7 days resulted for none of the samples in a loss of the HCV RNA load of more than 0.5 log10 IU/ml, indicating that one freeze-thaw cycle does not markedly affect the outcome. This was confirmed in a recent study by the Australian Red Cross Blood Service [14] showing that the detection of HCV RNA was not affected by storing plasma frozen at −30 °C for up to 3 years at high and low viral load levels, and that the detection of HCV RNA was not affected by up to three freeze-thaw cycles.
It remains unknown which specific factors have influenced the loss of HCV RNA in some of the patient plasma samples. A viral load loss ≥ 1 log10 IU/ml was independent of the viral load in the original sample (fig. 1, 2) and of the HCV genotype (data not shown). On the other hand, it has to be noted that in our study samples were tested in several runs, which could account for a higher variance of test results due to the inter-assay variance.
Overall, in a discussion on potentially extension of the maximal storage time of plasma samples for HCV RNA testing of cornea donors, different aspects have to be considered: At present, German legislation does not define a detection limit of the HCV RNA PCR used in the context of cornea donor testing [3,4]. Because of this lack of requirement, usually the detection limit required according to German legislation for testing blood donations (5,000 IU/ml = 3.7 log10 IU/ml HCV RNA) [15,16] is applied.
Of note, samples are pre-diluted 1:5 in negative plasma which increases the theoretical detection limit of the test to 75 IU/ml (15 IU/ml = 1.18 log10 IU/ml standard detection limit) and the detection limit for donor samples to 1,000 IU/ml (= 3.0 log10 IU/ml). Importantly, none of the 43 samples studied fell below this level, even after prolonged storage at 4 °C.
In the most extreme case of our study, a reduction of the HCV RNA load by 1.81 log10 IU/ml was observed, corresponding to a 64-fold reduction. Theoretically, in a plasma sample with an HCV load of 1,000 IU/ml (the required detection limit after pre-dilution) the viral load would be reduced to 16 IU/ml but would be still above the assay's detection limit. In this context the benefit of a 1:3 instead of 1:5 dilution has to be considered, because the sensitivity and reliability of results in samples with low viral load might be improved. On the other hand, in cases of low sample volume or in the presence of inhibitory factors a higher pre-dilution may have advantages. Along these lines, Schulze et al. [17] showed by testing plasma spiked with 5,000 IU/ml HCV RNA that all samples, which had been immediately stored at 5 °C for up to 120 h, were qualitatively detectable in a 1:96 dilution (simulating pool testing). While the use of pooled sample testing is common in the context of blood donors, it is not applicable for testing of individual cornea donors.
However, it is possible that a plasma sample with a starting HCV RNA load of less than 1,000 IU/ml could be missed after storage for 6-8 days at 4 °C. It is thus critical that all donors in parallel are serologically tested for anti-HCV antibodies. Accordingly, a chronically infected donor in most cases would be identified. Acute HCV infections prior to seroconversion typically show a rapidly increasing viral load, i.e., the time window with low RNA levels is therefore very short [18,19,20,21].
A potential limitation of our study design is that the samples used represent anti-HCV-positive samples, exclusively. It is conceivable that HCV antibodies might stabilize HCV particles. Therefore, future analyses with window phase samples may provide important additional insight into particle behavior and stability also in the absence of antibodies. Furthermore, it has to be noted that our validation was performed exclusively with plasma samples, and results cannot be transferred directly to serum samples. Interestingly, a study by Da Silva Cardoso et al. [22] suggests that the presence of blood cells and the type of blood sample (serum or plasma) has only a small effect on the HCV RNA concentration after storage at 4 °C.
Another relevant aspect to assess is that the transmission risk for the cornea recipient, in regard to blood-borne viruses like HCV, via a cornea transplant is nearly nonexistent because this tissue is rarely soaked with blood and additionally rinsed with saline prior to transplantation [23]. Remarkably, transplantation of several tissues from an HCV RNA-positive, antibody-negative donor resulted in an HCV infection in 8 out of 30 recipients. In contrast, the recipient of the cornea remained HCV-negative [24]. However, this has to be interpreted with caution. Heck et al. [25] were able to detect HCV RNA in 17 of 22 (77%) cornea samples of HCV-positive donors. None of the cornea samples were HCV RNA-positive in the absence of detection of HCV RNA in the blood sample, demonstrating the importance of routine HCV RNA PCR blood testing to reduce the transplantation-associated risk for HCV transmission.
