Abstract
Introduction
Advantages of vascular closure device over manual compression include patient comfort, early mobilisation and discharge, avoidance of interruption of anticoagulation, avoidance of local compression and its sequelae and less time constraint on staff. No published Indian data exist regarding Perclose Proglide suture mediated vascular closure device (SMC).
Aim
To study the 24 h and 30 day outcome of Perclose Proglide SMC retrospectively.
Study design
Retrospective observational study conducted in the Department of Cardiology, Government Medical College, Calicut, Kerala from June 2013 to June 2015.
Methodology
All consecutive patients with Perclose Proglide SMC deployment done by a single operator for achieving access site haemostasis where 24 h and 30 day post-procedure data were available were included. Major and minor complications, procedure success, device failure were predefined.
Results
323 patients were analysed. Procedure success rate was 99.7% (322/323). Transient oozing occurred in 44 patients (13.6%), minor and major complications occurred in 2% and 1.5% of patients respectively. Major complication included one case of retroperitoneal bleed, one access site infection, one pseudo aneurysm formation and two access site arterial stenosis. There was no death or complication requiring limb amputation. “Preclose” technique was used successfully in six patients. Primary device failure occurred in 12 cases which were tackled successfully with second Proglide in all except one.
Conclusion
Perclose Proglide SMC is a safe and effective method to achieve haemostasis up to 22F with less complication rate.
Abbreviations: PEVAR, percutaneous endovascular aneurysm repair; EVAR, endovascular aneurysm repair; TAVI, trans catheter aortic valve implantation; SMC, suture mediated closure; VCD, vascular closure device; MRSA, methicillin resistant Staphylococcus aureus; CFA, common femoral artery; RCT, randomised control trial
Keywords: Suture mediated vascular closure device, Perclose Proglide, EVAR, Preclose
1. Background
Common femoral artery is the most common arterial access site for percutaneous intervention in our institution, with manual compression as the most common method to achieve haemostasis. Although manual compression is the gold standard for achieving the haemostasis, use of vascular closure devices is becoming popular. The limitations of manual compression are the need to interrupt anticoagulation, repeat administration of local anaesthetic, patient's discomfort including prolonged recumbency, back pain, urinary retention and vasovagal reaction. The commonly used vascular closure devices are either collagen based (Angioseal, Vasoseal) or suture mediated (Perclose Prostar, Perclose AT, Perclose Proglide). The advantages of vascular closure devices are better patient comfort, less stress on staff, less nursing time for post-procedure monitoring, shorter time for haemostasis, early ambulation and early hospital discharge. 6F Perclose Proglide suture mediated closure device (Abbott Vascular Devices, Redwood City, CA, USA) is the most common vascular closure device used to achieve haemostasis in our cath lab. Major limitation of Proglide is the cost of the device. Literature review shows paucity of Indian data. Studies from abroad may not be applicable to our population and various published studies show different rates of complications. Our aim was to retrospectively study the outcome of Perclose Proglide suture mediated closure device (SMC) in Indian population.
2. Aim of study
To study the 24 h and 30 day outcome of 6F Perclose Proglide suture mediated closure device.
3. Methodology
3.1. Study design
Retrospective observational study from the Department of Cardiology, Government Medical College, Calicut, Kerala, India during June 2013 to June 2015.
3.2. Inclusion criteria
All patients undergoing percutaneous interventions in whom Perclose Proglide SMC was deployed to achieve haemostasis by a single operator, provided their 24 h and 30 day follow-up data regarding clinical outcome and access site are available.
3.3. Exclusion criteria
If the inclusion criteria are not satisfied.
3.4. Data collection
Baseline clinical data and 24 h post-procedure data were collected from the prospective institutional interventional database. For 30 day's clinical data, patients were contacted over telephone and advised to review with the follow-up data card. If clinical data regarding access site and ipsilateral limb are not available, patients were interviewed regarding access site symptoms or any hospitalisation for access site complication or ipsilateral claudication during the first 30 days after the procedure. If the patients were admitted for access site complications during the first month post-procedure, their clinical data are collected from the hospital case record or discharge summary.
