Abstract
Mounting evidence indicates that Wnt signaling is relevant to pathophysiology of diverse mental illnesses including schizophrenia, bipolar disorder, and autism spectrum disorder. In the 35 years since Wnt ligands were first described, animal studies have richly explored how downstream Wnt signaling pathways affect an array of neurodevelopmental processes and how their disruption can lead to both neurological and behavioral phenotypes. Recently, human induced pluripotent stem cell (hiPSC) models have begun to contribute to this literature while pushing it in increasingly translational directions. Simultaneously, large-scale human genomic studies are providing evidence that sequence variation in Wnt signal pathway genes contributes to pathogenesis in several psychiatric disorders. This article reviews neurodevelopmental and postneurodevelopmental functions of Wnt signaling, highlighting mechanisms, whereby its disruption might contribute to psychiatric illness, and then reviews the most reliable recent genetic evidence supporting that mutations in Wnt pathway genes contribute to psychiatric illness. We are proponents of the notion that studies in animal and hiPSC models informed by the human genetic data combined with the deep knowledge base and tool kits generated over the last several decades of basic neurodevelopmental research will yield near-term tangible advances in neuropsychiatry.
Keywords: Animal model, Autism, Bipolar disorder, Genes, Glycogen synthase kinase 3, Induced pluripotent stem cells, Lithium, Molecular psychiatry, Neurodevelopment, Schizophrenia, Wnt signaling
Introduction
The importance of uncovering the molecular etiologies of psychiatric disorders cannot be overstated. Millions of people in the US suffer from mental illness, corresponding to an annual economic cost of over USD 300 billion [1], and rates and proportional economic burden in other countries are likely to be similar. Although psychiatric disorders have complex multifactorial etiologies, mounting evidence indicates Wnt signaling as one mechanistic link between symptomatologically diverse mental illnesses, including schizophrenia (Scz), bipolar disorder (BD), and autism spectrum disorder (ASD). The idea that altered Wnt signaling contributes to mental illness originated in part from the discovery approximately 2 decades ago that the mood-stabilizing drug lithium inhibits glycogen synthase kinase 3 (GSK3) to mimic activation of the Wnt/β-catenin signaling pathway [2]. Even before that and increasingly since, animal studies have revealed how Wnt proteins and pathways govern a wide variety of neurodevelopmental processes and how their disruption can lead to both neurological and behavioral phenotypes. The advent of human induced pluripotent stem cell (hiPSC) models is contributing to further expansion of knowledge in this area. Simultaneously, recent large-scale human genomic studies in several psychiatric disorders, most notably ASD, provide strong genetic support for the participation of Wnt signaling in psychiatric pathophysiology.
While the general significance of Wnt signaling in psychiatry is now hard to deny, relating specific neurodevelopmental pathway roles to the pathogenesis of specific psychiatric disorders remains challenging. To start with, there is the heterogeneity of Wnt signaling itself: There are 19 separately encoded Wnt ligand loci within the genomes of mammals, encoding secreted proteins that activate either canonical or noncanonical Wnt pathways (explained further below) depending on the receptor and intracellular molecular context of responding cells. Wnt signals are transduced through diverse intracellular signaling proteins to affect transcription of target genes, cytoskeletal dynamics, and other cellular processes. Wnt signaling is active in the central nervous system (CNS) from its earliest developmental stages into adulthood, orchestrating a broad range of neurodevelopmental and some postneurodevelopmental processes including CNS regionalization, neural progenitor differentiation, neuronal migration, axon guidance, dendrite development, synaptogenesis, adult neurogenesis, and neural plasticity. Moreover, the heterogeneity of Wnt signaling intersects with the heterogeneity of psychiatric pathogenesis. In the preceding list, every neurodevelopmental and neuroplastic process has been suggested, if not empirically implicated, as a potential pathogenic factor in behavioral illnesses [3,4]. Given the broad array of Wnt pathway-influenced neurodevelopmental and postneurodevelopmental processes that could theoretically affect psychiatric pathology, it is understandable to feel daunted by the task of linking specific roles to pathogenesis in a way that can lead to therapeutic progress. But we believe that such pessimism is unwarranted. Instead, we believe that strategies combining contemporary human genomic findings with empirical studies in animal and hiPSC models, building on prior decades of basic research on Wnt signaling in neurodevelopment, can produce tangible, near-term advances in neuropsychiatry. The intent of this review is to facilitate this by framing Wnt signaling in the context of its various neurodevelopmental and postneurodevelopmental functions, highlighting mechanisms whereby its disruption might contribute to psychiatric illness.
Brief Overview of WNT Signaling
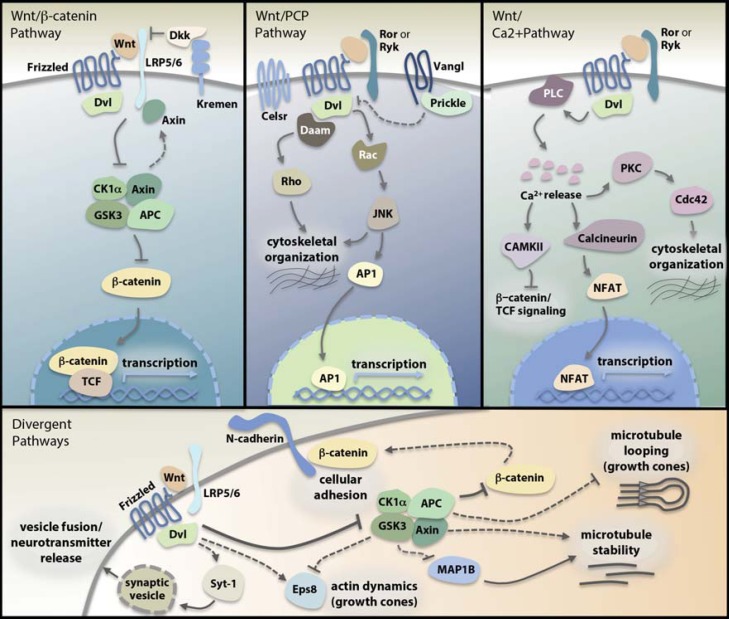
In this section, we will provide a general overview of Wnt pathways (Fig. 1), although greater detail can be found in reviews dedicated to pathway mechanisms [5,6,7,8,9]. Wnt proteins are secreted, cysteine-rich glycolipoproteins that function as paracrine signaling molecules to affect target cells [6,10]. Signaling pathways activated by Wnts govern diverse developmental processes and regulate adult tissue homeostasis through many cellular responses, including proliferation, differentiation, migration, and apoptosis. Although often discussed as linear pathways and referred to as either “canonical” (β-catenin-dependent) or “noncanonical” (β-catenin-independent), Wnt signaling can also be thought of more holistically as a network of interconnected biochemical cascades; that is, Wnt-receptor binding may simultaneously activate several cross-regulated intracellular pathways to elicit a coordinated change in cellular state [11,12,13].
Fig. 1.
Schematic summary of Wnt pathways (see text).
The originally described, and still molecularly best-characterized, “canonical” Wnt/β-catenin pathway hinges on regulated proteolysis of cytoplasmic β-catenin (Fig. 1a). In the absence of Wnt pathway activation, the vast majority of β-catenin protein in a cell is localized to the submembranous space where it complexes stably with cadherin proteins involved in cell adhesion at adherens junctions and synapses; free cytoplasmic β-catenin levels are kept low through constitutive activity of a multiprotein “β-catenin destruction complex” composed of the scaffold proteins axin and adenomatous polyposis coli (APC) plus the kinases casein kinase 1α (CK1α) and GSK31. Once bound to the complex, β-catenin is sequentially phosphorylated by CK1α and GSK3, leading to its ubiquitination and proteasome-mediated degradation. Binding of extracellular Wnt ligand to its co-receptors, Frizzled (Fzd) and low-density lipoprotein receptor-related protein 5 (LRP5) or LRP6, results in phosphorylation of the intracellular scaffold protein Dishevelled (Dvl) and its translocation to the receptor complex accompanied by axin, which is thereby removed from the destruction complex. These translocation events contribute to Wnt-induced Fzd/LRP5/6 clustering, formation of signalosomes comprised of axin, Dvl and a number of other proteins [15,16], and destabilization of the β-catenin destruction complex. Without the destruction complex to facilitate interactions between β-catenin and its 2 Wnt-pathway kinases, phosphorylation of β-catenin by CK1α and GSK3 is greatly reduced, and cytoplasmic β-catenin no longer efficiently degraded. β-Catenin that has not been phosphorylated by GSK3 accumulates and translocates to the nucleus where it interacts with members of the T-cell factor (TCF)/lymphoid enhancer factor family of transcription factors to regulate transcription of specific Wnt pathway target genes.
There are also divergent biochemical cascades that can be initiated simultaneously with canonical Wnt signaling by the same ligand-receptor interaction but that do not depend on β-catenin (Fig. 1d). An example is a pathway described in developing neurons in which dissociation of the destruction complex not only reduces phosphorylation of β-catenin but also phosphorylation of microtubule associated protein 1B (MAP1B), thereby leading to posttranslational changes in microtubule organization and stability important for growth cone guidance and presynapse formation [17,18]. Other molecular changes facilitated by divergent Wnt pathways include: Dvl binding of synaptic vesicle proteins to facilitate neurotransmitter release [19]; Dvl modulation of the actin-binding protein Eps8 (epidermal growth factor receptor pathway substrate 8) to regulate actin dynamics critical for axon growth [20]; dissociation of APC from plus ends of microtubules to facilitate growth cone mobility [21]; and formation of β-catenin/cadherin complexes at the plasma membrane to promote cell-cell adhesion.
Separate Wnt ligand-receptor interactions lead to other pathways. In the noncanonical Wnt/planar cell polarity (PCP) pathway, Wnt-Fzd binding, at least in vertebrates, can lead to downstream activation of c-Jun N-terminal kinase (JNK), RhoA and Rac1, causing changes in both actin and microtubule-associated cytoskeletal dynamics (Fig. 1b). Core components of this pathway include several transmembrane proteins unique to it, particularly Van Gogh/strabismus (Vangl) and Flamingo (Celsr1-3) – plus a set of submembranously localized scaffold proteins that partially overlap with the Wnt/β-catenin pathway, including Dvl, Diego, and Prickle. These transmembrane and submembranous components engage in both cooperative and competitive interactions leading to their polarized distribution within the cell; one of the critical roles of the pathway, for which it is named, is the establishment of transverse (planar) polarity of epithelial cells and regulation of intercalating “convergent-extension” cell movements during certain developmental processes.
In the noncanonical Wnt/Calcium (Ca2+) pathway, Wnt-Fzd binding regulates phospholipase C in a Dvl-dependent manner (Fig. 1c). This catalyzes an IP3/DAG-dependent increase in intracellular Ca2+ levels with consequent activation of protein kinase C (PKC), calcineurin, and Ca2+/calmodulin-dependent protein kinase II (CaMKII). Activated PKC phosphorylates the small GTPase Cdc42 regulating organization of the actin cytoskeleton. Calcineurin promotes nuclear import of nuclear factor of activated T-cells (NFAT), a transcription-activating protein. CAMKII activates a pathway that inhibits nuclear β-catenin, thereby inactivating Wnt/β-catenin transcriptional activity. The increase in cytosolic Ca2+ has also recently been linked to production of nitric oxide (NO) in neurons, which influences synaptic excitability through modulation of potassium (K+) channels [22].
Finally, in the Frizzled-nuclear import (FNI) pathway which thus far has only been described at the Drosophila melanogaster (fruit fly) neuromuscular junction [23,24,25], Wnt-Fzd binding causes internalization and cleavage of Fzd. Subsequent nuclear trafficking of a transcriptionally active Fzd cleavage fragment depends on its interaction with 7-PDZ-domain glutamate-receptor interacting protein (GRIP) [24,26].
Neural Plate Specification and Neural Tube Formation
Wnt signaling is required for proper patterning of the CNS in vertebrates beginning at the earliest stages of neural development [27,28], involving specification of the ectodermally derived neural plate and its subsequent folding to form the neural tube (Table 1). Although signaling defects during these early stages are often associated with gross neurological defects, when considering behavioral disorders and their treatment (as well as potential side effects of treatment), it is important to have a firm grasp of Wnt signaling at all stages of neural development.
Table 1.
Summary of Wnt pathway genes associated with neural plate specification and neural tube formation
| Process | Wnt pathway | Gene | Ref. | |
|---|---|---|---|---|
| Neural plate | Wnt/β-catenin | Wnt3A | [30, 31] | |
| specification | β-catenin | [30, 31] | ||
| Wnt8 | [29] | |||
| Tcf3 | [32] | |||
| Wnt8c | [33] | |||
| Dkk1 | [35, 36, 37] | |||
| Sp1 | [39] | |||
| Neural tube formation | Wnt/β-catenin | Axin | [53] | |
| Tcf3 | [54] | |||
| LRP6 | [55, 56, 57, 58, 59] | |||
| Wnt/PCP | Dact1 | [52] | ||
| Scribble | [41, 47, 50] | |||
| Celsr1 | [40, 47, 51] | |||
| Dvl1/2/3 | [44, 49] | |||
| Vangl1/2 | [43] | |||
Some of the first experiments implicating Wnt/β-catenin signaling in neural plate specification illustrated its caudalizing (posteriorizing) effects. Experiments using Xenopuslaevis (African clawed frog) animal caps demonstrated that ectopic expression of either Wnt3a or β-catenin induces posterior neural markers and suppresses rostral (anterior) neural markers, whereas expression of a dominant-negative form of Wnt8 inhibits posterior markers [29,30,31]. Subsequent genetic experiments showed the extent to which gross abnormalities can result from disrupted Wnt signaling at early developmental stages. This includes analysis of the Danio rerio (zebrafish) null mutation in Tcf3, called headless (hdl) because it leads to absence of anterior neural structures [32]. Similar posteriorizing effects are caused by transgenically overexpressing Wnt8c in Mus musculus (laboratory mice), leading to absence of the anterior forebrain [33]. During normal development, Wnt ligands secreted by the underlying paraxial dorsolateral mesoderm establish a signaling gradient that helps specify the anterior-posterior axis of the neural plate [34]. Expression of the secreted Wnt antagonist, Dickkopf1 (Dkk1), ensures anterior inhibition of Wnt signaling: loss of Dkk1 expression yields a posteriorized phenotype reminiscent of Wnt overexpression [35,36,37,38]. Additional gain-of-function and loss-of-function (LOF) analyses in zebrafish suggest Wnt/β-catenin signaling establishes posterior neuroectoderm identities at least in part by promoting expression of the Sp1 family transcription factors Sp5 and Sp5-like [39].
After specification, cells within the neural plate undergo morphogenetic movements giving rise to lateral upwellings of the neural plate called neural folds. The folds from each side eventually merge and fuse at the dorsal midline, converting the initially flat neural plate into a neural tube which develops further to become spinal cord and brain. Disruptions in this process cause neural tube defects (NTDs), which range in severity and include conditions such as spina bifida, anencephaly, and craniorachischisis. Mice with genetic disruptions in critical modulators of Wnt/PCP signaling, including Dact1 (Dapper, Frodo), Scribble (Scrib), cadherin EGF LAG seven-pass G-type receptor 1 (Celsr1), Dvl2, Vangl1, and Vangl2, have highly penetrant NTD phenotypes [40,41,42,43,44]. Importantly, human genomic studies of patients with NTDs have also revealed mutations in several of these same Wnt/PCP pathway genes [45,46,47,48,49,50,51,52]. A role for the Wnt/β-catenin pathway is indicated in this process as well. For example, mice with LOF mutations in either Axin1 or Tcf3 exhibit incomplete neural tube closure [53,54], and various mutations in LRP6 cause NTDs in mice [55,56,57], and are present in human NTD patients [58,59]. Recent evidence suggests defects in this case may be due in part to inappropriate fate specification of the laterally placed neural plate border cells governed by the Wnt/β-catenin pathway [60]. Furthermore, there may be a biochemical connection between the Wnt/β-catenin and Wnt/PCP pathways in facilitating neural tube closure. While the LRP6 receptor is generally thought to be dedicated to Wnt/β-catenin signaling, recent data show that NTD-associated LRP6 mutations decrease Wnt/β-catenin activity while simultaneously increasing Wnt/PCP activity [58]. This supports the notion that these seemingly separable Wnt pathways engage in reciprocal inhibition and other forms of cross talk to form integrated signaling networks involved in developmental decision-making [13,61]. Similar observations have been made in other neurodevelopmental contexts (cf. Neural Precursor Migration, below).
Brain Regionalization
At the stage of the early germinal neuroepithelium, Wnt genes and Wnt pathway modulators are expressed in overlapping spatial and temporal patterns [62,63,64] and are critical for establishing signaling centers that inform regional identities along the rostral-caudal and dorsal-ventral axes of the developing CNS [65,66,67](Table 2). Structural and functional impairments in the forebrain (derived from telencephalon and diencephalon), midbrain (derived from mesencephalon), and hindbrain (derived from metencephalon and myelencephalon) have all been associated with neuropsychiatric pathogenesis [68,69,70].
Table 2.
Summary of Wnt pathway genes associated with brain regionalization
| Pathway/brain region | Gene | Ref. |
|---|---|---|
| Uncharacterized | ||
| Forebrain | Wnt2b | [73, 74] |
| Wnt3a | [73, 74] | |
| Wnt5a | [73] | |
| Wnt7b | [75] | |
| Wnt8b | [75] | |
| Wnt/β-catenin | ||
| Forebrain | Lef/Tcf | [79] |
| Forebrain, midbrain, hindbrain | LRP6 | [57, 80, 89] |
| Midbrain, hindbrain | Wnt1 | [82, 83, 84, 91] |
| Midbrain, hindbrain, spinal cord | β-catenin | [85, 86] |
| Midbrain | Fzd3 | [87] |
| Fzd6 | [87] | |
Uncharacterized: genes characterized by expression data only.