In conclusion, our data for 43 HCV RNA-positive EDTA plasma samples show that a prolonged storage for up to 8 days (and thus considerably longer than the current standard of 72 h) at 4 °C may lead to decreasing HCV RNA loads, but this has no significant influence on the reliability of a qualitative HCV RNA detection to assess the suitability of a cornea donor in respect to this infectious agent. Beyond donor testing, the storage-associated reduction of HCV RNA loads may impact viral load monitoring of HCV patients under therapy.
Disclosure Statement
The authors declare that they have no conflicts of interest relevant to the manuscript submitted.
References
- 1.European Union Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Official Journal of the European Union. 2004;102:48–58. [Google Scholar]
- 2.European Union Commission Directive 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Official Journal of the European Union. 2006;38:40–52. [Google Scholar]
- 3.Verordnung über die Anforderungen an Qualität und Sicherheit der Entnahme von Geweben und deren Übertragung nach dem Transplantationsgesetz (TPG-Gewebeverordnung – TPG-GewV). Fassung vom 26. März 2008;BGBl. I 1998;512.
- 4.Paul-Ehrlich-Institut Beschluss der Bundesärztekammer vom 14.02.2014: Richtlinie zur Gewinnung von Spenderhornhäuten und zum Führen einer Augenhornhautbank. Dtsch Arztebl. 2014 DOI: 10.3238/arztebl.2014.rl_augenhornhautbank_01. [Google Scholar]
- 5.Heim A, Wagner D, Rothämel T, Hartmann U, Flik J, Verhagen W. Evaluation of serological screening of cadaveric sera for donor selection for cornea transplantation. J Med Virol. 1999;58:291–295. doi: 10.1002/(sici)1096-9071(199907)58:3<291::aid-jmv16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Miédougé M, Chatelut M, Mansuy JM, Rostaing L, Malecaze F, Sandres-Sauné K, Boudet F, Puel J, Abbal M, Izopet J. Screening of blood from potential organ and cornea donors for viruses. J Med Virol. 2002;66:571–575. doi: 10.1002/jmv.2183. [DOI] [PubMed] [Google Scholar]
- 7.Wilkemeyer I, Pruss A, Kalus U, Schroeter J. Comparative infectious serology testing of pre- and post-mortem blood samples from cornea donors. Cell Tissue Bank. 2012;13:447–452. doi: 10.1007/s10561-012-9326-0. [DOI] [PubMed] [Google Scholar]
- 8.José M, Curtu S, Gajardo R, Jorquera JI. The effect of storage at different temperatures on the stability of Hepatitis C virus RNA in plasma samples. Biologicals. 2003;31:1–8. doi: 10.1016/s1045-1056(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 9.Sener K, Yapar M, Bedir O, Gül C, Coskun O, Kubar A. Stability of hepatitis C virus RNA in blood samples by TaqMan real-time PCR. J Clin Lab Anal. 2010;24:134–138. doi: 10.1002/jcla.20354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gessoni G, Barin P, Valverde S, Giacomini A, Di Natale C, Orlandini E, Arreghini N, De Fusco G, Frigato A, Fezzi M, Antico F, Marchiori G. Biological qualification of blood units: considerations about the effects of sample's handling and storage on stability of nucleic acids. Transfus Apher Sci. 2004;30:197–203. doi: 10.1016/j.transci.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Grant PR, Kitchen A, Barbara JA, Hewitt P, Sims CM, Garson JA, Tedder RS. Effects of handling and storage of blood on the stability of hepatitis C virus RNA: implications for NAT testing in transfusion practice. Vox Sang. 2000;78:137–142. doi: 10.1159/000031171. [DOI] [PubMed] [Google Scholar]