4. Perclose Proglide SMC deployment-procedure1 (Supplementary video)
All the deployments were done by a single operator and the decision to deploy was at the discretion of the operator. Perclose Proglide SMC is a 6F suture based haemostatic device using polypropylene suture. The device consists of a plunger, handle, guide, and knot pusher. All Perclose Proglide insertions were done after ensuring vascular surgery back up. Pre-insertion fluoroscopy was used to evaluate local vessel wall calcification. Antibiotic prophylaxis was not used for device placement. Periprocedural antiplatelets and anticoagulants were used in accordance with the institutional protocol for the respective interventional procedure.
After the percutaneous procedure, under strict aseptic precaution catheter is removed and a 0.035 in. guide wire introduced through the sheath. Perclose Proglide device is tracked over the wire. Once the wire exit port of the device reaches the skin surface, the guide wire is removed and device is further advanced until pulsatile bleeding appears through the marker lumen side port which confirms the intravascular positioning. Subsequently the lever is pulled to deploy the “feet” within the arterial lumen. The device is gently pulled back to position the feet against the anterior arterial wall. As the plunger is depressed, two needles are deployed within the tissue track and directed towards the feet. Further depression of the plunger, advances the needles through the arterial wall and into the feet. The feet capture the needles, creating a suture loop. The device (containing the needles) is then removed, leaving behind the two suture tails. The longer tail is pulled steadily and preformed knot is pushed towards the arteriotomy site using the knot pusher provided to achieve haemostasis. The remaining free suture is then cut just above the knot using the blade embedded in the knot pusher, and a suitable dressing is placed on the wound. After the procedure distal flow is confirmed by examining the distal pulse. Manual compression is used if bleeding continues after placing the suture. Patients remained supine for 6 h after the procedure. They are reassessed at 18–24 h after the procedure for any complication and if there are no complications, the patients are discharged within 24 h.
5. Preclose technique
Perclose Proglide is used in the “Preclose” manner for large sized wounds. Wounds up to 14F shall be closed by two preplaced sutures and above 14F using three pre-placed sutures. For this, a femoral angiogram of the target vessel is performed after inserting a 5F/6F pigtail catheter through the opposite femoral artery or an upper limb artery. The loop of the pigtail catheter is placed at the target site of puncture with a clear visualisation of the loop “en face” in PA view. This angiogram is used as the landmark to ensure the puncture in the centre of the vessel wall anteriorly. Through the standard 18 gauge puncture needle a 0.035 J guide wire is introduced over which the first Proglide SMC is introduced. Guide wire is removed, SMC is rotated to 10 o’ clock position and first suture is placed. The 0.035 J guide wire is re introduced through the wire port over which the second Proglide SMC is introduced. This SMC is rotated to 2 o’ clock position and the second suture is placed. A third suture is placed in the 12 o’ clock position by a third device for >14F wounds. The final wire is retained for introducing the vascular sheath. After placing each suture the respective threads are carefully kept apart to prevent intertwining with the subsequently placed suture. A 7F vascular sheath is now introduced over the retained guide wire. This is followed by progressively larger dilators to enlarge the wound and finally the desired vascular sheath or device is introduced. Progressive dilatation helps to prevent an uncontrolled tear in the vessel wall. After completing the intervention procedure, the introduced vascular sheath or device is removed. Each of the preplaced sutures is now sequentially closed using the knot pusher. Distal pulse is examined after closing the wound to ensure the integrity of the arterial lumen.
6. Definitions used in the study
6.1. Major complication
Any death or loss of limb due to access site complication or any access site symptom immediately or within 30 days of Proglide deployment requiring blood transfusion, antibiotic usage or any percutaneous or surgical therapy to address the complication.
6.2. Minor complication
Persistent access site pain or bleeding which needed more than 5 min of local arterial compression and not meeting the criteria of major complication.