Rostrally, Wnt signaling is integrally involved in development of the forebrain [71], arguably the most physiologically complex structure in vertebrate anatomy. Wnt-mediated dorsal-ventral patterning in the telencephalon has been demonstrated by analysis of Foxg1-mediated transcriptional repression of Wnts in zebrafish, showing that loss of Wnt/β-catenin signaling prevents cortical specification [72]. Wnt expression (Wnt2b, Wnt3a, Wnt5a, Wnt7b, and Wnt8b) and β-catenin-dependent signaling activity is high in the cortical hem, a forebrain signaling center that instructs hippocampal development [73,74,75,76]. Consistent with a patterning role for Wnts secreted from this source, mutant mice with no functional Gli3, a transcription factor necessary for Wnt expression in the cortical hem, lack dorsomedial telencephalic Wnt/β-catenin signaling activity and fail to develop hippocampi [73,77,78]. This phenotype has been reproduced through inhibition of lymphoid enhancer factor/TCF signaling, underscoring the requirement for Wnt/β-catenin signaling in hippocampal development [79]. Finally, characterization of LRP6 mutant mice has not only confirmed a requirement for Wnt/β-catenin signaling in cortical and hippocampal patterning [80], but has further suggested a role in development of the dorsal thalamus and thalamocortical neural circuitry: only a small, disorganized dorsal thalamus lacking most major thalamic nuclei and thalamocortical projections develops in mice lacking LRP6 [81].
Impairing the Wnt/β-catenin pathway also disrupts midbrain and hindbrain regionalization. In mice, LOF mutations in Wnt1 lead to complete lack of midbrain and cerebellum [82,83,84]. Conditional deletion of β-catenin mimics these defects [85,86], LRP6 mutants partially mimic the Wnt1 mutant phenotype [57], and Fzd3/Fzd6 double mutants also display disruptions in midbrain development [87]. Moreover, several studies, including LRP6 mutant analysis, overexpression of β-catenin, and ablation of β-catenin support a requirement for Wnt/β-catenin signaling in neurogenesis of midbrain dopaminergic neurons in the mouse [88,89,90,91].
The Wnt/β-catenin pathway plays critical roles in patterning the most caudal component of the CNS, the developing spinal cord. For example, in the developing spinal cord of Gallus gallus (chick) embryos, ectopic expression of mutant-stabilized (dominant-active) β-catenin causes overexpression of neural patterning genes including Olig3 in neural precursor cells (NPCs) [92]. The presence of an Olig3 LOF mutation in the context of this dominant-active form of β-catenin leads to a decrease in a subset of dorsal interneurons, indicating that Wnt/β-catenin pathway-induced transcription of Olig3 is required for specification of these neuronal subtypes [92]. At the same time, Wnt/β-catenin signaling inhibits differentiation of oligodendrocytes in the developing ventral spinal cord, as evidenced by increased generation of these cells in response to ectopic expression of a Wnt antagonist [93].
Neural Stem and Precursor Cell Proliferation
Right from the discovery of the first Wnt gene in vertebrates, Wnt signaling has been associated with the regulation of cell division. The first vertebrate Wnt gene was identified because it acts as a mammary oncogene when pathogenically activated by a tumor virus in mice [94]. Since that time, a large number of Wnt genes and other core Wnt/β-catenin pathway components have been strongly implicated in oncogenesis – more specifically in mitogenesis – when improperly activated [95,96]. So, it is not surprising that one of the physiological roles of Wnt signaling in the CNS is to regulate NPC proliferation (Table 3).
Table 3.
Summary of Wnt pathway genes associated with neural precursor cell or neural stem cell proliferation
In fact, the gradient of NPC mitotic activity in the developing neural tube directly corresponds to a gradient of Wnt/β-catenin signaling (dorsal-high to ventral-low) established by Wnt1 and Wnt3a secreted from the dorsal midline [63,82,97]. As discussed earlier, genetic studies in mice have established that loss of Wnt1 prevents midbrain development [82,83], loss of Wnt3a prevents hippocampal development [98], and loss of both Wnt1 and Wnt3a together cause additional defects in the caudal diencephalon, rostral hindbrain, and cranial and spinal ganglia [99].
Experimental dissection of phenotypes resulting from loss of β-catenin or exposure to the Wnt antagonist Dkk1 in the developing telencephalon has demonstrated an interwoven role for Wnt/β-catenin signaling in both NPC proliferation and dorsal-ventral patterning [100,101]. Similarly, in the metencephalon, Wnt/β-catenin signaling is thought to regulate both the growth and differentiation of NPCs [102,103]. In toto, data support important roles for this pathway in both brain regionalization and cell division, revealing that many Wnt ligands are partially redundant in these processes.
The gross brain regionalization and proliferative defects caused by loss of Wnt1 and Wnt3a have led to experiments designed to dissect out functions of Wnt/β-catenin signaling specifically in NPC proliferation. Supporting a role in proliferation per se, studies in the chick neural tube have revealed that ectopic expression of Wnt1 significantly increased numbers of NPCs [97,104]. Similarly, NPC proliferation increased upon introduction of dominant-active β-catenin in the developing chick spinal cord, mouse neocortex or mouse cerebellum [97,103,105,106,107]. Conversely, expression of dominant-negative Tcf4 cell autonomously blocked entry of NPCs into the S phase in the developing chick spinal cord [97]. LOF analysis conducted through ablation of β-catenin in the mouse neocortical ventricular zone caused premature cell cycle exit and differentiation [108,109], while deletion of GSK3 led to increased proliferation and decreased differentiation of neural progenitors along the mouse neuraxis [110]. Further support comes from in utero electroporation of inhibitory RNA (RNAi) for disrupted in schizophrenia-1 (DISC1) or Dix domain containing-1 (Dixdc1), positive regulators of Wnt/β-catenin signaling; their reduction by RNAi reduces NPC proliferation in the developing mouse cerebral cortex [111]. Finally, studies in culture strongly support the critical importance of Wnt/β-catenin signaling in promoting neural stem cell (NSC) proliferation [103,112] and, coming full circle back to the proto-oncogenic effects of Wnt, hyperactivated Wnt/β-catenin signaling is a primary cause of the aggressive pediatric brain tumor, medulloblastoma [113]. In fact the cell-cycle regulator N-myc, itself a proto-oncogene, is a downstream effector of Wnt pathway-induced NPC proliferation: conditional deletion of N-myc prevents β-catenin-induced NPC proliferation in vitro [114]. In the concluding section of this review, we discuss the therapeutic implications of links between the Wnt/β-catenin pathway and cancer.
In the last decade, it has become increasingly clear that neural proliferation continues in some brain areas into adulthood; moreover, that disruptions in adult neurogenesis are linked to psychiatric disorders (as well as neurodegenerative diseases) [115,116], whereas enhancing neurogenesis may be an important mechanism of action for antidepressant and antipsychotic drugs [117,118,119,120]. Wnt signaling is involved in adult neurogenesis of the 2 identified NSC populations in the adult brain (reviewed in [121,122]), which reside within the subventricular zone (SVZ) of the lateral ventricles and subgranular zone (SGZ) of the hippocampal dentate gyrus (DG). Inhibition of Wnt/β-catenin signaling in cultured adult hippocampal progenitors depletes the number of multipotent progenitors, indicating a requirement of this pathway for NSC maintenance [123,124]. Similarly, in vivo inhibition of Wnt/β-catenin in the DG of adult mice or rats causes reduced NSC proliferation in the SGZ [123,125], and Wnt7a null mice exhibit decreased NSC proliferation in both the SVZ and SGZ [126]. Thus, Wnt signaling affects the proliferation of neural stem and progenitor cells throughout embryonic and adult life with potential relevance to both psychopathology and therapeutics.
Neural Precursor Migration
After their final mitotic division, most early NPCs undergo “radial” migration out of the ventricular zone in close apposition to the cytoneme-like projections of radial glia extending perpendicularly to the neural tube surface (Table 4). In doing so, migrating NPCs establish a basic layered architecture comprised of mantle (gray matter) and marginal (white matter) zones throughout the CNS. The spinal cord retains this basic 2-layer organization, whereas the brain's more complex neural architecture arises from far more complicated and highly derived patterns of NPC migration. For instance, in the developing neocortex, sequential waves of NPC migration give rise to 6 cortical layers populated by neurons with distinctive molecular signatures, functional properties, connectivity patterns, and synaptic targets. Moreover, some NPCs residing in circumscribed regions of the SVZ give rise to specialized neuronal subtypes that disperse more broadly in the cortex via additional migratory mechanisms (such as cortical GABAergic interneurons that arise from the ventrally located ganglionic eminences and travel to their final destinations via tangential migration followed by either inward or outward radial migration [127]). Changes in the temporal or spatial program of NPC migration affect the coordinated development of presynaptic and postsynaptic connections, thereby disrupting neural networks. On this basis, it can be expected to lead to both neurologic diseases (e.g., epilepsy) and psychiatric disorders (e.g., behavioral/cognitive/emotional dysregulation).
Table 4.
Summary of Wnt pathway genes associated with neural precursor migration
Wnt signaling has been linked to neural and glial cell migration in diverse systems. This includes the lateral line primordium, a group of cells giving rise to a sensory organ in zebrafish, and in which Wnt/β-catenin signaling influences the expression of chemokine receptors necessary for proper cell migration [128]. In cultured glioblastoma cells, Wnt5a signals through a noncanonical pathway to affect expression of matrix metalloproteinase 2 (MMP-2) [129], a protein that facilitates migration (tumor invasion) via degradation of extracellular matrix components. In both mice and humans, Wnt signaling participates in the migration of oligodendrocyte precursor cells that develop into the myelinating cells of the CNS, again by regulating expression of a chemokine receptor (Cxcr4) [130]. The Wnt/PCP pathway also plays a major role in the extensive migrations of neural crest cells [131,132] that give rise to most of the peripheral nervous system (PNS) and also contribute critical cell types to many other tissues in all vertebrates.
Studies over the last 5 years have revealed that Wnt signaling participates in migration of NPCs in the developing cerebral cortex and cerebellum. Analyses of pyramidal neuron precursors in the ventricular zone of developing mouse neocortex have demonstrated critical interconnected roles of the Wnt/PCP and Wnt/β-catenin pathways in the regulation of NPC migration. Experiments using both constitutive and conditional genetic approaches to target Wnt5a-mediated PCP signaling and β-catenin/TCF-dependent Wnt signaling support a model in which the Wnt/PCP pathway inhibits the Wnt/β-catenin pathway to promote transcriptional activation of ephrin-B1, a transmembrane protein critical for pyramidal precursor migration [133]. In addition to its effects on NPC proliferation, reduction of DISC1, a positive regulator of Wnt signaling, disrupts the tangential migration of cortical interneurons [134,135], at least in part by influencing filamentous F-actin assembly critical for filopodial and lamellopodial dynamics [134]. Similarly, knockdown of Tcf4 in cortical progenitor cells impairs the formation of actin-rich leading processes, thereby inhibiting neuronal migration [136]. In vitro cell migration assays using mouse NSCs indicate that Wnt/β-catenin signaling also promotes cell migration via cross talk with prostaglandin E2 (PGE2) signaling. This pathway leads to transcriptional upregulation of Ctnnb1, Ptgs2, Ccnd1, Mmp9 [137] – all of which, likely not coincidentally, have been linked to the etiopathology of ASD and Scz [138,139,140,141,142,143,144,145].
Axon Pathfinding
As neurons migrate to their final destination, a next critical phase of their maturation is the formation, elongation, and targeting of a (typically single) axon (Table 5). Depending on the neuronal subtype and its function, this highly specialized presynaptic neurite may extend over extremely long anatomical distances to connect with cellular targets locally, in another region of the CNS, in the PNS, or even outside the nervous system proper. The development of functional neural circuits governing thought and behavior relies in large measure on precisely guided outgrowth and pathfinding of axons to reach specific target cells. On this basis, dysregulated axon guidance is a candidate neurodevelopmental mechanism that could conceptually contribute to a wide range of behavioral disorders [146,147,148,149,150,151].
Table 5.
Summary of Wnt pathway genes associated with axon pathfinding
| Cell type | Pathway (regulatory target) | Gene | Ref. |
|---|---|---|---|
| Ventral midbrain DA neurons | Wnt/β-catenin | Wnt7a | [173] |
| Cerebellar granule cells | Divergent “canonical” (MAP1B) | Wnt7a | [169, 170, 171] |
| DRG neurons | Divergent “canonical” (Eps8) | Dvl | [20] |
| Unknown (GSK3-dependent) | Wnt3 | [172] | |
| Spinal commissural neurons | Wnt/PCP | Wnt4 | [158] |
| Wnt5a | [158, 159] | ||
| Wnt7b | [158] | ||
| Fzd3 | [158, 159] | ||
| Celsr3 | [159] | ||
| Vangl2 | [159] | ||
| Dvl1 | [159] | ||
| Brainstem monoaminergic neurons | Wnt/PCP | Fzd3 | [157] |
| Celsr3 | [157] | ||
| Vangl2 | [157] | ||
| Prethalamic neurons | Wnt/PCP | Fzd3 | [161] |
| Celsr3 | [161] | ||
| Cortical neurons (corticospinal and callosal axons) | Wnt/Ca2+ | Wnt5a | [155, 174, 175, 176, 177] |
| Ryk | [174, 175, 176, 177] | ||
Divergent “canonical”: divergent Wnt pathway genes.
The developing axons of many neurons receive positional information in the form of extracellular Wnt gradients [152,153]. The growing axon is tipped with a highly motile lamellopodial and filopodial structure called the growth cone responsible for detecting and responding to extracellular guidance cues that direct axon outgrowth. As receptors on the surface interact with extracellular cues, the growth cone rapidly translates extracellular gradients into intracellular signals driving changes in cytoskeletal organization to directionally guide axon extension [154]. Wnt receptors expressed on growth cones couple extracellular Wnt gradients to downstream pathways mediating directed axon outgrowth [155,156,157].
Wnt/PCP pathway-mediated axon guidance was initially observed in spinal cord commissural neurons [158]. In the vertebrate spinal cord, commissural neurons first project axons ventrally toward the floor plate, where they turn and move rostrally or caudally after crossing the midline (“postcrossing”). Analyses using open book explants of developing Rattus norvegicus (rat) spinal cords showed that postcrossing commissural axons are directed by gradients of Wnt4, Wnt5a, and Wnt7b along the rostral-caudal axis [158]. This effect was at least partially dependent on Fzd3-mediated Wnt/PCP signaling; loss of Fzd3 caused postcrossing commissural neurons to project randomly, while loss of LRP6 had no effect on guidance of commissural neurons [158]. Genetic studies demonstrated that other core Wnt/PCP pathway components are also required for commissural axon guidance, including Celsr3, Vangl2, and Dvl1 [159]. Further experiments using rat spinal cord open book explants as well as dissociated cultures of commissural neurons have revealed a unique molecular mechanism governing Wnt/PCP pathway-related growth cone guidance [159]. Dvl1, a protein usually associated with Fzd activation, inhibits Fzd3-mediated PCP signaling by promoting Fzd3 phosphorylation, preventing its internalization and concomitant intracellular signal transduction [159]. However, in response to Fzd3-Wnt5a binding, Vangl2 antagonizes the inhibitory effect of Dvl1, preventing Fzd3 phosphorylation and promoting transduction of the Wnt5a signal via JNK activation [159]. This mechanism allows for a localized gradient-sensitive response of the growth cone to extracellular Wnt5a.
In addition to spinal cord commissural neurons, the Wnt/PCP pathway facilitates axon guidance in many other neuronal subtypes [156]. In mice, genetic deletion of Fzd3 caused aberrant axon guidance in subpopulations of cranial and spinal motor neurons [160], and deletion of Celsr3, Vangl2, or Fzd3 each caused axon pathfinding defects in monoaminergic neurons of the developing brainstem [157]. Furthermore, conditional deletion of either Fzd3 or Celsr3 in subpopulations of neurons in the early ventral telencephalon and prethalamus led to disrupted thalamocortical axon guidance [161,162]. Interestingly, malfunctions in brainstem monoaminergic neurons and thalamocortical interactions have been associated with Scz, BD, and ASD [163,164,165,166,167,168].
Instead of the Wnt/PCP pathway, Wnt7a is thought to regulate axon guidance through activation of both Wnt/β-catenin and distinctly divergent Wnt pathways in different neuronal subtypes. In cerebellar granule cells, recombinant Wnt7a expression induced axonal spreading, axonal branching, and increased growth cone size and complexity in vitro [169,170,171]. Deletion of Wnt7a in mice caused simpler, less mature glomerular rosettes [170], a structural hallmark of the cerebellar mossy fiber-granule cell synapse. Investigation of the Wnt7a signaling mechanism indicated that, rather than phosphorylation of β-catenin, axon growth mediated by Wnt7a depends on regulation of GSK3-mediated phosphorylation of microtubule-associated proteins including MAP1B to affect cytoskeletal changes [17]. This is an example of a divergent Wnt signaling pathway using upstream components of the Wnt/β-catenin pathway but not transcriptional regulation via β-catenin to achieve its effects [18]. Another instance of pathway divergence was identified using cultured dorsal root ganglion (DRG) neurons; Dvl directly binds the actin-binding protein Eps8, which increases F-actin abundance in DRG growth cones and enables lamellar protrusion [20]. In this study, GSK3 inhibition blocked Wnt-mediated Eps8 activity, indicating involvement of upstream components of the Wnt/β-catenin pathway [20] although stabilized β-catenin does not appear to play a role. Perhaps this is the mechanism by which Wnt3, produced by motor neurons, increases axon branching and growth cone size in neurotrophin-3-responsive DRG sensory neurons – there is evidence these phenotypes are GSK3-dependent [172], though the downstream events remain unclear. In contrast, dopaminergic neurons within the developing ventral midbrain do require Wnt7a-mediated activation of β-catenin transcriptional activity to ensure their axons reach appropriate forebrain targets [173].
