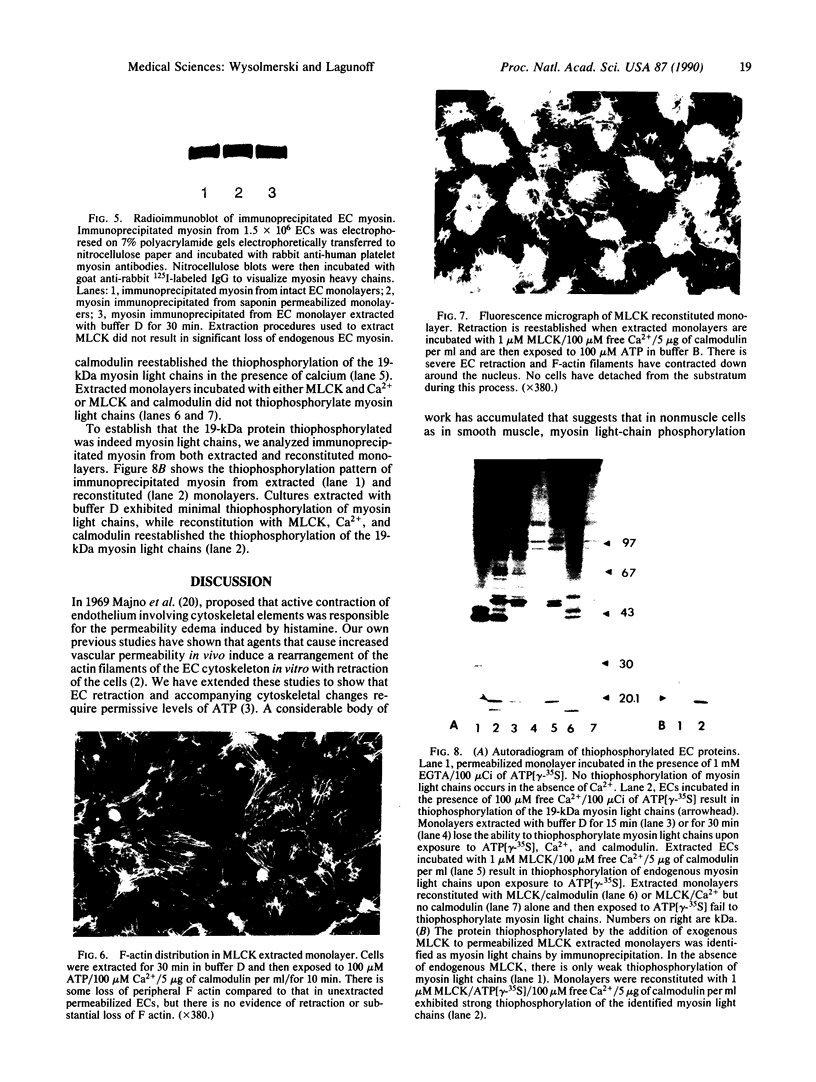
Abstract
Permeabilized bovine pulmonary artery endothelial cell monolayers were used to investigate the mechanism of endothelial cell retraction. Postconfluent endothelial cells permeabilized with saponin retracted upon exposure to ATP and Ca2+. Retraction was accompanied by thiophosphorylation of 19,000-Da myosin light chains when adenosine 5'-[gamma-[35S]thio]triphosphate was included in the medium. Both retraction and thiophosphorylation of myosin light chains exhibited a graded quantitative dependence on Ca2+. When permeabilized monolayers were extracted in buffer D containing 100 mM KCl and 30 mM MgCl2 for 30 min, the cells failed to retract upon exposure to ATP and Ca2+, and no thiophosphorylation of myosin light chains occurred. The ability both to retract and to thiophosphorylate myosin light chains was restored by the addition to the permeabilized, extracted cells of myosin light-chain kinase and calmodulin together but not by either alone. These studies indicate that endothelial cell retraction, as does smooth muscle contraction, depends on myosin light-chain kinase phosphorylation of myosin light chains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol. 1982 May;242(5):C404–C408. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- Broschat K. O., Stidwill R. P., Burgess D. R. Phosphorylation controls brush border motility by regulating myosin structure and association with the cytoskeleton. Cell. 1983 Dec;35(2 Pt 1):561–571. doi: 10.1016/0092-8674(83)90190-3. [DOI] [PubMed] [Google Scholar]
- Del Vecchio P. J., Smith J. R. Expression of angiotensin-converting enzyme activity in cultured pulmonary artery endothelial cells. J Cell Physiol. 1981 Sep;108(3):337–345. doi: 10.1002/jcp.1041080307. [DOI] [PubMed] [Google Scholar]
- Holzapfel G., Wehland J., Weber K. Calcium control of actin-myosin based contraction in triton models of mouse 3T3 fibroblasts is mediated by the myosin light chain kinase (MLCK)-calmodulin complex. Exp Cell Res. 1983 Oct;148(1):117–126. doi: 10.1016/0014-4827(83)90192-1. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kargacin G. J., Fay F. S. Physiological and structural properties of saponin-skinned single smooth muscle cells. J Gen Physiol. 1987 Jul;90(1):49–73. doi: 10.1085/jgp.90.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Adelstein R. S. The heavy chain of smooth muscle myosin is phosphorylated in aorta cells. J Biol Chem. 1988 Jan 25;263(3):1099–1102. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laposata M., Dovnarsky D. K., Shin H. S. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983 Sep;62(3):549–556. [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Owaribe K., Hayashi H., Hatano S. Ca2+-dependent contraction of human lung fibroblasts treated with Triton X-100: a role of Ca2+-calmodulin-dependent phosphorylation of myosin 20,000-dalton light chain. Cell Motil. 1984;4(5):315–331. doi: 10.1002/cm.970040503. [DOI] [PubMed] [Google Scholar]
- Porrello K., Burnside B. Regulation of reactivated contraction in teleost retinal cone models by calcium and cyclic adenosine monophosphate. J Cell Biol. 1984 Jun;98(6):2230–2238. doi: 10.1083/jcb.98.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Wysolmerski R. B., Lagunoff D. Inhibition of endothelial cell retraction by ATP depletion. Am J Pathol. 1988 Jul;132(1):28–37. [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski R., Lagunoff D. The effect of ethchlorvynol on cultured endothelial cells. A model for the study of the mechanism of increased vascular permeability. Am J Pathol. 1985 Jun;119(3):505–512. [PMC free article] [PubMed] [Google Scholar]