6.3. Procedure success
Achievement of complete haemostasis with or without compression, i.e. use of light, non-arterial compression of less than 5 min.
6.4. Device failure
Failure to place suture correctly inspite of proper technique and final haemostasis achieved with manual arterial compression.
7. Results
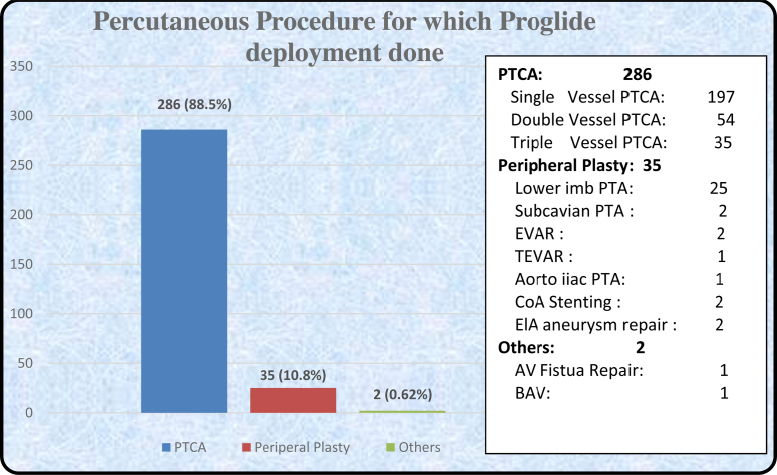
342 Perclose Proglide SMC deployed patients were identified, 19 were excluded due to various reasons and 323 were accepted for final analysis (Table 4). Baseline characteristics and percutaneous procedure details are given in Table 1, Table 2 and Fig. 1.
Table 4.
Selection of study population.
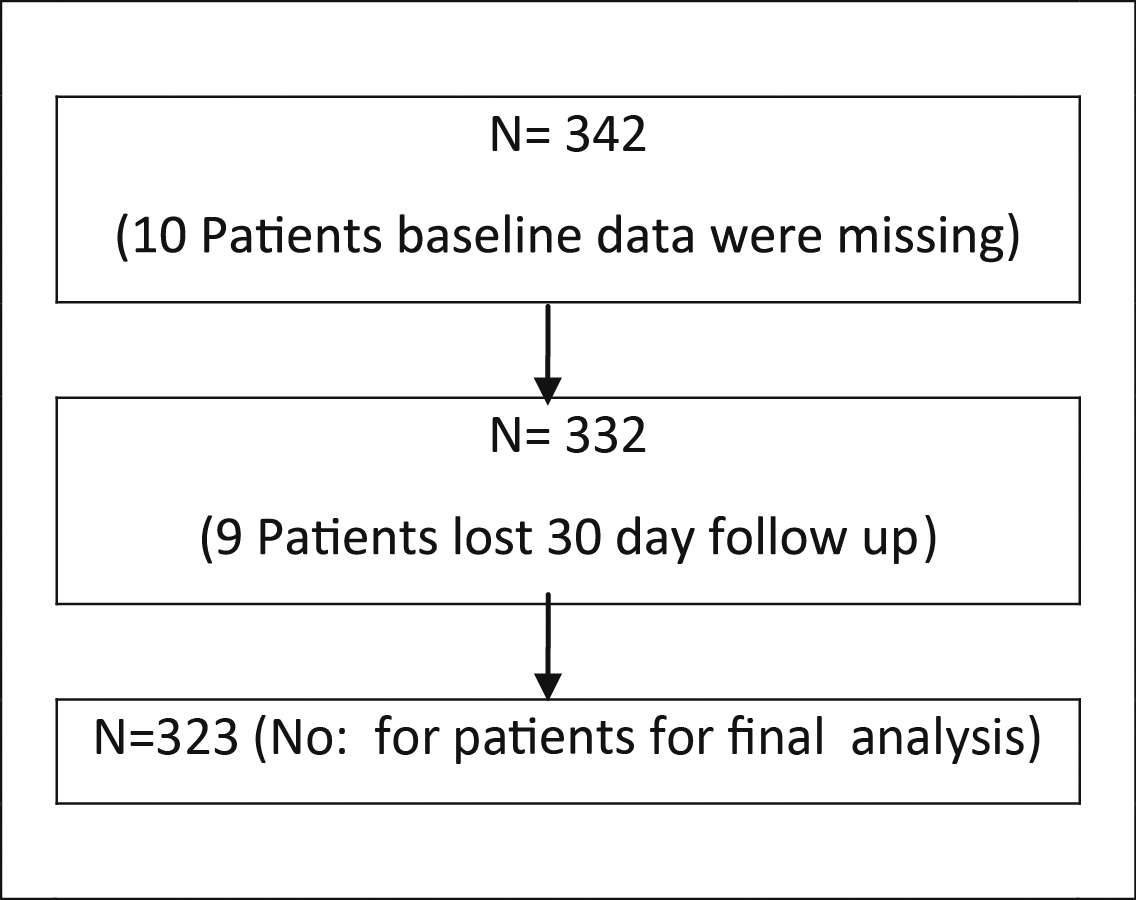
Table 1.