Wnt5a is thought to signal through the Wnt/Ca2+ pathway to exert its effect as a repulsive cue for postcrossing corticospinal axons descending from the cerebral cortex along the dorsal spinal cord [174] as well as for cortical axons projecting across the corpus callosum [155]. Corticospinal axons only become sensitive to Wnt-facilitated repulsion after midline crossing, coincident with expression of the Ryk receptor [174]. In vitro studies using mouse cortical explants indicated that Wnt5a repels Ryk-expressing axons [155]. Moreover, when the interaction between Ryk and Wnt5a was blocked by injection of a Ryk antibody [174] or by RNAi-mediated knockdown of Ryk in developing Mesocricetus auratus (hamster) cortical slices [175], postcrossing axons projected randomly, indicating that Ryk is required for their guidance. In vitro analysis of cortical neurons and in vivo analysis of cortical slices showed that the Wnt5a-Ryk interaction causes fluctuations in intracellular calcium [175,176,177]. Moreover, knockdown of Ryk [175] or exposure to CaMKII inhibitors [175,177] caused defects in axon outgrowth and guidance. Taken together, these data suggest that the Wnt5a/Ryk interaction initiates a Wnt/Ca2+ signaling pathway to regulate axon growth and guidance of corticospinal and callosal axons.
Dendritogenesis
Dendritogenesis involves a process called arborization (or ramification) by which dendrites extend from neuronal cell bodies and elaborate to form intricate branching patterns (Table 6). Time-lapse imaging has revealed dendritic arborization to be a highly dynamic process involving extension and retraction of fine filopodial branches, only a fraction of which persist to become enduring components of the dendritic arbor [178,179,180]. The complex networks of dendritic arbors greatly expand the surface area and reach of each neuron allowing reception of electrical inputs from a correspondingly greatly expanded set of axon terminals. Moreover, while inhibitory synapses primarily form along dendritic shafts or directly on the neuronal soma, a large proportion of excitatory axon terminals synapse at dendritic spines – small protrusions decorating the length of dendrites on “spiny” neurons – most of which are themselves excitatory (glutamatergic) pyramidal projection neurons. Dendritic spines are highly dynamic and exhibit a striking degree of experience-dependent structural and functional plasticity [181], as do synapses in general (discussed in the following section). Unsurprisingly, impaired dendrite arborization, spine morphogenesis and dendritic plasticity have been implicated in the pathology of neurodevelopmental and neuropsychiatric disorders through both postmortem neuropathological analyses of affected individuals and studies in animal models [182,183,184,185,186]. In this section, we review Wnt-based studies relating to dendrite development, while the subsequent section focuses on how synapses form between the elaborated (postsynaptic) dendrites and (presynaptic) axon terminals.
Table 6.
Summary of Wnt pathway genes associated with dendritogenesis
| Cell type | Pathway (regulatory target) | Gene | Ref. |
|---|---|---|---|
| Cortical pyramidal neurons | Wnt/β-catenin | TCF4a | [189] |
| Wnt2 | [191] | ||
| Hippocampal pyramidal neurons | Wnt/β-catenin | Wnt2 | [190] |
| Divergent “canonical” (catenin/cadherin) | β-catenin | [187] | |
| Wnt/PCP | Vangl2 | [197, 198, 199] | |
| Wnt7b | [194] | ||
| Dvl | [194] | ||
| Rho | [194] | ||
| JNK | [194] | ||
| Dact1 | [195] | ||
| Cortical GABAergic interneurons | Wnt/PCP | Dact1 | [196, 197] |
| Hippocampal pyramidal neurons | Wnt/Ca2+ | Wnt7a | [206] |
Divergent “canonical”: divergent Wnt pathway genes.
Also affects glutamatergic synapse density – unclear if a primary effect or secondary to dendrite phenotype.
Dendritogenesis in the hippocampus is promoted by Wnt/β-catenin signaling, although not via transcriptional regulation of target genes. Instead, Wnt-mediated stabilization of β-catenin leads to increases in functional β-catenin/cadherin complexes at the membrane [187], facilitating adhesive interactions between presynaptic and postsynaptic elements required for dendrite growth, synapse formation, and maintenance of dendritic arbors [188]. This mechanism was uncovered through analysis of primary rat hippocampal neurons that display increased dendritic branching in direct proportion to the amount of stabilized β-catenin expressed, an effect potentiated by addition of other catenin/cadherin complex members and inhibited by addition of the secreted Wnt antagonist Dkk-1. In this study, expression of dominant-negative forms of Tcf that uncouple Wnt signaling from transcriptional activity had no bearing on dendritic branching [187]. In sum, the effector mechanism implicated by these experiments is that Wnt-dependent β-catenin stabilization facilitates dendritogenesis by directly enabling growing dendrites to make connections with other neurons through enhancement of catenin/cadherin complexes, as opposed to transcriptional changes. In contrast, a recent study has shown that in differentiating mouse cortical neurons, TCF4 restricts dendritic branching by directly regulating the expression of Neurexin loci [189]. Given the general mechanism of Wnt/β-catenin signaling, in which stabilized β-catenin binds to TCFs in the nucleus to switch their transcriptional activity, this finding suggests the possibility that a strictly canonical Wnt signaling pathway may promote dendritogenesis in postmitotic neurons by regulating transcription of Neurexin genes.
In several neuron subtypes, Wnt2 activates the Wnt/β-catenin pathway to mediate dendrite growth in response to upstream triggering events or molecules. For example, an activity-dependent N-methyl-D-aspartate receptor (NMDAR)-mediated Ca2+ signaling pathway leads to transcriptional upregulation of Wnt2 in cultured hippocampal neurons, which then stimulates increased dendritic arborization [190]. Very similarly, brain-derived neurotrophic factor (BDNF), a signaling molecule that promotes dendrite growth and spine formation in most (if not all) differentiating neurons, exerts effects in primary mouse cortical neurons through transcriptional upregulation of Wnt2 [191]; exposure to secreted or cytoplasmic Wnt inhibitors reduced the size of dendritic arbors and prevented BDNF-induced increases in spine density [191]. Wnt2-induced dendrite development is also implicated as a molecular mechanism in neurodevelopmental toxicity caused by the environmental pollutant polychlorinated biphenyl-95 (PCB-95) [183]. Experiments using primary rat hippocampal neurons showed that PCB-95 acts on ryanodine receptors (Ryr) in the endoplasmic reticulum to cause inappropriately high Ca2+ release into the cytosol, leading to cAMP response element binding (CREB)-dependent transcription of Wnt2, which then stimulates the growth of more elaborate dendritic arbors with decreased activity-dependent plasticity [183]. This example is especially noteworthy given the well-accepted but poorly understood interwoven effects of genetics and environment (and indeed other factors – e.g. “nature vs. nurture”) in the pathology of mental illness [192,193]. Specifically, this work empirically demonstrates mechanistic interplay between an environmental factor (i.e., exposure to PCB-95) and a signaling molecule encoded in the genome (WNT2) – with neurodevelopmental consequences (increased but less plastic dendritic arborization) that could plausibly contribute to behavioral symptoms manifesting as a psychiatric condition.
There is also evidence that Wnt/PCP signaling regulates dendrite formation. Hippocampal cultures derived from mice lacking Dvl1 have reduced dendritic arbors, whereas exposure to Wnt7b or overexpression of Dvl1, both normally expressed in the hippocampus, cause increased dendritic branching [194]. Inhibition of downstream modulators of the PCP pathway (Rho and JNK) prevented Dvl1-facilitated outgrowth, whereas manipulation of GSK3 activity had no effect [194]. Further support for the role of Wnt/PCP signaling has come from analysis of Dact1 mutant mice, in which both hippocampal pyramidal neurons [195] and cortical interneurons have simpler dendritic arbors [196,197]. This same phenotype has been observed in hippocampal neurons derived from Vangl2 mutant mice [197], as well as in cultured hippocampal neurons subjected to transient knockdown of Vangl2 [198,199]. Dact1 and Vangl2 are both modulators of Wnt/PCP signaling that function upstream of small GTPases [195,200,201] to influence cytoskeletal changes necessary for dendrite arborization and spine development [202,203,204,205]. Vangl2 also affects dendritogenesis through direct interactions with N-cadherin [198,199], indicating a potential bidirectional mechanism downstream of Wnt/PCP signaling during dendrite development, as well as potential cross talk with the aforementioned promotion of catenin/cadherin complexes contributing to dendritogenesis downstream of the Wnt/β-catenin pathway.
Finally, the Wnt/Ca2+ pathway facilitates dendrite development in the hippocampus. Similar to Wnt7b, Wnt7a is required for hippocampal dendrite development in vivo; however, in this case available data suggest signaling through the Wnt/Ca2+ pathway modulator CaMKII [206]. Wnt7a exposure leads to rapid activation of a postsynaptic CAMKII-reporter in dendritic spines. Moreover, CaMKII inhibitors or Ca2+ chelators prevent Wnt7a-mediated dendrite growth [206]. It is worth noting that while this study provides compelling evidence that Wnt7a activates the Wnt/Ca2+ pathway in dendritic spine formation, this does not preclude that other Wnt pathways may be involved as well. The same caveat applies to most of the foregoing evidence about Wnt signaling across neurodevelopmental events. In many cases, although a single “pathway” may have been experimentally explored by a specific study, other pathways have not been excluded from the process, and more than one Wnt-dependent mechanism or indeed a network of molecularly interconnected biochemical cascades may in fact be involved.
Synapse Formation and Function
As dendrites undergo arborization, they form connections with axon terminals to create synapses, specialized connections that permit rapid electrochemical communication between neurons (Table 7). For a dendritic filopodia to establish a synapse with an axon terminal, the 2 elements must form an initial adhesive interaction that is then replaced by critical synaptic components through a developmentally coordinated process. The elements of this process include presynaptic protein clustering in axonal terminal boutons, postsynaptic protein accumulation in apposing dendritic spines, and transsynaptic dialogue (i.e., intercellular signaling) to coordinate development of the 2 sides of the synapse belonging to different neurons. Considering the significant roles that Wnt signaling plays in axon and dendrite development, it comes as no surprise that Wnts are also important for synapse formation and function. In fact, Wnt7a, Wnt5a, Wnt3, Wnt3a, and Wnt2 all have established roles in synapse development and plasticity [21,169,170,172,206,207,208,209,210,211,212].
Table 7.
Summary of Wnt pathway genes associated with synapse formation and function
| Cell type | Synaptic process | Pathway (regulatory target) | Gene | Ref. |
|---|---|---|---|---|
| DRG neurons | Synapse formation | Divergent “canonical” (APC to MT +ends) | Wnt3a, APC | [21] |
| Granule cell mossy fiber synapse | Synaptic protein clustering | Divergent “canonical” (TCF-independent) | Wnt7a | [169, 170, 207, 213] |
| Excitatory function | Unknown | Wnt7a, Dvl | [207] | |
| Hippocampal pyramidal neurons | Synaptic plasticity (LTP) | Wnt/β-catenin | Wnt3a | [209] |
| Synaptic protein clustering | Wnt/PCP | Wnt5a, Ror1, Ror2 | [211, 222, 223] [223] | |
| Excitatory function | Wnt/Ca2+ and possibly Wnt/PCP | Wnt5a, Ror2 | [22, 208, 212, 216, 217] | |
| Inhibitory function | Wnt/CA2+ | Wnt5a | [210] | |
| Excitatory function | Dvl-dependent synaptic vesicle release and possibly Wnt/Ca2+ | Wnt7a, Dvl | [19, 206] | |
| Synapse formation | Wnt/PCP (N-cadherin) | Vangl2 | [198, 199] | |
| Postsynaptic assembly | Wnt/PCP (PSD-95) | Vangl2 | [198, 228] | |
| Prickle2 | [227] | |||
| Synapse formation and function (synaptic vesicle release) | Wnt/PCP (Syn1) | Prickle1 | [229] | |
| Hippocampal and cortical pyramidal neurons | Synapse formation | Wnt/PCP | Dact1 | [226] |
| Forebrain pyramidal neurons | Synapse formation | Wnt/PCP | Vangl2 | [197] |
Divergent “canonical”: divergent Wnt pathway genes.
Accumulating data suggest that Wnt7a signals bidirectionally through divergent pathways to influence both presynaptic and postsynaptic assembly, as well as to promote excitatory synapse function. In fact, Wnt7a was the first Wnt protein to be associated with synaptogenesis when it was found to be expressed in granule cells of the developing mouse cerebellum at the time when these cells form synapses with mossy fiber axons [169,170]. Further examination led to the discovery that soluble Wnt7a is sufficient to induce axon remodeling and synapsin-1 clustering (requisite for presynaptic function) in cultured mossy fibers, and that these effects are mimicked by GSK3 inhibition [170]. Moreover, granule cell-conditioned medium phenocopies the effect of Wnt7a on cultured mossy fibers, and this effect is inhibited by addition of a Wnt antagonist [170]. These data support that Wnt7a secreted from granule cells is required to induce presynaptic formation in mossy fibers, and that the pathway downstream involves GSK3-dependent activity. Ablation of Wnt7a in mice led to disruption of glomerular rosettes and delay of synapsin-1 clustering [170]. Dvl1 null mice exhibit the same phenotype, while Wnt7a/Dvl1 double mutant mice show even simpler glomerular rosettes [207]. Postsynaptically, exposure to Wnt7a increased clustering of PSD95, the major scaffold protein of the excitatory postsynaptic density [206,213].
Electrophysiological recordings of cerebellar slices have further indicated that Wnt7a/Dvl1 double mutant mice have a diminished frequency of miniature excitatory postsynaptic currents (mEPSCs) [207]. In studies using cultured hippocampal neurons, Wnt7a was found to increase the frequency of mEPSCs without affecting miniature inhibitory postsynaptic currents [206]. The mechanisms involved are not restricted to neurodevelopment: a pathway downstream of Wnt7a modulates EPSCs through effects on the synaptic vesicle fusion process [19]. Specifically, Wnt7a at the synapse promotes interaction of Dvl1 with synaptotagmin-1 (Syt-1), SNAP25, and syntaxin – facilitating Ca2+-induced vesicle-presynaptic membrane fusion and neurotransmitter release in an activity-dependent manner [19].
Given the preceding sections, it will come as no surprise that Wnt7a signals through multiple pathways to fulfill its synapse-promoting functions. While Dvl1 and GSK3 are required for the Wnt7a-mediated promotion of synaptogenesis, a mutant form of β-catenin lacking the Tcf binding domain does not phenocopy loss of Wnt7a in hippocampal neurons [214]. This indicates that Tcf-dependent transcription is not required for Wnt7a-mediated effects on synapse formation. Additionally, an apparent absence of JNK and CaMKII activation in the presence of Wnt7a in hippocampal neurons initially suggested that neither the Wnt/PCP nor Wnt/Ca2+ pathways were utilized [215]. However, a more recent study found significant activation of CaMKII activity in hippocampal neurons upon treatment with Wnt7a, and moreover that inhibition of CaMKII prevents Wnt7a-mediated increases of mEPSCs [206]. This finding does not exclude the model of facilitated neurotransmitter release mediated by Wnt7a-dependent interactions of Dvl1 with synaptic vesicle proteins, but it does suggest participation of additional mechanisms downstream of Dvl1. Wnt7a-mediated axonal remodeling in cerebellar mossy fibers is associated with changes in microtubule organization [170], reminiscent of earlier findings involving the inhibition of GSK3-mediated phosphorylation of MAP1B in axon remodeling. A reasonable synthesis of these data is that Wnt7a promotes synapse formation and function by influencing cytoskeletal changes downstream of the Wnt/Ca2+ pathway and also by modulating excitatory neurotransmitter release in a Dvl-dependent manner, perhaps via multiple downstream cascades.
Wnt5a similarly acts through at least 2 pathways to facilitate processes required for synapse formation and function, including excitatory and inhibitory synaptic transmission and presynaptic and postsynaptic protein clustering. The Wnt/Ca2+ pathway was first implicated when Wnt5a was shown to trigger increased calcium concentrations in dendritic processes of cultured hippocampal neurons and increased amplitude of field excitatory postsynaptic potentials in rat hippocampal slices; both effects can be inhibited by calcium channel blockers [212]. It was later found that Wnt5a facilitates fast excitatory glutamatergic synaptic transmission by potentiating NMDAR currents [208]. Wnt5a-evoked Ca2+ release from Ryr channels is required for NO production, which directly increases NMDAR assembly at the postsynaptic membrane [216]. Wnt-mediated NMDAR potentiation is impeded by addition of calcium chelators, PKC inhibitors and JNK inhibitors [208], reaffirming a requirement for the Wnt/Ca2+ pathway while also suggesting a role for the Wnt/PCP pathway. To modulate NMDAR synaptic transmission in hippocampal neurons, Wnt5a signals through receptor tyrosine-kinase-like orphan receptor 2 (Ror2) [217], a well-characterized Wnt receptor implicated in both Wnt/Ca2+ and Wnt/PCP pathways [218,219]. This Wnt5a-Ror2-Ca2+-NO pathway also regulates hippocampal excitability by inhibiting voltage-gated K+ currents (Kv currents) [22]. Kv channels play key roles in the spiking patterns of hippocampal neurons [220], and are thus important regulators of synaptic plasticity [221]. In addition to excitatory transmission, Wnt5a is implicated in inhibitory synaptic transmission through the Wnt/Ca2+, but not the Wnt/PCP, pathway. Experiments using cultured rat hippocampal neurons showed that Wnt5a facilitates fast inhibitory γ-aminobutyric acid (GABA)-ergic synaptic transmission through induction of surface expression and clustering of GABAA receptors in a CaMKII-, but not JNK-dependent manner [210]. Finally, Wnt5a induces postsynaptic clustering of PSD95 in cultured rat hippocampal neurons [211,222] by binding Ror1-Ror2 receptor complexes [223] to activate the JNK-dependent Wnt/PCP pathway [211]. Interestingly, while Wnt5a increases postsynaptic clustering in short-term experiments, long exposures (over 12 h) also increase presynaptic clustering and synaptic contacts [222].