- 12.de Moreau de Gerbehaye AI, Bodéus M, Robert A, Horsmans Y, Goubau P. Stable hepatitis C virus RNA detection by RT-PCR during four days storage. BMC Infect Dis. 2002;2:22. doi: 10.1186/1471-2334-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gessoni G, Barin P, Frigato A, Fezzi M, de Fusco G, Arreghini N, Galli P, Marchiori G. The stability of hepatitis C virus RNA after storage at +4 degrees C. J Viral Hepat. 2000;7:283–286. doi: 10.1046/j.1365-2893.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 14.Allison KM, Faddy HM, Margaritis A, Ismay S, Marks DC. The impact on blood donor screening for human immunodeficiency virus, hepatitis C virus, and hepatitis B virus using plasma from frozen-thawed plasma preparation tubes. Transfusion. 2016;56:449–456. doi: 10.1111/trf.13372. [DOI] [PubMed] [Google Scholar]
- 15.Bekanntmachung des Paul-Ehrlich-Instituts zur beabsichtigten Auflage zur Verminderung des Risikos von Hepatitis C-Virus Kontaminationen in mittels Plasmapherese gewonnenem Frischplasma (vom 16. Juli 1999) Bundesanzeiger. 1999;139:12469. [Google Scholar]
- 16.Paul-Ehrlich-Institut Notification: Prevention of adverse effects of medicinal products Imposition of conditions on the marketing authorisations for cellular blood components and fresh frozen plasma. 2013. http://www.pei.de/SharedDocs/Downloads/vigilanz/haemovigilanz/bescheide/2013-01-07-implementation-minimum-standards-blood-screening-tests.pdf?_blob= publicationFile&v=1 (last accessed October 18, 2016).
- 17.Schulze TJ, Weiss C, Luhm J, Brockmann C, Görg S, Hennig H. Preanalytical stability of HIV-1 and HCV RNA: impact of storage and plasma separation from cells on blood donation testing by NAT. Transfus Med. 2011;21:99–106. doi: 10.1111/j.1365-3148.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann E, Neumann AU, Schmidt JM, Zeuzem S. Hepatitis C virus kinetics. Antivir Ther. 2000;5:85–90. [PubMed] [Google Scholar]
- 19.Nübling CM, Unger G, Chudy M, Raia S, Löwer J. Sensitivity of HCV core antigen and HCV RNA detection in the early infection phase. Transfusion. 2002;42:1037–1045. doi: 10.1046/j.1537-2995.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- 20.Glynn SA, Kleinman SH, Wright DJ, Busch MP. NHLBI Retrovirus Epidemiology Donor Study. International application of the incidence rate/window period model. Transfusion. 2002;42:966–972. doi: 10.1046/j.1537-2995.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 21.Glynn SA, Wright DJ, Kleinman SH, Hirschkorn D, Tu Y, Heldebrant C, Smith R, Giachetti C, Gallarda J, Busch MP. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005;45:994–1002. doi: 10.1111/j.1537-2995.2005.04390.x. [DOI] [PubMed] [Google Scholar]
- 22.Da Silva Cardoso M, Koerner K, Kubanek B. PCR screening in the routine of blood banking of the German Red Cross blood transfusion service of Baden-Wuerttemberg. Infusions Ther Transfusion Med. 1998;25:116–120. [Google Scholar]
- 23.Pruss A, Caspari G, Krüger DH, Blümel J, Nübling CM, Gürtler L, Gerlich WH. Tissue donation and virus safety: more nucleic acid amplification testing is needed. Transpl Infect Dis. 2010;12:375–386. doi: 10.1111/j.1399-3062.2010.00505.x. [DOI] [PubMed] [Google Scholar]
- 24.Tugwell BD, Patel PR, Williams IT, Hedberg K, Chai F, Nainan OV, Thomas AR, Woll JE, Bell BP, Cieslak PR. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143:648–654. doi: 10.7326/0003-4819-143-9-200511010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Heck E, Dingrando A, Proctor C, Cavanagh HD. Viral HCV RNA reactivity of corneal cells in plasma HCV nucleic acid-positive eye donors. Cornea. 2013;32:506–507. doi: 10.1097/ICO.0b013e318268d6d6. [DOI] [PubMed] [Google Scholar]