Baseline characteristics.
| Baseline characteristics (N = 323) | ||
|---|---|---|
| 1 | Age (years) | 36–76 (mean ± SD = 55 ± 9) |
| 2 | Sex | Male: 257 (79.6%) Female: 66 (20.4%) |
| 3 | Diabetes mellitus | 137 (42%) |
| 4 | Hypertension | 126 (39%) |
| 5 | Dyslipidemia | 131 (40%) |
| 6 | Smoking | 154 (48%) |
| 7 | Chronic kidney disease | 0 |
| 8 | Lower limb peripheral vascular disease | 25 (7.7%) |
Table 2.
Percutaneous procedure details.
| Percutaneous procedure details (N = 323) | |
|---|---|
| Access site | CFA: 327 (100%) |
| Antiplatelet loading dose | Aspirin + Clopidogrel: 304 (94%) Aspirin + Prasugrel: 19 (6%) |
| Periprocedure antiplatelets | Tirofiban: 14 (4.3%) |
| Periprocedure anticoagulation | Heparin 10,000 IU: 96 (30%) 7500 IU: 227 (70%) |
| Sheath size | 7F = 316, 11F = 1, 14F = 3, 21F = 1, 22F = 2 |
| Perclose Proglide SMC deployment technique | Preclose: 6 Postclose: 317 |
Fig. 1.
Percutaneous procedures for which Perclose Proglide deployment done.
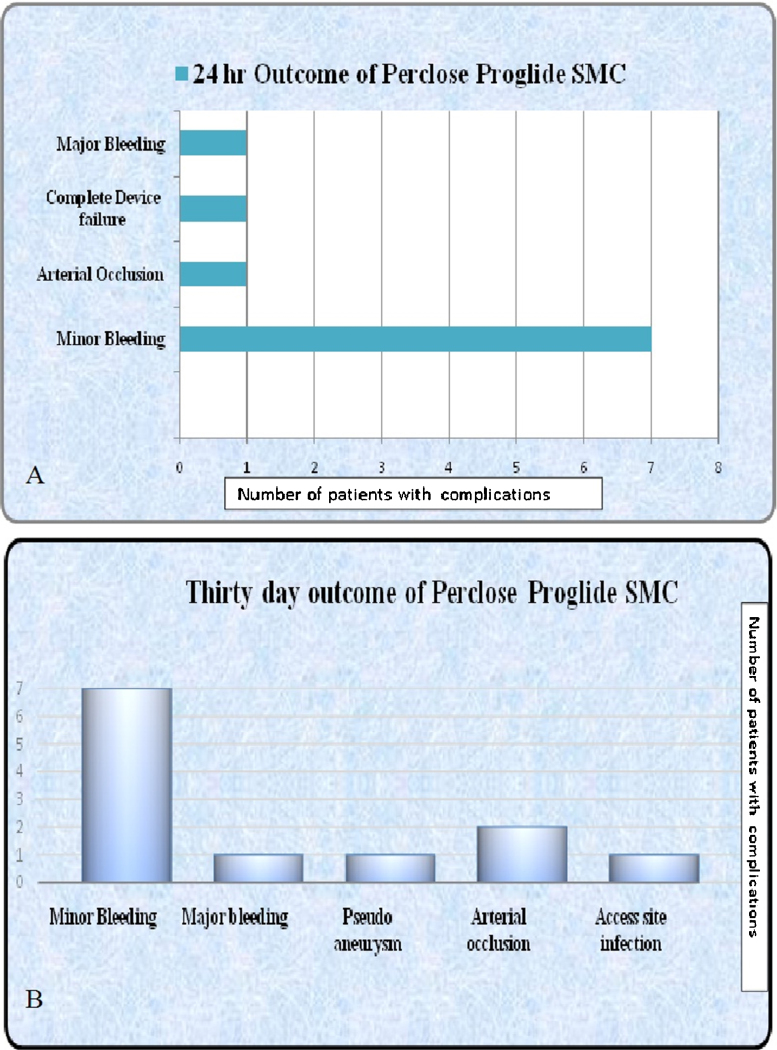
Analysis of 24 h data (Fig. 2A) showed that Perclose Proglide SMC procedure was successful in the first attempt in 311 patients. In remaining 12 cases a second device was successful in all except one in whom haemostasis was achieved with manual arterial compression for 30 min. Final procedure success rate was 