Wnt3a can signal through a divergent Wnt/β-catenin pathway to mediate synapse formation by decreasing the amount of APC associated with microtubule plus ends, thereby promoting formation of microtubule loops in axon growth cones [21]. Microtubule looping contributes to the architecture of axonal boutons and is important for their formation [224,225]. There is also provocative evidence for Wnt/β-catenin pathway involvement in synaptic plasticity: tetanic stimulation of mouse hippocampal slices sufficient to cause long-term potentiation (LTP) led to NMDAR-dependent presynaptic release of Wnt3a, which in turn caused β-catenin accumulation and target gene regulation in postsynaptic neurons [209]. Additional evidence that Wnt/β-catenin signaling is involved in LTP was provided by experiments in which LTP was impaired in the presence of a secreted Wnt antagonist; conversely, LTP was enhanced by application of a specific GSK3 inhibitor, mimicking activation of the Wnt/β-catenin pathway [209].
We have framed the aforementioned studies in the context of specific Wnt ligands, but there have been numerous studies illustrating the roles that downstream Wnt pathway components play in synapse formation and function. For example, a role for the Wnt/PCP pathway in synaptogenesis, mechanistically separate from roles in dendritogenesis and spine formation, has been supported by analyses of differential genetic and rescue effects of neurodevelopmental phenotypes in forebrain neurons from Dact1 and Vangl2 knockout mice [197,226]. Biochemical and molecular analyses revealed that Vangl2 binds directly to N-cadherin [198,199], the transmembrane protein that facilitates the initial critical adhesive event between presynaptic and postsynaptic elements, to mediate its endocytic removal from the membrane [199]. Vangl2 and Prickle2 also bind and promote postsynaptic clustering of PSD95, critical for maintenance of synaptic integrity [199,227,228]. Prickle1 was found to regulate synaptogenesis and synaptic vesicle trafficking through a direct interaction with synapsin1 (Syn1) [229]; the synapsin protein family have important roles in synapse formation and neurotransmitter release and have previously been implicated in the pathogenesis of psychiatric disorders [230,231,232,233,234,235,236]. Indeed, Prickle1+/- mice exhibit ASD-like behaviors, and expression of a mutant form of Prickle in cultured cells phenocopies the vesicle trafficking defect observed with aberrant Syn1 function [229]. While upstream Wnt ligands involved in these events remain to be identified, these studies provide additional evidence that components of the Wnt/PCP pathway are critical contributors to synapse formation and function.
Human Genomic Studies
There are numerous studies, primarily in gene-targeted mouse lines, demonstrating that genetic disruption in Wnt pathway genes can affect complex behavior, including in assays of sociability, repetitive behaviors and vocalizations that could be relevant to the cardinal symptoms of ASD [237,238,239,240,241,242], sensory processing and prepulse inhibition that could be relevant to symptoms of Scz [243,244,245], and motivation, impulsivity, anxiety and activity patterns that could be relevant to symptoms of BD and other affective and anxiety disorders [246,247,248](Table 8). These data clearly demonstrate behavioral phenotypes in these animal models, but controversy remains over the relevance of such findings to the genetic etiology of psychiatric disorders – that is, it remains unclear whether such data have predictive relevance for involvement of the corresponding genes in clinically defined behavioral disorders in the human population. On this basis we do not emphasize these types of animal behavioral data in this review except where the corresponding gene is separately linked to psychiatric disease by strong human genetic data.
Table 8.
Summary of Wnt pathway genes associated with ASD, SCZ, and BD and corresponding human genomic analyses
| Pathway/gene | Disorder | Type of genetic evidence | Ref. |
|---|---|---|---|
| Canonical Wnt/β-catenin | |||
| CHD8 | ASD | WES, CGA, sequencing of balanced chromosomal abnormalities | [261, 262, 263, 264] |
| SCZ | WES | [262] | |
| CTNNB1 (β-catenin) | ASD | WES | [263, 271] |
| SCZ | CGA | [145] | |
| TCF7 | ASD | WES | [261] |
| WNT7A | ASD | WGS | [268] |
| BD | CGA | [296] | |
| DISC1 | ASD | WGS | [260] |
| SCZ | LA | [274] | |
| BD | LA | [297, 298, 299, 300] | |
| TCF4 | ASD | GWAS, sequencing of balanced chromosomal abnormalities | [272, 273] |
| BD | GWAS, CGA | [301, 302] | |
| SCZ | GWAS | [253] | |
| WNT2 | ASD | GWAS, LA | [266] |
| WNT3 | ASD | “NETBAG” analysis of de novo CNV data | [267] |
| APC | ASD | LA, CGA | [241, 269, 270] |
| FZD3 | SCZ | LA | [291, 292, 293] |
| HMG2L1 | BD | LA | [303] |
| SFRP1 | SCZ | LA | [291, 292, 293] |
| DKK4 | SCZ | LA, CGA | [290, 291, 292, 293] |
| PTEN | ASD | CGA | [278, 279, 280] |
| WNT1 | ASD | CGA | [240] |
| WNT2B | BD | CGA | [296] |
| DKK1 | SCZ | CGA | [289] |
| KREMEN1 | SCZ | CGA | [289] |
| PPARD | BD | CGA | [296] |
| Noncanonical Wnt | |||
| PRICKLE1 | ASD | WES | [282] |
| SESTD1 | BD | GWAS | [305] |
| PRICKLE2 | ASD | CGA | [237] |
Noncanonical Wnt: Wnt/PCP pathway genes; WGS, whole genome sequencing; WES, whole exome sequencing; LA, linkage analysis; GWAS, genome-wide association study; CGA, candidate gene approach.
Through human genomic studies of psychiatric disorders, a picture is emerging of overlapping genetic etiologies: many genetic variants and loci are implicated across several psychiatric disorders [249,250,251,252,253]. Wnt pathway-associated genes are among hundreds of loci that have been identified in this effort. In this section, we discuss these genes in the context of individual disorders, focusing on ASD, Scz, and BD, for which the most reliable genetic data exist because of their relatively high heritability. However, before summarizing the Wnt pathway loci implicated in each of these 3 psychiatric disorders, it is important to establish context by providing an introduction to the genetics of complex disorders and describing the different classes of genetic studies and the type of evidence they offer.
As with every multifactorial medical disorder with a heritable component, the genetic contribution to psychiatric disorders is complex and can be subdivided into several categories. One of the simplest classification schemes is to separate “common” genetic variation from “rare” (and de novo) genetic variation. Common variants arose in the human genome many generations ago and have since expanded such that they are now present in ≥1% of the total population or a well-defined subpopulation (e.g., ethnic group). Being common, these variants are likely to be either nondeleterious or to have small incremental effects on disease pathogenesis. By contrast, rare variants may have large effects on pathogenesis but have either arisen de novo in an individual still alive today or recently enough in the expansion of the human population that they have not yet been eliminated despite being deleterious. Most current models for complex diseases in psychiatry assume that common variants in aggregate are the major contributor to disease susceptibility in the general population. For example, a 2014 analysis of a large Swedish epidemiological sample estimated that narrow-sense heritability (the ratio of additive genetic variation to total phenotypic variation) in ASD is 52.4%, with common variation accounting for almost 50% and rare/de novo mutations accounting for only 2.6% of total disease burden [254]. Models influenced by recent large-scale genome-wide sequencing studies estimate a larger proportion of disease burden arising from rare or de novo variants [255,256] – but even so, they continue to posit that common variation accounts for the lion's share of overall genetic susceptibility in ASD. At this time, there is little reason to expect that the relative proportion of burden arising from rare versus common variation will be substantially different in other psychiatric disorders.
The different types of human genetic studies used to identify variants linked to disease susceptibility have varying degrees of statistical rigor. In general, the strongest current evidence is provided by large-scale genome-wide sequencing analysis in which every locus of the genome is sequenced in large numbers (i.e., thousands) of patients and controls. High statistical standards are used to ferret out recurrent LOF mutations that are strongly overrepresented in affected versus unaffected individuals and to correct for multiple comparisons (i.e., finding significance at a single locus while simultaneously examining every locus in the genome). While these studies reveal loci that are definitively causal, so far they have only identified rare causes that account for a small percentage of disease burden in the overall population. Another powerful type of genetic study, linkage analysis, relies on pedigrees to identify strong associations between disease and specific genetic variations within a family of related individuals. Similar to genome-wide sequencing, these studies have so far most successfully identified rare causes and are limited by the availability and discovery of informative pedigrees. The genome-wide association study (GWAS) is a method with the potential to provide data for contributions from common variants with high statistical rigor. GWAS takes advantage of common single-nucleotide polymorphisms (SNPs) used for genotyping to identify variants associated with disease by assuming linkage disequilibrium between SNPs and proximal loci. GWAS should ultimately identify the loci harboring the common sequence variation that in aggregate contributes to the majority of genetic disease burden in psychiatry. Although to date most GWAS findings in psychiatry have been modest due to a need for much larger sample sizes given small effect sizes and large numbers of contributory loci, an example of a recent high-impact study that used GWAS information combined with creative and targeted follow-up has linked common variation at the C4 gene in the major histocompatibility complex to Scz, pointing to changes in immune molecule-driven synaptic pruning as a specific pathogenic mechanism [257]. It is to be expected that ever-expanding human genetic sample sizes and increasingly sophisticated statistical algorithms will lead to many more GWAS-guided advances of this kind in the coming decade. Finally, candidate gene sequencing approaches traditionally have tried to identify susceptibility loci by investigating a single or small number of loci in relatively limited sample and control datasets (i.e., n <103) buttressing this by functional considerations derived from pharmacology, animal models, or other basic research findings. Given the low statistical power of this genetic approach, combined with the recent realization that there is a much higher-than-expected rate of rare and de novo nucleotide variation in the human exome, the significance of such findings is increasingly called into question pending stronger evidence derived from one of the other types of genetic study described above.
Autism Spectrum Disorder
The genomic analysis of ASD, a behaviorally defined neurodevelopmental disorder with a genetic contribution estimated to be upwards of 50% (in some studies as high as 95%) [254,258,259,260], has led to the identification of a number of Wnt/β-catenin and Wnt/PCP pathway loci. Significantly, the single locus with the highest frequency of de novo LOF mutations in genome-wide sequencing studies of ASD is chromodomain helicase DNA-binding protein 8 (CHD8), encoding a DNA helicase that co-regulates many Wnt/β-catenin transcriptional targets [261,262,263,264,265]. Other genetic evidence supports roles for several core Wnt/β-catenin pathway loci in the genetics of ASD including WNT1 [240], WNT2[266], WNT3[267], WNT7A[268], APC [241,269,270], CTNNB1 (β-catenin) [263,271], TCF4 [272,273], and TCF7[261]. This review has highlighted the critical roles these genes play during neural development. Outside the “core” of the pathway, other molecules implicated in pathway modulation with compelling genetic evidence for involvement in ASD include DISC1[268], PTEN, and the Prickle genes. Our neurodevelopmental discussion of DISC1, a Wnt pathway scaffold protein first identified in affected members of a large Scottish pedigree with high incidence of various psychiatric disorders [274], emphasized its roles in neural precursor proliferation and migration. However, there is also evidence that DISC1 is required for development of glutamatergic synapses [275,276], although it is not yet clear if this function is Wnt-dependent. The gene encoding PTEN, a cytoplasmic protein that suppresses Wnt/β-catenin signaling in the developing mouse cortex [277], has been repeatedly identified as a high-risk ASD susceptibility gene in human genomic studies [278,279,280,281]. Finally, single nucleotide variants of the Wnt/PCP pathway genes PRICKLE1 and PRICKLE2 were identified in whole exome sequencing of affected families [237,282]. Mutations in PRICKLE1 and PRICKLE2 have previously been associated with epilepsy [283,284], a condition highly comorbid with ASD [285]. Both genes encode proteins with roles in synaptogenesis; PRICKLE1 is important for Syn1 function in the presynapse [229], while PRICKLE2 interacts with PSD95 and NMDA receptors in the postsynapse [227].
Schizophrenia
Wnt/β-catenin loci have also been prominent in large-scale genomic analyses of Scz, a disorder with an estimated heritability of ∼65-80% [286,287,288]. In fact, some Wnt/β-catenin pathway loci have emerged as shared risk factors for Scz and ASD, including DISC1[274], TCF4 [253,273], CTNNB1[145], and CHD8[262]. Other Wnt genes implicated in Scz include the secreted Wnt antagonists DKK1 [289] and DKK4 [290], as well as the receptor required for DKK-mediated Wnt antagonism KREMEN1 [289]. Moreover, DKK4, along with FZD3 and Secreted Frizzled-Related Protein 1 (SFRP1), reside on chromosome 8p, a genomic region repeatedly implicated in psychiatric genetic studies over the past 2 decades [291,292,293].
Bipolar Disorder
Genomic analysis of BD, with an estimated heritability of ∼60-85% [286,294,295], has also uncovered disruptions in Wnt/β-catenin pathway loci, including WNT7A and WNT2B[296], DISC1[297,298,299,300], and TCF7L2 (TCF4) [301,302]. Other genes that help regulate the Wnt/β-catenin signaling have also been identified. For example, a family-based study of SNPs found a prominent Wnt/β-catenin target gene, Peroxisome Proliferator-Activated Receptor-Delta (PPARD), to be the most significantly associated SNP [296]. Another family-based SNP mapping study found a high mobility group (HMG) box family member, HMG2L1, to be significantly associated with BD [303], and there is emerging evidence that HMG2L1 functions as a negative regulator of Wnt/β-catenin signaling [304].
With regard to noncanonical Wnt signaling in BD, a recent large GWAS study identified a single locus containing a noncoding SNP with strong linkage to lithium-responsive BD: SEC14 and spectrin domain containing 1 (SESTD1) [305]. Sestd1, expressed both during neurodevelopment and in the mature CNS [306], has recently been shown to directly interact with Dvl2, Dact1, and Vangl2 in a PCP-related signaling cascade during embryonic development [307].
Concluding Remarks: Therapeutic Considerations
Lithium, which came into systematic use as a mood stabilizer shortly after World War II [308], is the oldest psychiatric drug in Western medicine with folk roots that may stretch back to antiquity [309]. It remains one of the staples of treatment in BD, with a significant subclass of patients exquisitely responsive to it. Lithium also remains in a class by itself as the only drug with a highly selective therapeutic utility in the treatment of mood swings; other drugs used for this indication were designed and are used for other indications – i.e. as anticonvulsants, antipsychotics, etc. – with side effect profiles and neurotransmitter impacts in line with these other drug classes. No medications have been devised based on an understanding of the molecular action of lithium with the intent to duplicate or improve upon it. This is despite the fact that in some otherwise responsive BD patients, the use of lithium is limited by its side effect profile, particularly a progressive nephrotoxicity that occurs with chronic treatment. So, there is certainly an unmet clinical need and a substantial potential market for such a pharmaceutical – yet no such drug has been intentionally developed or marketed.
The deficit of new drugs based on lithium is partly due to a complex mechanism of action; lithium affects several enzymes and cellular pathways simultaneously, making its most relevant molecular mechanism in the treatment of BD difficult to disentangle [310]. Nonetheless, it has become increasingly clear over the last 2 decades that one of the relevant (though by no means exclusive) mechanisms of action of lithium within the CNS is inhibition of GSK3, which mimics activation of Wnt/β-catenin signaling [311]. Lithium, a monovalent cation, directly inhibits GSK3, a metalloenzyme, by replacing divalent Mg in its structure. Lithium can similarly substitute for Mg in other metalloproteins; its relative specificity for GSK3 and a few other enzymes (such as inositol monophosphatase and inositol polyphosphate 1-phosphatase in the phosphoinositide pathway) stems from biophysical idiosyncrasies – the distribution of positive charges, bulky coordinating residues, and solvent accessibility – of the metal-binding pocket in lithium-sensitive metalloproteins [312]. But lithium is far from the only psychiatric drug to impact GSK3: the mood stabilizer/anticonvulsant valproic acid (VPA) [313,314,315], diverse antipsychotics [316,317,318,319,320], antidepressants including tricyclics and selective serotonin reuptake inhibitors [321,322,323,324], as well as the novel acute antidepressant ketamine [325], all modulate GSK3 as one of their many downstream effects. Many orally administrable molecules with specific and selective inhibitory activity on GSK3 are commercially available and have been widely used in basic scientific studies including in live animal models, with little evidence of toxicity [326,327,328,329,330]. So, the absence of psychiatric drugs intentionally designed to impact this pathway does not reflect an absence of evidence for efficacy or a difficulty in chemically creating such a drug – more likely it reflects another major consideration: reluctance to develop and market a novel psychiatric drug deliberately designed to act on a pathway with links to cancer.
In fact, the extensive clinical experience with lithium itself provides evidence that such concerns regarding cancer risk are likely overblown. In over 60 years of systematic use in patients across the globe, no links between lithium therapy and oncogenesis have emerged; if anything, there is evidence that chronic administration of lithium has an oncoprotective effect in humans [331,332]. Over a similar period, extensive basic science investigations in animal models and cell lines have provided little evidence that pharmaceutical activation of the Wnt pathway at the level of the plasma membrane or cytoplasm – i.e. at or above biochemical GSK3 regulation – leads to cancer [333,334]. That said, as expected for a pathway with such diverse developmental functions there is reason to proceed with caution based on potentially adverse developmental consequences of manipulating Wnt signaling, particularly in utero. Indeed, extensive clinical experience has demonstrated that maternal use of either lithium or VPA is associated with the increased incidence of some birth defects. In the case of VPA, this includes an increased risk to the newborn for cognitive defects and ASD [335,336]. In the case of lithium exposure, the data suggesting adverse neurodevelopmental consequences in the newborn is complicated: although in some studies children who experienced prenatal lithium exposure had normal neurological, cognitive, and behavioral outcomes [337,338], at least 1 study found that prenatal exposure led to transient neurodevelopmental differences that resolved by 1 year of age [339]. Prenatal lithium exposure is however clearly associated with an increased risk for cardiovascular defects, particularly Ebstein's anomaly, a malformation of the tricuspid valve (in one recent study 0.6% of unexposed children experienced cardiovascular anomalies compared to 4.1% of lithium-exposed children, although ∼60% of the lithium exposure cases resolved spontaneously) [340]. For both VPA and lithium, it is not clear if associated birth defects are caused by the drugs’ activity on GSK3 and Wnt/β-catenin signaling, or if they may be a consequence of the drugs’ effects on other molecular targets during development. Regardless, these clinical data do suggest caution is warranted before new drugs intentionally targeting GSK3 or the Wnt/β-catenin pathway are administered to pregnant women.
On the other hand, several recent studies in mouse models and hiPSCs support that modulation of the Wnt/β-catenin pathway has therapeutic potential in psychiatry. Mice with combined mutations in 2 Dvl genes (Dvl1 and Dvl3) have adult behavioral abnormalities reminiscent of ASD (increased repetitive behaviors and decreased social behavior), along with accelerated early brain growth similar to a subgroup of ASD patients. During prenatal development, these animals have hypoactivity of a β-catenin-dependent transcriptional cascade in cortical NPCs, with a resultant hyperproliferation phenotype. Prenatal administration of a specific GSK3 inhibitor to these animals corrected developmental brain growth abnormalities as well as adult ASD-like behavioral phenotypes [341]. A parallel study examined cellular and molecular defects in hiPSC lines derived from ASD patients with accelerated early brain growth, and similarly found a hyperproliferative NPC phenotype responsive to lithium [342]. Finally, cortical pyramidal neurons from a mouse line mutant for the neuronally expressed Wnt/β-catenin pathway activator and DISC1 partner Dixdc1 have reduced dendritic spine and excitatory synapse density correlating with phenotypes in the affective domain (behavioral despair) and social domain (reciprocal social interaction); administration of either lithium or a selective GKS3 inhibitor corrects both the neurodevelopmental and behavioral phenotypes in these animals [343]. This supports that a major neurodevelopmental/neuroplastic target of lithium – and of other psychiatric drugs that either directly or indirectly modulate GSK3 – is the formation, stability, and/or turnover of dendritic spines and glutamatergic synapses [325,344,345]. The same study also provided evidence supporting a “goldilocks principle” for Wnt signaling in these processes at the dendritic spine and synapse, showing that either too much or too little signal pathway activation is similarly deleterious [343]. This is consistent with the established importance of Wnt pathway dosage and the role of Wnts as morphogens in other developmental contexts [75,121,197,346,347,348,349]. Together, the recent work in animals and hIPSCs underscores how experimental model systems in which genetic, signaling, neurodevelopmental, circuit, and behavioral phenotypes are simultaneously characterized and correlated can be used to test hypotheses and potentially to develop and test novel psychopharmaceuticals that modulate Wnt/β-catenin signaling.
With regard to GSK3-independent (i.e., noncanonical) Wnt pathways, there is also reason to be optimistic. Naturally occurring and manufactured compounds that specifically inhibit the Wnt/PCP pathway have been identified [350], opening the door for development of new agents that can selectively inhibit or activate this pathway via similar molecular mechanisms [351]. In closing, as each of the Wnt pathways – even the most divergent pathways discussed in the preceding sections – is biochemically characterized, they provide promising targets for rational drug exploration and development in psychiatry with a clear path forward through the use of animal and hiPSC models as prescribed above.
Disclosure Statement
The authors have no conflicts of interest to report.
Acknowledgments
This study was supported by a NARSAD Independent Investigator Award (B.N.R.C.) and NIMH T32 #5T32MH089920 (K.A.M./B.N.R.C.).
Footnotes
In mammals, there are two GSK3 isoenzymes encoded by the GSK3α and GSK3β loci. As these isoforms are biochemically redundant in Wnt/β-catenin signaling and as there are no known pharmacological compounds that selectively block α vs. β, they will not be distinguished further in this review [14].
References
- 1.Insel TR. Assessing the economic costs of serious mental illness. Am J Psychiatry. 2008;165:663–665. doi: 10.1176/appi.ajp.2008.08030366. [DOI] [PubMed] [Google Scholar]
- 2.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroon T, Sierksma MC, Meredith RM. Investigating mechanisms underlying neurodevelopmental phenotypes of autistic and intellectual disability disorders: a perspective. Front Syst Neurosci. 2013;7:75. doi: 10.3389/fnsys.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 7.Seshadri S, Kamiya A, Yokota Y, Prikulis I, Kano S, Hayashi-Takagi A, Stanco A, Eom TY, Rao S, Ishizuka K, Wong P, Korth C, Anton ES, Sawa A. Disrupted-in-Schizophrenia-1 expression is regulated by beta-site amyloid precursor protein cleaving enzyme-1-neuregulin cascade. Proc Natl Acad Sci USA. 2010;107:5622–5627. doi: 10.1073/pnas.0909284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 9.Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol. 2009;1:a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Kestler HA, Kuhl M. From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci. 2008;363:1333–1347. doi: 10.1098/rstb.2007.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 14.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 16.Fiedler M, Mendoza-Topaz C, Rutherford TJ, Mieszczanek J, Bienz M. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating beta-catenin. Proc Natl Acad Sci USA. 2011;108:1937–1942. doi: 10.1073/pnas.1017063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 18.Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol. 2004;164:243–253. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciani L, Marzo A, Boyle K, Stamatakou E, Lopes DM, Anane D, McLeod F, Rosso SB, Gibb A, Salinas PC. Wnt signalling tunes neurotransmitter release by directly targeting Synaptotagmin-1. Nat Commun. 2015;6:8302. doi: 10.1038/ncomms9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamatakou E, Hoyos-Flight M, Salinas PC. Wnt signalling promotes actin dynamics during axon remodelling through the actin-binding protein Eps8. PLoS One. 2015;10:e0134976. doi: 10.1371/journal.pone.0134976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purro SA, Ciani L, Hoyos-Flight M, Stamatakou E, Siomou E, Salinas PC. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J Neurosci. 2008;28:8644–8654. doi: 10.1523/JNEUROSCI.2320-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parodi J, Montecinos-Oliva C, Varas R, Alfaro IE, Serrano FG, Varas-Godoy M, Munoz FJ, Cerpa W, Godoy JA, Inestrosa NC. Wnt5a inhibits K+ currents in hippocampal synapses through nitric oxide production. Mol Cell Neurosci. 2015;68:314–322. doi: 10.1016/j.mcn.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Budnik V, Salinas PC. Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol. 2011;21:151–159. doi: 10.1016/j.conb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosca TJ, Schwarz TL. Drosophila Importin-alpha2 is involved in synapse, axon and muscle development. PLoS One. 2010;5:e15223. doi: 10.1371/journal.pone.0015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 28.Yaguchi J, Takeda N, Inaba K, Yaguchi S. Cooperative Wnt-nodal signals regulate the patterning of anterior neuroectoderm. PLoS Genet. 2016;12:e1006001. doi: 10.1371/journal.pgen.1006001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang AG, Papalopulu N, Goulding MD, Kintner C. Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from posterior nonaxial mesoderm. Dev Biol. 1999;212:366–380. doi: 10.1006/dbio.1999.9319. [DOI] [PubMed] [Google Scholar]
- 30.McGrew LL, Hoppler S, Moon RT. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech Dev. 1997;69:105–114. doi: 10.1016/s0925-4773(97)00160-3. [DOI] [PubMed] [Google Scholar]
- 31.McGrew LL, Lai CJ, Moon RT. Specification of the anteroposterior neural axis through synergistic interaction of the Wnt signaling cascade with noggin and follistatin. Dev Biol. 1995;172:337–342. doi: 10.1006/dbio.1995.0027. [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popperl H, Schmidt C, Wilson V, Hume CR, Dodd J, Krumlauf R, Beddington RS. Misexpression of Cwnt8C in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development. 1997;124:2997–3005. doi: 10.1242/dev.124.15.2997. [DOI] [PubMed] [Google Scholar]
- 34.Elkouby YM, Elias S, Casey ES, Blythe SA, Tsabar N, Klein PS, Root H, Liu KJ, Frank D. Mesodermal Wnt signaling organizes the neural plate via Meis3. Development. 2010;137:1531–1541. doi: 10.1242/dev.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 36.Kazanskaya O, Glinka A, Niehrs C. The role of Xenopus dickkopf1 in prechordal plate specification and neural patterning. Development. 2000;127:4981–4992. doi: 10.1242/dev.127.22.4981. [DOI] [PubMed] [Google Scholar]
- 37.Zakin L, Reversade B, Virlon B, Rusniok C, Glaser P, Elalouf JM, Brulet P. Gene expression profiles in normal and Otx2-/- early gastrulating mouse embryos. Proc Natl Acad Sci USA. 2000;97:14388–14393. doi: 10.1073/pnas.011513398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, Aizawa S, Matsui Y, Matsuo I. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 40.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 41.Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, Arkell R, Stanier P, Copp AJ. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Ding Y, Lei YP, Yang XY, Xie GM, Wen J, Cai CQ, Li H, Chen Y, Zhang T, Wu BL, Jin L, Chen Y, Wang HY. Identification of novel rare mutations of DACT1 in human neural tube defects. Hum Mutat. 2012;33:1450–1455. doi: 10.1002/humu.22121. [DOI] [PubMed] [Google Scholar]
- 43.Torban E, Patenaude AM, Leclerc S, Rakowiecki S, Gauthier S, Andelfinger G, Epstein DJ, Gros P. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci USA. 2008;105:3449–3454. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 45.Kibar Z, Salem S, Bosoi CM, Pauwels E, De Marco P, Merello E, Bassuk AG, Capra V, Gros P. Contribution of VANGL2 mutations to isolated neural tube defects. Clin Genet. 2011;80:76–82. doi: 10.1111/j.1399-0004.2010.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen S, Zhu H, Lu W, Mitchell LE, Shaw GM, Lammer EJ, Finnell RH. Planar cell polarity pathway genes and risk for spina bifida. Am J Med Genet A. 2010;152A:299–304. doi: 10.1002/ajmg.a.33230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson A, Escuin S, Doudney K, Vekemans M, Stevenson RE, Greene ND, Copp AJ, Stanier P. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat. 2012;33:440–447. doi: 10.1002/humu.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Marco P, Merello E, Piatelli G, Cama A, Kibar Z, Capra V. Planar cell polarity gene mutations contribute to the etiology of human neural tube defects in our population. Birth Defects Res A Clin Mol Teratol. 2014;100:633–641. doi: 10.1002/bdra.23255. [DOI] [PubMed] [Google Scholar]
- 49.De Marco P, Merello E, Consales A, Piatelli G, Cama A, Kibar Z, Capra V. Genetic analysis of disheveled 2 and disheveled 3 in human neural tube defects. J Mol Neurosci. 2013;49:582–588. doi: 10.1007/s12031-012-9871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei Y, Zhu H, Duhon C, Yang W, Ross ME, Shaw GM, Finnell RH. Mutations in planar cell polarity gene SCRIB are associated with spina bifida. PLoS One. 2013;8:e69262. doi: 10.1371/journal.pone.0069262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lei Y, Zhu H, Yang W, Ross ME, Shaw GM, Finnell RH. Identification of novel CELSR1 mutations in spina bifida. PLoS One. 2014;9:e92207. doi: 10.1371/journal.pone.0092207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, Ding Y, Lei YP, Yang XY, Xie GM, Wen J, Cai CQ, Li H, Chen Y, Zhang T, Wu BL, Jin L, Chen Y, Wang HY. Identification of novel rare mutations of DACT1 in human neural tube defects. Hum Mutat. 2012;33:1450–1455. doi: 10.1002/humu.22121. [DOI] [PubMed] [Google Scholar]
- 53.Perry WL, Vasicek TJ, Lee JJ, Rossi JM, Zeng L, Zhang T, Tilghman SM, Costantini F. Phenotypic and molecular analysis of a transgenic insertional allele of the mouse Fused locus. Genetics. 1995;141:321–332. doi: 10.1093/genetics/141.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu CI, Hoffman JA, Shy BR, Ford EM, Fuchs E, Nguyen H, Merrill BJ. Function of Wnt/beta-catenin in counteracting Tcf3 repression through the Tcf3-beta-catenin interaction. Development. 2012;139:2118–2129. doi: 10.1242/dev.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carter M, Chen X, Slowinska B, Minnerath S, Glickstein S, Shi L, Campagne F, Weinstein H, Ross ME. Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc Natl Acad Sci USA. 2005;102:12843–12848. doi: 10.1073/pnas.0501963102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kokubu C, Heinzmann U, Kokubu T, Sakai N, Kubota T, Kawai M, Wahl MB, Galceran J, Grosschedl R, Ozono K, Imai K. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development. 2004;131:5469–5480. doi: 10.1242/dev.01405. [DOI] [PubMed] [Google Scholar]
- 57.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 58.Allache R, Lachance S, Guyot MC, De Marco P, Merello E, Justice MJ, Capra V, Kibar Z. Novel mutations in Lrp6 orthologs in mouse and human neural tube defects affect a highly dosage-sensitive Wnt non-canonical planar cell polarity pathway. Hum Mol Genet. 2014;23:1687–1699. doi: 10.1093/hmg/ddt558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei Y, Fathe K, McCartney D, Zhu H, Yang W, Ross ME, Shaw GM, Finnell RH. Rare LRP6 variants identified in spina bifida patients. Hum Mutat. 2015;36:342–349. doi: 10.1002/humu.22750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimura-Yoshida C, Mochida K, Ellwanger K, Niehrs C, Matsuo I. Fate specification of neural plate border by canonical Wnt signaling and Grhl3 is crucial for neural tube closure. EBioMedicine. 2015;2:513–527. doi: 10.1016/j.ebiom.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H. Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development. 1993;118:439–448. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- 63.Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 64.Fischer T, Guimera J, Wurst W, Prakash N. Distinct but redundant expression of the Frizzled Wnt receptor genes at signaling centers of the developing mouse brain. Neuroscience. 2007;147:693–711. doi: 10.1016/j.neuroscience.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 65.Cavodeassi F, Houart C. Brain regionalization: of signaling centers and boundaries. Dev Neurobiol. 2012;72:218–233. doi: 10.1002/dneu.20938. [DOI] [PubMed] [Google Scholar]
- 66.Quinlan R, Graf M, Mason I, Lumsden A, Kiecker C. Complex and dynamic patterns of Wnt pathway gene expression in the developing chick forebrain. Neural Dev. 2009;4:35. doi: 10.1186/1749-8104-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inestrosa NC, Varela-Nallar L. Wnt signalling in neuronal differentiation and development. Cell Tissue Res. 2015;359:215–223. doi: 10.1007/s00441-014-1996-4. [DOI] [PubMed] [Google Scholar]
- 68.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Villanueva R. The cerebellum and neuropsychiatric disorders. Psychiatry Res. 2012;198:527–532. doi: 10.1016/j.psychres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 70.Greene JG. Gene expression profiles of brain dopamine neurons and relevance to neuropsychiatric disease. J Physiol. 2006;575:411–416. doi: 10.1113/jphysiol.2006.112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison-Uy SJ, Pleasure SJ. Wnt signaling and forebrain development. Cold Spring Harb Perspect Biol. 2012;4:a008094. doi: 10.1101/cshperspect.a008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danesin C, Peres JN, Johansson M, Snowden V, Cording A, Papalopulu N, Houart C. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev Cell. 2009;16:576–587. doi: 10.1016/j.devcel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 74.Mangale VS, Hirokawa KE, Satyaki PR, Gokulchandran N, Chikbire S, Subramanian L, Shetty AS, Martynoga B, Paul J, Mai MV, Li Y, Flanagan LA, Tole S, Monuki ES. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- 76.Subramanian L, Tole S. Mechanisms underlying the specification, positional regulation, and function of the cortical hem. Cereb Cortex. 2009;19(suppl 1):i90–i95. doi: 10.1093/cercor/bhp031. [DOI] [PubMed] [Google Scholar]