99.7% (322/323). Immediately after deployment, 44 (13.6%) patients had mild oozing requiring manual non-arterial compression of less than 5 min. Oozing is not considered as a complication as per the definition. Seven cases (2%) required 15–20 min of arterial compression. Retroperitoneal haematoma with haemoglobin drop of 3 g/d1 occurred in a middle-aged woman (0.3%) and was managed conservatively with three units of packed cell transfusion. She was discharged from the hospital after 5 days.
Fig. 2.
(A and B) 24 h and 30 day outcomes of Perclose Proglide SMC.
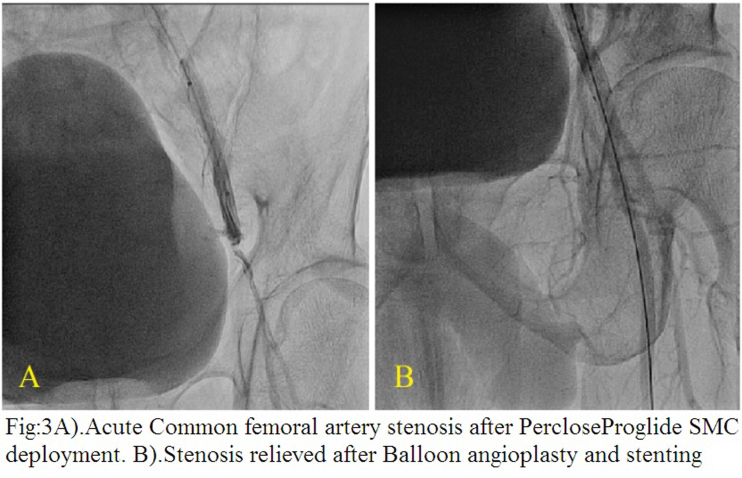
Acute arterial stenosis with slowing of distal flow occurred during Proglide deployment in one case (supplementary Fig. 3A and B). The patient was a middle-aged man who underwent PTA with stenting for aorto iliac disease through bilateral common femoral (CFA) and left brachial arterial access. Patient complained of pain over the left leg immediately after suture placement at left CFA with Proglide SMC. Check angiogram of left CFA through the brachial access showed acute subtotal occlusion at the Proglide deployed site and was immediately corrected by balloon dilatation and stenting as there was a tendency to recoil after plain balloon dilatation. No mortality or complication requiring amputation occurred in the first 24 h.