- 77.Fotaki V, Price DJ, Mason JO. Wnt/beta-catenin signaling is disrupted in the extra-toes (Gli3(Xt/Xt)) mutant from early stages of forebrain development, concomitant with anterior neural plate patterning defects. J Comp Neurol. 2011;519:1640–1657. doi: 10.1002/cne.22592. [DOI] [PubMed] [Google Scholar]
- 78.Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- 79.Galceran J, Miyashita-Lin EM, Devaney E, Rubenstein JL, Grosschedl R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127:469–482. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- 80.Zhou CJ, Zhao C, Pleasure SJ. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou CJ, Pinson KI, Pleasure SJ. Severe defects in dorsal thalamic development in low-density lipoprotein receptor-related protein-6 mutants. J Neurosci. 2004;24:7632–7639. doi: 10.1523/JNEUROSCI.2123-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 83.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 84.Thomas KR, Musci TS, Neumann PE, Capecchi MR. Swaying is a mutant allele of the proto-oncogene Wnt-1. Cell. 1991;67:969–976. doi: 10.1016/0092-8674(91)90369-a. [DOI] [PubMed] [Google Scholar]
- 85.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 86.Chilov D, Sinjushina N, Rita H, Taketo MM, Makela TP, Partanen J. Phosphorylated beta-catenin localizes to centrosomes of neuronal progenitors and is required for cell polarity and neurogenesis in developing midbrain. Dev Biol. 2011;357:259–268. doi: 10.1016/j.ydbio.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 87.Stuebner S, Faus-Kessler T, Fischer T, Wurst W, Prakash N. Fzd3 and Fzd6 deficiency results in a severe midbrain morphogenesis defect. Dev Dyn. 2010;239:246–260. doi: 10.1002/dvdy.22127. [DOI] [PubMed] [Google Scholar]
- 88.Alves dos Santos MT, Smidt MP. En1 and Wnt signaling in midbrain dopaminergic neuronal development. Neural Dev. 2011;6:23. doi: 10.1186/1749-8104-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castelo-Branco G, Andersson ER, Minina E, Sousa KM, Ribeiro D, Kokubu C, Imai K, Prakash N, Wurst W, Arenas E. Delayed dopaminergic neuron differentiation in Lrp6 mutant mice. Dev Dyn. 2010;239:211–221. doi: 10.1002/dvdy.22094. [DOI] [PubMed] [Google Scholar]
- 90.Tang M, Villaescusa JC, Luo SX, Guitarte C, Lei S, Miyamoto Y, Taketo MM, Arenas E, Huang EJ. Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J Neurosci. 2010;30:9280–9291. doi: 10.1523/JNEUROSCI.0860-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderegg A, Lin HP, Chen JA, Caronia-Brown G, Cherepanova N, Yun B, Joksimovic M, Rock J, Harfe BD, Johnson R, Awatramani R. An Lmx1b-miR135a2 regulatory circuit modulates Wnt1/Wnt signaling and determines the size of the midbrain dopaminergic progenitor pool. PLoS Genet. 2013;9:e1003973. doi: 10.1371/journal.pgen.1003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zechner D, Muller T, Wende H, Walther I, Taketo MM, Crenshaw EB, 3rd, Treier M, Birchmeier W, Birchmeier C. Bmp and Wnt/beta-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev Biol. 2007;303:181–190. doi: 10.1016/j.ydbio.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 93.Shimizu T, Kagawa T, Wada T, Muroyama Y, Takada S, Ikenaka K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev Biol. 2005;282:397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 94.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 95.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25:254–264. doi: 10.1016/j.ceb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 98.Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 99.Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 100.Gulacsi AA, Anderson SA. Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat Neurosci. 2008;11:1383–1391. doi: 10.1038/nn.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gunhaga L, Marklund M, Sjodal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- 102.Selvadurai HJ, Mason JO. Wnt/beta-catenin signalling is active in a highly dynamic pattern during development of the mouse cerebellum. PLoS One. 2011;6:e23012. doi: 10.1371/journal.pone.0023012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pei Y, Brun SN, Markant SL, Lento W, Gibson P, Taketo MM, Giovannini M, Gilbertson RJ, Wechsler-Reya RJ. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development. 2012;139:1724–1733. doi: 10.1242/dev.050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dickinson ME, Krumlauf R, McMahon AP. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- 105.Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- 106.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 107.Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A. Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol. 2007;309:285–297. doi: 10.1016/j.ydbio.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woodhead GJ, Mutch CA, Olson EC, Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci. 2006;26:12620–12630. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 110.Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci USA. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DeSouza RM, Jones BR, Lowis SP, Kurian KM. Pediatric medulloblastoma – update on molecular classification driving targeted therapies. Front Oncol. 2014;4:176. doi: 10.3389/fonc.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuwahara A, Hirabayashi Y, Knoepfler PS, Taketo MM, Sakai J, Kodama T, Gotoh Y. Wnt signaling and its downstream target N-myc regulate basal progenitors in the developing neocortex. Development. 2010;137:1035–1044. doi: 10.1242/dev.046417. [DOI] [PubMed] [Google Scholar]
- 115.Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. doi: 10.1016/j.brainres.2016.01.023. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 116.Kang E, Wen Z, Song H, Christian KM, Ming GL. Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Biol. 2016;8:a019026. doi: 10.1101/cshperspect.a019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. 2004;29:196–205. [PMC free article] [PubMed] [Google Scholar]
- 118.Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K. Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J Neurosci Res. 2007;85:3574–3585. doi: 10.1002/jnr.21455. [DOI] [PubMed] [Google Scholar]
- 119.Lau WM, Qiu G, Helmeste DM, Lee TM, Tang SW, So KF, Tang SW. Corticosteroid decreases subventricular zone cell proliferation, which could be reversed by paroxetine. Restor Neurol Neurosci. 2007;25:17–23. [PubMed] [Google Scholar]
- 120.Schreiber R, Newman-Tancredi A. Improving cognition in schizophrenia with antipsychotics that elicit neurogenesis through 5-HT(1A) receptor activation. Neurobiol Learn Mem. 2014;110:72–80. doi: 10.1016/j.nlm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 121.Choe Y, Pleasure SJ, Mira H. Control of adult neurogenesis by short-range morphogenic-signaling molecules. Cold Spring Harb Perspect Biol. 2015;8:a018887. doi: 10.1101/cshperspect.a018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Varela-Nallar L, Inestrosa NC. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci. 2013;7:100. doi: 10.3389/fncel.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 124.Wexler EM, Paucer A, Kornblum HI, Palmer TD, Geschwind DH. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–1141. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12:31–40. doi: 10.1038/ncb2001. sup pp 31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rubenstein JL. Annual research review: development of the cerebral cortex: implications for neurodevelopmental disorders. J Child Psychol Psychiatry. 2011;52:339–355. doi: 10.1111/j.1469-7610.2010.02307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aman A, Piotrowski T. Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell. 2008;15:749–761. doi: 10.1016/j.devcel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 129.Kamino M, Kishida M, Kibe T, Ikoma K, Iijima M, Hirano H, Tokudome M, Chen L, Koriyama C, Yamada K, Arita K, Kishida S. Wnt-5a signaling is correlated with infiltrative activity in human glioma by inducing cellular migration and MMP-2. Cancer Sci. 2011;102:540–548. doi: 10.1111/j.1349-7006.2010.01815.x. [DOI] [PubMed] [Google Scholar]
- 130.Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, Fancy SP. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351:379–384. doi: 10.1126/science.aad3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mayor R, Theveneau E. The role of the non-canonical Wnt-planar cell polarity pathway in neural crest migration. Biochem J. 2014;457:19–26. doi: 10.1042/BJ20131182. [DOI] [PubMed] [Google Scholar]
- 132.De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- 133.Boitard M, Bocchi R, Egervari K, Petrenko V, Viale B, Gremaud S, Zgraggen E, Salmon P, Kiss JZ. Wnt signaling regulates multipolar-to-bipolar transition of migrating neurons in the cerebral cortex. Cell Rep. 2015;10:1349–1361. doi: 10.1016/j.celrep.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 134.Steinecke A, Gampe C, Nitzsche F, Bolz J. DISC1 knockdown impairs the tangential migration of cortical interneurons by affecting the actin cytoskeleton. Front Cell Neurosci. 2014;8:190. doi: 10.3389/fncel.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Steinecke A, Gampe C, Valkova C, Kaether C, Bolz J. Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons. J Neurosci. 2012;32:738–745. doi: 10.1523/JNEUROSCI.5036-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen T, Wu Q, Zhang Y, Lu T, Yue W, Zhang D. Tcf4 controls neuronal migration of the cerebral cortex through regulation of Bmp7. Front Mol Neurosci. 2016;9:94. doi: 10.3389/fnmol.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wong CT, Ahmad E, Li H, Crawford DA. Prostaglandin E2 alters Wnt-dependent migration and proliferation in neuroectodermal stem cells: implications for autism spectrum disorders. Cell Commun Signal. 2014;12:19. doi: 10.1186/1478-811X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chow ML, Pramparo T, Winn ME, Barnes CC, Li HR, Weiss L, Fan JB, Murray S, April C, Belinson H, Fu XD, Wynshaw-Boris A, Schork NJ, Courchesne E. Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages. PLoS Genet. 2012;8:e1002592. doi: 10.1371/journal.pgen.1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoo HJ, Cho IH, Park M, Cho E, Cho SC, Kim BN, Kim JW, Kim SA. Association between PTGS2 polymorphism and autism spectrum disorders in Korean trios. Neurosci Res. 2008;62:66–69. doi: 10.1016/j.neures.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 140.Kim JE, Shin MS, Seo TB, Ji ES, Baek SS, Lee SJ, Park JK, Kim CJ. Treadmill exercise ameliorates motor disturbance through inhibition of apoptosis in the cerebellum of valproic acid-induced autistic rat pups. Mol Med Rep. 2013;8:327–334. doi: 10.3892/mmr.2013.1518. [DOI] [PubMed] [Google Scholar]
- 141.Abdallah MW, Michel TM. Matrix metalloproteinases in autism spectrum disorders. J Mol Psychiatry. 2013;1:16. doi: 10.1186/2049-9256-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lepeta K, Kaczmarek L. Matrix metalloproteinase-9 as a novel player in synaptic plasticity and schizophrenia. Schizophr Bull. 2015;41:1003–1009. doi: 10.1093/schbul/sbv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB, Haroutunian V. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology. 2008;33:2993–3009. doi: 10.1038/npp.2008.19. [DOI] [PubMed] [Google Scholar]
- 144.Wei J, Hemmings GP. A study of a genetic association between the PTGS2/PLA2G4A locus and schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2004;70:413–415. doi: 10.1016/j.plefa.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 145.Levchenko A, Davtian S, Freylichman O, Zagrivnaya M, Kostareva A, Malashichev Y. Beta-catenin in schizophrenia: possibly deleterious novel mutation. Psychiatry Res. 2015;228:843–848. doi: 10.1016/j.psychres.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 146.Hussman JP, Chung RH, Griswold AJ, Jaworski JM, Salyakina D, Ma D, Konidari I, Whitehead PL, Vance JM, Martin ER, Cuccaro ML, Gilbert JR, Haines JL, Pericak-Vance MA. A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism. Mol Autism. 2011;2:1. doi: 10.1186/2040-2392-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suda S, Iwata K, Shimmura C, Kameno Y, Anitha A, Thanseem I, Nakamura K, Matsuzaki H, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, Koizumi K, Higashida H, Takei N, Mori N. Decreased expression of axon-guidance receptors in the anterior cingulate cortex in autism. Mol Autism. 2011;2:14. doi: 10.1186/2040-2392-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu Y, Yang H, Bi Y, Zhang Y, Zhen C, Xie S, Qin H, He J, Liu L, Liu Y. Positive association between NTNG1 and schizophrenia in Chinese Han population. J Genet. 2011;90:499–502. doi: 10.1007/s12041-011-0112-8. [DOI] [PubMed] [Google Scholar]
- 149.Wilcox JA, Quadri S. replication of NTNG1 association in schizophrenia. Psychiatr Genet. 2014;24:266–268. doi: 10.1097/YPG.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 150.Eastwood SL, Harrison PJ. Decreased mRNA expression of netrin-G1 and netrin-G2 in the temporal lobe in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:933–945. doi: 10.1038/sj.npp.1301457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001;55:569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 152.Hollis ER., 2nd Axon guidance molecules and neural circuit remodeling after spinal cord injury. Neurotherapeutics. 2016;13:360–369. doi: 10.1007/s13311-015-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol. 2012;4:a008003. doi: 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guan KL, Rao Y. Signalling mechanisms mediating neuronal responses to guidance cues. Nat Rev Neurosci. 2003;4:941–956. doi: 10.1038/nrn1254. [DOI] [PubMed] [Google Scholar]
- 155.Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, Stacker SA, Cooper HM. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chai G, Goffinet AM, Tissir F. Celsr3 and Fzd3 in axon guidance. Int J Biochem Cell Biol. 2015;64:11–14. doi: 10.1016/j.biocel.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 157.Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, Zou Y, Pasterkamp RJ. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30:16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 159.Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell. 2011;20:177–191. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hua ZL, Smallwood PM, Nathans J. Frizzled3 controls axonal development in distinct populations of cranial and spinal motor neurons. Elife. 2013;2:e01482. doi: 10.7554/eLife.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feng J, Xian Q, Guan T, Hu J, Wang M, Huang Y, So KF, Evans SM, Chai G, Goffinet AM, Qu Y, Zhou L. Celsr3 and Fzd3 organize a pioneer neuron scaffold to steer growing thalamocortical axons. Cereb Cortex. 2016;26:3323–3334. doi: 10.1093/cercor/bhw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hua ZL, Jeon S, Caterina MJ, Nathans J. Frizzled3 is required for the development of multiple axon tracts in the mouse central nervous system. Proc Natl Acad Sci USA. 2014;111:E3005–E3014. doi: 10.1073/pnas.1406399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Skold M, Kallstrand J, Nehlstedt S, Nordin A, Nielzen S, Holmberg J, Adolfsson R. Thalamocortical abnormalities in auditory brainstem response patterns distinguish DSM-IV bipolar disorder type I from schizophrenia. J Affect Disord. 2014;169:105–111. doi: 10.1016/j.jad.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 164.Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98. doi: 10.3389/fpsyt.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nair A, Treiber JM, Shukla DK, Shih P, Muller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- 167.Van den Heuvel DM, Pasterkamp RJ. Getting connected in the dopamine system. Prog Neurobiol. 2008;85:75–93. doi: 10.1016/j.pneurobio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 168.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 170.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 171.Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- 172.Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 173.Fernando CV, Kele J, Bye CR, Niclis JC, Alsanie W, Blakely BD, Stenman J, Turner BJ, Parish CL. Diverse roles for Wnt7a in ventral midbrain neurogenesis and dopaminergic axon morphogenesis. Stem Cells Dev. 2014;23:1991–2003. doi: 10.1089/scd.2014.0166. [DOI] [PubMed] [Google Scholar]
- 174.Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, Zou Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- 175.Hutchins BI, Li L, Kalil K. Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev Neurobiol. 2011;71:269–283. doi: 10.1002/dneu.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hutchins BI, Li L, Kalil K. Wnt-induced calcium signaling mediates axon growth and guidance in the developing corpus callosum. Sci Signal. 2012;5:pt1. doi: 10.1126/scisignal.2002523. [DOI] [PubMed] [Google Scholar]
- 177.Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- 179.Wu GY, Zou DJ, Rajan I, Cline H. Dendritic dynamics in vivo change during neuronal maturation. J Neurosci. 1999;19:4472–4483. doi: 10.1523/JNEUROSCI.19-11-04472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- 181.Lai KO, Ip NY. Structural plasticity of dendritic spines: the underlying mechanisms and its dysregulation in brain disorders. Biochim Biophys Acta. 2013;1832:2257–2263. doi: 10.1016/j.bbadis.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 182.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wayman GA, Bose DD, Yang D, Lesiak A, Bruun D, Impey S, Ledoux V, Pessah IN, Lein PJ. PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ Health Perspect. 2012;120:1003–1009. doi: 10.1289/ehp.1104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jiang M, Ash RT, Baker SA, Suter B, Ferguson A, Park J, Rudy J, Torsky SP, Chao HT, Zoghbi HY, Smirnakis SM. Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J Neurosci. 2013;33:19518–19533. doi: 10.1523/JNEUROSCI.1745-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 187.Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- 188.Tan ZJ, Peng Y, Song HL, Zheng JJ, Yu X. N-cadherin-dependent neuron-neuron interaction is required for the maintenance of activity-induced dendrite growth. Proc Natl Acad Sci USA. 2010;107:9873–9878. doi: 10.1073/pnas.1003480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.D'Rozario M, Zhang T, Waddell EA, Zhang Y, Sahin C, Sharoni M, Hu T, Nayal M, Kutty K, Liebl F, Hu W, Marenda DR. Type I bHLH proteins daughterless and Tcf4 restrict neurite branching and synapse formation by repressing neurexin in postmitotic neurons. Cell Rep. 2016;15:386–397. doi: 10.1016/j.celrep.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 191.Hiester BG, Galati DF, Salinas PC, Jones KR. Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation. Mol Cell Neurosci. 2013;56:115–127. doi: 10.1016/j.mcn.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci. 2014;8:19. doi: 10.3389/fnins.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Falkai P, Schmitt A. Investigation of the influence of environmental factors in severe psychiatric diseases: epigenetics as new research tool. Fortschr Neurol Psychiatr. 2013;81:367. doi: 10.1055/s-0033-1335965. [DOI] [PubMed] [Google Scholar]
- 194.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 195.Okerlund ND, Kivimae S, Tong CK, Peng IF, Ullian EM, Cheyette BN. Dact1 is a postsynaptic protein required for dendrite, spine, and excitatory synapse development in the mouse forebrain. J Neurosci. 2010;30:4362–4368. doi: 10.1523/JNEUROSCI.0354-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Arguello A, Cheyette BN. Dapper Antagonist of Catenin-1 (Dact1) contributes to dendrite arborization in forebrain cortical interneurons. Commun Integr Biol. 2013;6 doi: 10.4161/cib.26656. e26656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Okerlund N, Stanley R, Cheyette B. The planar cell polarity transmembrane protein Vangl2 promotes dendrite, spine and glutamatergic synapse formation in the mammalian forebrain. Mol Neuropsychiatry. 2016;2:107–114. doi: 10.1159/000446778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hagiwara A, Yasumura M, Hida Y, Inoue E, Ohtsuka T. The planar cell polarity protein Vangl2 bidirectionally regulates dendritic branching in cultured hippocampal neurons. Mol Brain. 2014;7:79. doi: 10.1186/s13041-014-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nagaoka T, Ohashi R, Inutsuka A, Sakai S, Fujisawa N, Yokoyama M, Huang YH, Igarashi M, Kishi M. The Wnt/planar cell polarity pathway component Vangl2 induces synapse formation through direct control of N-cadherin. Cell Rep. 2014;6:916–927. doi: 10.1016/j.celrep.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 200.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 201.Lindqvist M, Horn Z, Bryja V, Schulte G, Papachristou P, Ajima R, Dyberg C, Arenas E, Yamaguchi TP, Lagercrantz H, Ringstedt T. Vang-like protein 2 and Rac1 interact to regulate adherens junctions. J Cell Sci. 2010;123:472–483. doi: 10.1242/jcs.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Negishi M, Katoh H. Rho family GTPases and dendrite plasticity. Neuroscientist. 2005;11:187–191. doi: 10.1177/1073858404268768. [DOI] [PubMed] [Google Scholar]
- 204.Van Aelst L, Cline HT. Rho GTPases and activity-dependent dendrite development. Curr Opin Neurobiol. 2004;14:297–304. doi: 10.1016/j.conb.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 205.Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- 206.Ciani L, Boyle KA, Dickins E, Sahores M, Anane D, Lopes DM, Gibb AJ, Salinas PC. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca2+/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci USA. 2011;108:10732–10737. doi: 10.1073/pnas.1018132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cerpa W, Gambrill A, Inestrosa NC, Barria A. Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J Neurosci. 2011;31:9466–9471. doi: 10.1523/JNEUROSCI.6311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 210.Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M, Inestrosa NC. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci. 2010;30:8411–8420. doi: 10.1523/JNEUROSCI.5736-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen X, Nelson CD, Li X, Winters CA, Azzam R, Sousa AA, Leapman RD, Gainer H, Sheng M, Reese TS. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011;31:6329–6338. doi: 10.1523/JNEUROSCI.5968-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- 216.Munoz FJ, Godoy JA, Cerpa W, Poblete IM, Huidobro-Toro JP, Inestrosa NC. Wnt-5a increases NO and modulates NMDA receptor in rat hippocampal neurons. Biochem Biophys Res Commun. 2014;444:189–194. doi: 10.1016/j.bbrc.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 217.Cerpa W, Latorre-Esteves E, Barria A. RoR2 functions as a noncanonical Wnt receptor that regulates NMDAR-mediated synaptic transmission. Proc Natl Acad Sci USA. 2015;112:4797–4802. doi: 10.1073/pnas.1417053112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 220.Lien CC, Martina M, Schultz JH, Ehmke H, Jonas P. Gating, modulation and subunit composition of voltage-gated K+ channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol. 2002;538:405–419. doi: 10.1113/jphysiol.2001.013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- 222.Varela-Nallar L, Parodi J, Farias GG, Inestrosa NC. Wnt-5a is a synaptogenic factor with neuroprotective properties against Abeta toxicity. Neurodegener Dis. 2012;10:23–26. doi: 10.1159/000333360. [DOI] [PubMed] [Google Scholar]
- 223.Paganoni S, Bernstein J, Ferreira A. Ror1-Ror2 complexes modulate synapse formation in hippocampal neurons. Neuroscience. 2010;165:1261–1274. doi: 10.1016/j.neuroscience.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Galjart N. CLIPs and CLASPs and cellular dynamics. Nat Rev Mol Cell Biol. 2005;6:487–498. doi: 10.1038/nrm1664. [DOI] [PubMed] [Google Scholar]
- 225.Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Arguello A, Yang X, Vogt D, Stanco A, Rubenstein JL, Cheyette BN. Dapper antagonist of catenin-1 cooperates with Dishevelled-1 during postsynaptic development in mouse forebrain GABAergic interneurons. PLoS One. 2013;8:e67679. doi: 10.1371/journal.pone.0067679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hida Y, Fukaya M, Hagiwara A, Deguchi-Tawarada M, Yoshioka T, Kitajima I, Inoue E, Watanabe M, Ohtsuka T. Prickle2 is localized in the postsynaptic density and interacts with PSD-95 and NMDA receptors in the brain. J Biochem. 2011;149:693–700. doi: 10.1093/jb/mvr023. [DOI] [PubMed] [Google Scholar]
- 228.Yoshioka T, Hagiwara A, Hida Y, Ohtsuka T. Vangl2, the planar cell polarity protein, is complexed with postsynaptic density protein PSD-95 [corrected] FEBS Lett. 2013;587:1453–1459. doi: 10.1016/j.febslet.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 229.Paemka L, Mahajan VB, Skeie JM, Sowers LP, Ehaideb SN, Gonzalez-Alegre P, Sasaoka T, Tao H, Miyagi A, Ueno N, Takao K, Miyakawa T, Wu S, Darbro BW, Ferguson PJ, Pieper AA, Britt JK, Wemmie JA, Rudd DS, Wassink T, El-Shanti H, Mefford HC, Carvill GL, Manak JR, Bassuk AG. PRICKLE1 interaction with SYNAPSIN I reveals a role in autism spectrum disorders. PLoS One. 2013;8:e80737. doi: 10.1371/journal.pone.0080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Provenzano G, Pangrazzi L, Poli A, Sgado P, Berardi N, Bozzi Y. Reduced phosphorylation of synapsin I in the hippocampus of Engrailed-2 knockout mice, a model for autism spectrum disorders. Neuroscience. 2015;286:122–130. doi: 10.1016/j.neuroscience.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 231.Greco B, Manago F, Tucci V, Kao HT, Valtorta F, Benfenati F. Autism-related behavioral abnormalities in synapsin knockout mice. Behav Brain Res. 2013;251:65–74. doi: 10.1016/j.bbr.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Molinaro L, Hui P, Tan M, Mishra RK. Role of presynaptic phosphoprotein synapsin II in schizophrenia. World J Psychiatry. 2015;5:260–272. doi: 10.5498/wjp.v5.i3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen Q, Che R, Wang X, O'Neill FA, Walsh D, Tang W, Shi Y, He L, Kendler KS, Chen X. Association and expression study of synapsin III and schizophrenia. Neurosci Lett. 2009;465:248–251. doi: 10.1016/j.neulet.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dyck BA, Skoblenick KJ, Castellano JM, Ki K, Thomas N, Mishra RK. Behavioral abnormalities in synapsin II knockout mice implicate a causal factor in schizophrenia. Synapse. 2009;63:662–672. doi: 10.1002/syn.20643. [DOI] [PubMed] [Google Scholar]
- 235.Saviouk V, Moreau MP, Tereshchenko IV, Brzustowicz LM. Association of synapsin 2 with schizophrenia in families of Northern European ancestry. Schizophr Res. 2007;96:100–111. doi: 10.1016/j.schres.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen Q, He G, Wang XY, Chen Q, Liu XM, Gu ZZ, Liu J, Li KQ, Wang SJ, Zhu SM, Feng GY, He L. Positive association between synapsin II and schizophrenia. Biol Psychiatry. 2004;56:177–181. doi: 10.1016/j.biopsych.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 237.Sowers LP, Loo L, Wu Y, Campbell E, Ulrich JD, Wu S, Paemka L, Wassink T, Meyer K, Bing X, El-Shanti H, Usachev YM, Ueno N, Manak JR, Shepherd AJ, Ferguson PJ, Darbro BW, Richerson GB, Mohapatra DP, Wemmie JA, Bassuk AG. Disruption of the non-canonical Wnt gene PRICKLE2 leads to autism-like behaviors with evidence for hippocampal synaptic dysfunction. Mol Psychiatry. 2013;18:1077–1089. doi: 10.1038/mp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 239.Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav. 2004;3:51–62. doi: 10.1046/j.1601-183x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 240.Martin PM, Yang X, Robin N, Lam E, Rabinowitz JS, Erdman CA, Quinn J, Weiss LA, Hamilton SP, Kwok PY, Moon RT, Cheyette BN. A rare WNT1 missense variant overrepresented in ASD leads to increased Wnt signal pathway activation. Transl Psychiatry. 2013;3:e301. doi: 10.1038/tp.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mohn JL, Alexander J, Pirone A, Palka CD, Lee SY, Mebane L, Haydon PG, Jacob MH. Adenomatous polyposis coli protein deletion leads to cognitive and autism-like disabilities. Mol Psychiatry. 2014;19:1133–1142. doi: 10.1038/mp.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dong F, Jiang J, McSweeney C, Zou D, Liu L, Mao Y. Deletion of CTNNB1 in inhibitory circuitry contributes to autism-associated behavioral defects. Hum Mol Genet. 2016;25:2738–2751. doi: 10.1093/hmg/ddw131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Koshimizu H, Fukui Y, Takao K, Ohira K, Tanda K, Nakanishi K, Toyama K, Oshima M, Taketo MM, Miyakawa T. Adenomatous polyposis coli heterozygous knockout mice display hypoactivity and age-dependent working memory deficits. Front Behav Neurosci. 2011;5:85. doi: 10.3389/fnbeh.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kivimae S, Martin PM, Kapfhamer D, Ruan Y, Heberlein U, Rubenstein JL, Cheyette BN. Abnormal behavior in mice mutant for the Disc1 binding partner, Dixdc1. Transl Psychiatry. 2011;1:e43. doi: 10.1038/tp.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Amar S, Jones BC, Nadri C, Kozlovsky N, Belmaker RH, Agam G. Genetic correlational analysis of glycogen synthase kinase-3 beta and prepulse inhibition in inbred mice. Genes Brain Behav. 2004;3:178–180. doi: 10.1111/j.1601-183X.2004.00065.x. [DOI] [PubMed] [Google Scholar]
- 246.O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gould TD, O'Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 249.Crespi B, Stead P, Elliot M. Evolution in health and medicine Sackler colloquium: comparative genomics of autism and schizophrenia. Proc Natl Acad Sci USA. 2010;107(suppl 1):1736–1741. doi: 10.1073/pnas.0906080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Goes FS, Pirooznia M, Parla JS, Kramer M, Ghiban E, Mavruk S, Chen YC, Monson ET, Willour VL, Karchin R, Flickinger M, Locke AE, Levy SE, Scott LJ, Boehnke M, Stahl E, Moran JL, Hultman CM, Landen M, Purcell SM, Sklar P, Zandi PP, McCombie WR, Potash JB. Exome sequencing of familial bipolar disorder. JAMA Psychiatry. 2016;73:590–597. doi: 10.1001/jamapsychiatry.2016.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Doherty JL, Owen MJ. Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Med. 2014;6:29. doi: 10.1186/gm546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chow TJ, Tee SF, Yong HS, Tang PY. Genetic association of TCF4 and AKT1 gene variants with the age at onset of schizophrenia. Neuropsychobiology. 2016;73:233–240. doi: 10.1159/000446285. [DOI] [PubMed] [Google Scholar]
- 254.Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, Mahajan M, Manaa D, Pawitan Y, Reichert J, Ripke S, Sandin S, Sklar P, Svantesson O, Reichenberg A, Hultman CM, Devlin B, Roeder K, Buxbaum JD. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, Murtha MT, Bal VH, Bishop SL, Dong S, Goldberg AP, Jinlu C, Keaney JF, 3rd, Klei L, Mandell JD, Moreno-De-Luca D, Poultney CS, Robinson EB, Smith L, Solli-Nowlan T, Su MY, Teran NA, Walker MF, Werling DM, Beaudet AL, Cantor RM, Fombonne E, Geschwind DH, Grice DE, Lord C, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87:1215–1233. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon K, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE; Schizophrenia Working Group of the Psychiatric Genomics Consortium, Daly MJ, Carroll MC, Stevens B, McCarroll SA. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tick B, Bolton P, Happe F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57:585–595. doi: 10.1111/jcpp.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, Gillan N, Hallett V, Lietz S, Garnett T, Ronald A, Plomin R, Rijsdijk F, Happe F, Bolton P. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry. 2015;72:415–423. doi: 10.1001/jamapsychiatry.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee YH, Grabowska E, Dalkic E, Wang Z, Marks S, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y, Mistry M, Pavlidis P, Solomon R, Ghiban E, Antoniou E, Kelleher E, O'Brien C, Donohoe G, Gill M, Morris DW, McCombie WR, Corvin A. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry. 2014;19:652–658. doi: 10.1038/mp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, Witherspoon K, Gerdts J, Baker C, Vulto-van Silfhout AT, Schuurs-Hoeijmakers JH, Fichera M, Bosco P, Buono S, Alberti A, Failla P, Peeters H, Steyaert J, Vissers LE, Francescatto L, Mefford HC, Rosenfeld JA, Bakken T, O'Roak BJ, Pawlus M, Moon R, Shendure J, Amaral DG, Lein E, Rankin J, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158:263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Durak O, Gao F, Kaeser-Woo YJ, Rueda R, Martorell AJ, Nott A, Liu CY, Watson LA, Tsai LH. Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat Neurosci. 2016;19:1477–1488. doi: 10.1038/nn.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wassink TH, Piven J, Vieland VJ, Huang J, Swiderski RE, Pietila J, Braun T, Beck G, Folstein SE, Haines JL, Sheffield VC. Evidence supporting WNT2 as an autism susceptibility gene. Am J Med Genet. 2001;105:406–413. doi: 10.1002/ajmg.1401. [DOI] [PubMed] [Google Scholar]
- 267.Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Turner TN, Hormozdiari F, Duyzend MH, McClymont SA, Hook PW, Iossifov I, Raja A, Baker C, Hoekzema K, Stessman HA, Zody MC, Nelson BJ, Huddleston J, Sandstrom R, Smith JD, Hanna D, Swanson JM, Faustman EM, Bamshad MJ, Stamatoyannopoulos J, Nickerson DA, McCallion AS, Darnell R, Eichler EE. Genome sequencing of autism-affected families reveals disruption of putative noncoding regulatory DNA. Am J Hum Genet. 2016;98:58–74. doi: 10.1016/j.ajhg.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Barber JC, Ellis KH, Bowles LV, Delhanty JD, Ede RF, Male BM, Eccles DM. Adenomatous polyposis coli and a cytogenetic deletion of chromosome 5 resulting from a maternal intrachromosomal insertion. J Med Genet. 1994;31:312–316. doi: 10.1136/jmg.31.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhou XL, Giacobini M, Anderlid BM, Anckarsater H, Omrani D, Gillberg C, Nordenskjold M, Lindblom A. Association of adenomatous polyposis coli (APC) gene polymorphisms with autism spectrum disorder (ASD) Am J Med Genet B Neuropsychiatr Genet. 2007;144B:351–354. doi: 10.1002/ajmg.b.30415. [DOI] [PubMed] [Google Scholar]
- 271.Krumm N, O'Roak BJ, Shendure J, Eichler EE. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014;37:95–105. doi: 10.1016/j.tins.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lotan A, Fenckova M, Bralten J, Alttoa A, Dixson L, Williams RW, van der Voet M. Neuroinformatic analyses of common and distinct genetic components associated with major neuropsychiatric disorders. Front Neurosci. 2014;8:331. doi: 10.3389/fnins.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Talkowski ME, Rosenfeld JA, Blumenthal I, Pillalamarri V, Chiang C, Heilbut A, Ernst C, Hanscom C, Rossin E, Lindgren AM, Pereira S, Ruderfer D, Kirby A, Ripke S, Harris DJ, Lee JH, Ha K, Kim HG, Solomon BD, Gropman AL, Lucente D, Sims K, Ohsumi TK, Borowsky ML, Loranger S, Quade B, Lage K, Miles J, Wu BL, Shen Y, et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012;149:525–537. doi: 10.1016/j.cell.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 275.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Maher BJ, LoTurco JJ. Disrupted-in-schizophrenia (DISC1) functions presynaptically at glutamatergic synapses. PLoS One. 2012;7:e34053. doi: 10.1371/journal.pone.0034053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Chen Y, Huang WC, Sejourne J, Clipperton-Allen AE, Page DT. Pten mutations alter brain growth trajectory and allocation of cell types through elevated beta-catenin signaling. J Neurosci. 2015;35:10252–10267. doi: 10.1523/JNEUROSCI.5272-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.O'Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, Carvill G, Kumar A, Lee C, Ankenman K, Munson J, Hiatt JB, Turner EH, Levy R, O'Day DR, Krumm N, Coe BP, Martin BK, Borenstein E, Nickerson DA, Mefford HC, Doherty D, Akey JM, Bernier R, Eichler EE, Shendure J. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Spinelli L, Black FM, Berg JN, Eickholt BJ, Leslie NR. Functionally distinct groups of inherited PTEN mutations in autism and tumour syndromes. J Med Genet. 2015;52:128–134. doi: 10.1136/jmedgenet-2014-102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Frazier TW, Embacher R, Tilot AK, Koenig K, Mester J, Eng C. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol Psychiatry. 2015;20:1132–1138. doi: 10.1038/mp.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF, Herman GE. Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res. 2010;3:137–141. doi: 10.1002/aur.132. [DOI] [PubMed] [Google Scholar]
- 282.Cukier HN, Dueker ND, Slifer SH, Lee JM, Whitehead PL, Lalanne E, Leyva N, Konidari I, Gentry RC, Hulme WF, Booven DV, Mayo V, Hofmann NK, Schmidt MA, Martin ER, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Exome sequencing of extended families with autism reveals genes shared across neurodevelopmental and neuropsychiatric disorders. Mol Autism. 2014;5:1. doi: 10.1186/2040-2392-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bosoi CM, Capra V, Allache R, Trinh VQ, De Marco P, Merello E, Drapeau P, Bassuk AG, Kibar Z. Identification and characterization of novel rare mutations in the planar cell polarity gene PRICKLE1 in human neural tube defects. Hum Mutat. 2011;32:1371–1375. doi: 10.1002/humu.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tao H, Manak JR, Sowers L, Mei X, Kiyonari H, Abe T, Dahdaleh NS, Yang T, Wu S, Chen S, Fox MH, Gurnett C, Montine T, Bird T, Shaffer LG, Rosenfeld JA, McConnell J, Madan-Khetarpal S, Berry-Kravis E, Griesbach H, Saneto RP, Scott MP, Antic D, Reed J, Boland R, Ehaideb SN, El-Shanti H, Mahajan VB, Ferguson PJ, Axelrod JD, et al. Mutations in prickle orthologs cause seizures in flies, mice, and humans. Am J Hum Genet. 2011;88:138–149. doi: 10.1016/j.ajhg.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Buckley AW, Holmes GL. Epilepsy and Autism. Cold Spring Harb Perspect Med. 2016;6:a022749. doi: 10.1101/cshperspect.a022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 288.Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12–17. [PubMed] [Google Scholar]
- 289.Aleksic B, Kushima I, Ito Y, Nakamura Y, Ujike H, Suzuki M, Inada T, Hashimoto R, Takeda M, Iwata N, Ozaki N. Genetic association study of KREMEN1 and DKK1 and schizophrenia in a Japanese population. Schizophr Res. 2010;118:113–117. doi: 10.1016/j.schres.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 290.Proitsi P, Li T, Hamilton G, Di Forti M, Collier D, Killick R, Chen R, Sham P, Murray R, Powell J, Lovestone S. Positional pathway screen of wnt signaling genes in schizophrenia: association with DKK4. Biol Psychiatry. 2008;63:13–16. doi: 10.1016/j.biopsych.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 291.Pulver AE, Lasseter VK, Kasch L, Wolyniec P, Nestadt G, Blouin JL, Kimberland M, Babb R, Vourlis S, Chen H, et al. Schizophrenia: a genome scan targets chromosomes 3p and 8p as potential sites of susceptibility genes. Am J Med Genet. 1995;60:252–260. doi: 10.1002/ajmg.1320600316. [DOI] [PubMed] [Google Scholar]
- 292.Kendler KS, MacLean CJ, O'Neill FA, Burke J, Murphy B, Duke F, Shinkwin R, Easter SM, Webb BT, Zhang J, Walsh D, Straub RE. Evidence for a schizophrenia vulnerability locus on chromosome 8p in the Irish Study of High-Density Schizophrenia Families. Am J Psychiatry. 1996;153:1534–1540. doi: 10.1176/ajp.153.12.1534. [DOI] [PubMed] [Google Scholar]
- 293.Tabares-Seisdedos R, Rubenstein JL. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry. 2009;14:563–589. doi: 10.1038/mp.2009.2. [DOI] [PubMed] [Google Scholar]
- 294.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- 295.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 296.Zandi PP, Belmonte PL, Willour VL, Goes FS, Badner JA, Simpson SG, Gershon ES, McMahon FJ, DePaulo JR, Jr, Potash JB, Bipolar Disorder Phenome Group, National Institute of Mental Health Genetics Initiative Bipolar Disorder Consortium Association study of Wnt signaling pathway genes in bipolar disorder. Arch Gen Psychiatry. 2008;65:785–793. doi: 10.1001/archpsyc.65.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Hennah W, Thomson P, McQuillin A, Bass N, Loukola A, Anjorin A, Blackwood D, Curtis D, Deary IJ, Harris SE, Isometsa ET, Lawrence J, Lonnqvist J, Muir W, Palotie A, Partonen T, Paunio T, Pylkko E, Robinson M, Soronen P, Suominen K, Suvisaari J, Thirumalai S, St Clair D, Gurling H, Peltonen L, Porteous D. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol Psychiatry. 2009;14:865–873. doi: 10.1038/mp.2008.22. [DOI] [PubMed] [Google Scholar]
- 298.Palo OM, Antila M, Silander K, Hennah W, Kilpinen H, Soronen P, Tuulio-Henriksson A, Kieseppa T, Partonen T, Lonnqvist J, Peltonen L, Paunio T. Association of distinct allelic haplotypes of DISC1 with psychotic and bipolar spectrum disorders and with underlying cognitive impairments. Hum Mol Genet. 2007;16:2517–2528. doi: 10.1093/hmg/ddm207. [DOI] [PubMed] [Google Scholar]
- 299.Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH, Malhotra AK. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Millar JK, Christie S, Anderson S, Lawson D, Hsiao-Wei Loh D, Devon RS, Arveiler B, Muir WJ, Blackwood DH, Porteous DJ. Genomic structure and localisation within a linkage hotspot of Disrupted In Schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol Psychiatry. 2001;6:173–178. doi: 10.1038/sj.mp.4000784. [DOI] [PubMed] [Google Scholar]
- 301.Cuellar-Barboza AB, Winham SJ, McElroy SL, Geske JR, Jenkins GD, Colby CL, Prieto ML, Ryu E, Cunningham JM, Frye MA, Biernacka JM. Accumulating evidence for a role of TCF7L2 variants in bipolar disorder with elevated body mass index. Bipolar Disord. 2016;18:124–135. doi: 10.1111/bdi.12368. [DOI] [PubMed] [Google Scholar]
- 302.Winham SJ, Cuellar-Barboza AB, Oliveros A, McElroy SL, Crow S, Colby C, Choi DS, Chauhan M, Frye M, Biernacka JM. Genome-wide association study of bipolar disorder accounting for effect of body mass index identifies a new risk allele in TCF7L2. Mol Psychiatry. 2014;19:1010–1016. doi: 10.1038/mp.2013.159. [DOI] [PubMed] [Google Scholar]
- 303.Potash JB, Buervenich S, Cox NJ, Zandi PP, Akula N, Steele J, Rathe JA, Avramopoulos D, Detera-Wadleigh SD, Gershon ES, DePaulo JR, Jr, Feinberg AP, McMahon FJ, Consortium NGIBD. Gene-based SNP mapping of a psychotic bipolar affective disorder linkage region on 22q12.3:association with HMG2L1 and TOM1. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:59–67. doi: 10.1002/ajmg.b.30574. [DOI] [PubMed] [Google Scholar]
- 304.Yamada M, Ohkawara B, Ichimura N, Hyodo-Miura J, Urushiyama S, Shirakabe K, Shibuya H. Negative regulation of Wnt signalling by HMG2L1, a novel NLK-binding protein. Genes Cells. 2003;8:677–684. doi: 10.1046/j.1365-2443.2003.00666.x. [DOI] [PubMed] [Google Scholar]
- 305.Song J, Bergen SE, Di Florio A, Karlsson R, et al. Genome-wide association study identifies SESTD1 as a novel risk gene for lithium-responsive bipolar disorder. Mol Psychiatry. 2016;21:1290–1297. doi: 10.1038/mp.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Miehe S, Bieberstein A, Arnould I, Ihdene O, Rutten H, Strubing C. The phospholipid-binding protein SESTD1 is a novel regulator of the transient receptor potential channels TRPC4 and TRPC5. J Biol Chem. 2010;285:12426–12434. doi: 10.1074/jbc.M109.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yang X, Cheyette BN. SEC14 and spectrin domains 1 (Sestd1) and Dapper antagonist of catenin 1 (Dact1) scaffold proteins cooperatively regulate the Van Gogh-like 2 (Vangl2) four-pass transmembrane protein and planar cell polarity (PCP) pathway during embryonic development in mice. J Biol Chem. 2013;288:20111–20120. doi: 10.1074/jbc.M113.465427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11(suppl 2):4–9. doi: 10.1111/j.1399-5618.2009.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gerdtz J. Mental illness and the Roman physician: the legacy of Soranus of Ephesus. Hosp Community Psychiatry. 1994;45:485–487. doi: 10.1176/ps.45.5.485. [DOI] [PubMed] [Google Scholar]
- 310.Lenox RH, Wang L. Molecular basis of lithium action: integration of lithium-responsive signaling and gene expression networks. Mol Psychiatry. 2003;8:135–144. doi: 10.1038/sj.mp.4001306. [DOI] [PubMed] [Google Scholar]
- 311.O'Brien WT, Klein PS. Validating GSK3 as an in vivo target of lithium action. Biochem Soc Trans. 2009;37:1133–1138. doi: 10.1042/BST0371133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Dudev T, Lim C. Competition between Li+ and Mg2+ in metalloproteins. Implications for lithium therapy. J Am Chem Soc. 2011;133:9506–9515. doi: 10.1021/ja201985s. [DOI] [PubMed] [Google Scholar]
- 313.Boku S, Nakagawa S, Masuda T, Nishikawa H, Kato A, Takamura N, Omiya Y, Kitaichi Y, Inoue T, Kusumi I. Valproate recovers the inhibitory effect of dexamethasone on the proliferation of the adult dentate gyrus-derived neural precursor cells via GSK-3beta and beta-catenin pathway. Eur J Pharmacol. 2014;723:425–430. doi: 10.1016/j.ejphar.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 314.Long ZM, Zhao L, Jiang R, Wang KJ, Luo SF, Zheng M, Li XF, He GQ. Valproic acid modifies synaptic structure and accelerates neurite outgrowth via the glycogen synthase kinase-3beta signaling pathway in an Alzheimer's disease model. CNS Neurosci Ther. 2015;21:887–897. doi: 10.1111/cns.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- 316.Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 317.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kang UG, Seo MS, Roh MS, Kim Y, Yoon SC, Kim YS. The effects of clozapine on the GSK-3-mediated signaling pathway. FEBS Lett. 2004;560:115–119. doi: 10.1016/S0014-5793(04)00082-1. [DOI] [PubMed] [Google Scholar]
- 319.Roh MS, Seo MS, Kim Y, Kim SH, Jeon WJ, Ahn YM, Kang UG, Juhnn YS, Kim YS. Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exp Mol Med. 2007;39:353–360. doi: 10.1038/emm.2007.39. [DOI] [PubMed] [Google Scholar]
- 320.Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 321.Hui J, Zhang J, Kim H, Tong C, Ying Q, Li Z, Mao X, Shi G, Yan J, Zhang Z, Xi G. Fluoxetine regulates neurogenesis in vitro through modulation of GSK-3beta/beta-catenin signaling. Int J Neuropsychopharmacol. 2014;18:pyu099. doi: 10.1093/ijnp/pyu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Liu R, Dang W, Jianting M, Su C, Wang H, Chen Y, Tan Q. Citalopram alleviates chronic stress induced depression-like behaviors in rats by activating GSK3beta signaling in dorsal hippocampus. Brain Res. 2012;1467:10–17. doi: 10.1016/j.brainres.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 324.Joaquim HP, Talib LL, Forlenza OV, Diniz BS, Gattaz WF. Long-term sertraline treatment increases expression and decreases phosphorylation of glycogen synthase kinase-3B in platelets of patients with late-life major depression. J Psychiatr Res. 2012;46:1053–1058. doi: 10.1016/j.jpsychires.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 325.Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kozikowski AP, Gaisina IN, Yuan H, Petukhov PA, Blond SY, Fedolak A, Caldarone B, McGonigle P. Structure-based design leads to the identification of lithium mimetics that block mania-like effects in rodents. possible new GSK-3beta therapies for bipolar disorders. J Am Chem Soc. 2007;129:8328–8332. doi: 10.1021/ja068969w. [DOI] [PubMed] [Google Scholar]
- 327.Seto S, Yumoto K, Okada K, Asahina Y, Iwane A, Iwago M, Terasawa R, Shreder KR, Murakami K, Kohno Y. Quinolone derivatives containing strained spirocycle as orally active glycogen synthase kinase 3beta (GSK-3beta) inhibitors for type 2 diabetics. Bioorg Med Chem. 2012;20:1188–1200. doi: 10.1016/j.bmc.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 328.Lange C, Mix E, Frahm J, Glass A, Muller J, Schmitt O, Schmole AC, Klemm K, Ortinau S, Hubner R, Frech MJ, Wree A, Rolfs A. Small molecule GSK-3 inhibitors increase neurogenesis of human neural progenitor cells. Neurosci Lett. 2011;488:36–40. doi: 10.1016/j.neulet.2010.10.076. [DOI] [PubMed] [Google Scholar]
- 329.Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 331.Huang RY, Hsieh KP, Huang WW, Yang YH. Use of lithium and cancer risk in patients with bipolar disorder: population-based cohort study. Br J Psychiatry. 2016;209:393–399. doi: 10.1192/bjp.bp.116.181362. [DOI] [PubMed] [Google Scholar]
- 332.Martinsson L, Westman J, Hallgren J, Osby U, Backlund L. Lithium treatment and cancer incidence in bipolar disorder. Bipolar Disord. 2016;18:33–40. doi: 10.1111/bdi.12361. [DOI] [PubMed] [Google Scholar]
- 333.Gustin JP, Karakas B, Weiss MB, Abukhdeir AM, Lauring J, Garay JP, Cosgrove D, Tamaki A, Konishi H, Konishi Y, Mohseni M, Wang G, Rosen DM, Denmeade SR, Higgins MJ, Vitolo MI, Bachman KE, Park BH. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc Natl Acad Sci USA. 2009;106:2835–2840. doi: 10.1073/pnas.0813351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Michaelis M, Doerr HW, Cinatl J., Jr Valproic acid as anti-cancer drug. Curr Pharm Des. 2007;13:3378–3393. [PubMed] [Google Scholar]
- 335.Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH, Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Roullet FI, Lai JK, Foster JA. In utero exposure to valproic acid and autism – a current review of clinical and animal studies. Neurotoxicol Teratol. 2013;36:47–56. doi: 10.1016/j.ntt.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 337.Galbally M, Roberts M, Buist A, Perinatal Psychotropic Review Group Mood stabilizers in pregnancy: a systematic review. Aust N Z J Psychiatry. 2010;44:967–977. doi: 10.3109/00048674.2010.506637. [DOI] [PubMed] [Google Scholar]
- 338.van der Lugt NM, van de Maat JS, van Kamp IL, Knoppert-van der Klein EA, Hovens JG, Walther FJ. Fetal, neonatal and developmental outcomes of lithium-exposed pregnancies. Early Hum Dev. 2012;88:375–378. doi: 10.1016/j.earlhumdev.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 339.Kozma C. Neonatal toxicity and transient neurodevelopmental deficits following prenatal exposure to lithium: another clinical report and a review of the literature. Am J Med Genet A. 2005;132A:441–444. doi: 10.1002/ajmg.a.30501. [DOI] [PubMed] [Google Scholar]
- 340.Diav-Citrin O, Shechtman S, Tahover E, Finkel-Pekarsky V, Arnon J, Kennedy D, Erebara A, Einarson A, Ornoy A. Pregnancy outcome following in utero exposure to lithium: a prospective, comparative, observational study. Am J Psychiatry. 2014;171:785–794. doi: 10.1176/appi.ajp.2014.12111402. [DOI] [PubMed] [Google Scholar]
- 341.Belinson H, Nakatani J, Babineau BA, Birnbaum RY, Ellegood J, Bershteyn M, McEvilly RJ, Long JM, Willert K, Klein OD, Ahituv N, Lerch JP, Rosenfeld MG, Wynshaw-Boris A. Prenatal beta-catenin/Brn2/Tbr2 transcriptional cascade regulates adult social and stereotypic behaviors. Mol Psychiatry. 2016;21:1417–1433. doi: 10.1038/mp.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Marchetto MC, Belinson H, Tian Y, Freitas BC, Fu C, Vadodaria KC, Beltrao-Braga PC, Trujillo CA, Mendes AP, Padmanabhan K, Nunez Y, Ou J, Ghosh H, Wright R, Brennand KJ, Pierce K, Eichenfield L, Pramparo T, Eyler LT, Barnes CC, Courchesne E, Geschwind DH, Gage FH, Wynshaw-Boris A, Muotri AR. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry. doi: 10.1038/mp.2016.95. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Martin PM, Stanley RE, Ross AP, Freitas AE, Moyer CE, Brumback AC, Iafrati J, Stapornwongkul KS, Dominguez S, Kivimae S, Mulligan KA, Pirooznia M, McCombie WR, Potash JB, Zandi PP, Purcell SM, Sanders SJ, Zuo Y, Sohal VS, Cheyette BN. DIXDC1 contributes to psychiatric susceptibility by regulating dendritic spine and glutamatergic synapse density via GSK3 and Wnt/beta-catenin signaling. Mol Psychiatry. doi: 10.1038/mp.2016.184. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Higgins GA, Allyn-Feuer A, Barbour E, Athey BD. A glutamatergic network mediates lithium response in bipolar disorder as defined by epigenome pathway analysis. Pharmacogenomics. 2015;16:1547–1563. doi: 10.2217/pgs.15.106. [DOI] [PubMed] [Google Scholar]
- 345.Kondratiuk I, Leski S, Urbanska M, Biecek P, Devijver H, Lechat B, Van Leuven F, Kaczmarek L, Jaworski T. GSK-3beta and MMP-9 cooperate in the control of dendritic spine morphology. Mol Neurobiol. doi: 10.1007/s12035-015-9625-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Suriben R, Kivimae S, Fisher DA, Moon RT, Cheyette BN. Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet. 2009;41:977–985. doi: 10.1038/ng.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–2649. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- 348.Kalkman HO. A review of the evidence for the canonical Wnt pathway in autism spectrum disorders. Mol Autism. 2012;3:10. doi: 10.1186/2040-2392-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 350.Zhang Y, Yeh JR, Mara A, Ju R, Hines JF, Cirone P, Griesbach HL, Schneider I, Slusarski DC, Holley SA, Crews CM. A chemical and genetic approach to the mode of action of fumagillin. Chem Biol. 2006;13:1001–1009. doi: 10.1016/j.chembiol.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sundberg TB, Darricarrere N, Cirone P, Li X, McDonald L, Mei X, Westlake CJ, Slusarski DC, Beynon RJ, Crews CM. Disruption of Wnt planar cell polarity signaling by aberrant accumulation of the MetAP-2 substrate Rab37. Chem Biol. 2011;18:1300–1311. doi: 10.1016/j.chembiol.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]