Apart from the 24 h outcome, 30 day's follow-up (Fig. 2B) showed one diabetic perimenopausal woman who developed access site MRSA infection 1 week after the procedure. She subsequently developed pseudoaneurysm of CFA. This was managed surgically and 3 weeks of parenteral antibiotic therapy. Another patient developed right lower limb claudication following Perclose Proglide SMC 2 weeks after the procedure and Doppler evaluation showed critical stenosis at the site of suture placement and was managed by balloon angioplasty. There was no mortality or complication requiring amputation during the 30 days follow-up. We have used “Preclose technique” in six patients without any complication during the 24 h or 30 days follow-up. Overall outcome of Perclose Proglide SMC, their complications and management are summarised in Table 3.
Table 3.
Outcome of Perclose Proglide SMC.
| Results | ||
|---|---|---|
| Outcome of Perclose Proglide SMC | Treatment given | |
| Minor complication | ||
| Minor bleeding | 7 (2%) | Compression |
| Major complication | 5 (1.5%) | |
| Major Bleeding | 1 (0.3%) | Blood transfusion |
| Pseudo aneurysm | 1 (0.3%) | Surgical correction |
| Local infection | 1 (0.3%) | IV antibiotics followed by surgical correction as complicated by pseudo aneurysm |
| Arterial occlusion | 2 (0.6%) | Balloon dilatation with stenting in first case Balloon dilatation only in other case |
| Others | ||
| Primary device failure | 12 (3.7%) | Eleven case managed with second device |
| Complete device failure | 1 (0.3%) | Manual compression |
| Oozing | 44 (13.6%) | Non-arterial compression |
8. Discussion
There is paucity of Indian data regarding the use of vascular closure devices. We attempted to assess the 24 h and 30 days outcome of suture mediated closure device, the “Perclose Proglide” (Abbott Vascular Devices, Redwood City, CA, USA) SMC. The operator used Perclose Proglide in 22% of the cases during the study period. The device could be effectively used in both (preclose manner) for 14F and larger arteriotomies and (in post-close manner) for up to 11F arteriotomies with high procedural success (99.7%), low complication rates and no mortality or limb loss. Although SMC devices have not established superiority over manual compression in small puncture wounds there are certain distinct advantages. In large puncture wounds as in EVAR, aortic valvotomy and TAVI they definitely reduce the need for open surgical arteriotomy and its ensuing morbidity and cost involved in employing the surgical team.
Vascular closure devices are classified as passive and active devices. Passive devices include haemostatic pads (Chitoseal, Neptune pads) and Compression devices (Femostop). Active devices include collagen based (Vasoseal, Angioseal), clip based (StarClose) or suture based devices (Perclose Proglide, Prostar, X-Site, Super Stitch). Suture mediated vascular closure devices deploy sutures to achieve haemostasis with a knot made either by an in built device mechanism or manually which is advanced towards the puncture site to achieve closure of arteriotomy.
The Perclose system (Perclose Inc., Redwood City, CA, USA) introduced in 1994 was the first suture-mediated device to be approved by the FDA. Subsequently various designs were developed like Perclose AT, Prostar XL, Closer S and Proglide. Perclose Proglide introduced in 2004 is the latest generation suture mediated device from Abbot Vascular devices and FDA approved this device in 2013. Perclose Proglide offers improvements in the ease of knot delivery, trimming of the suture and polypropylene monofilaments sutures which are non-inflammatory and with high tensile strength. Each type of device is meant for a particularly sized2 arteriotomy wound like X-SITE for 6F, Super Stitch for 6–8F, Perclose AT for 5–8F, Prostar for 8–10F and Proglide for 5–21F sheath sizes. For Perclose Proglide, recommendation is to use two or more devices in a “preclosure”1 manner if puncture size ≥8F.
Older generation SMCs deploy needle from inside the vessels and are associated with vascular complications and involve complex steps for knot making and its advancement towards the site. Advantages of newer devices are needle deployment from outside the vessel, automated knot forming mechanism, inbuilt quick cut suture trimming mechanism, and easy knot advancement towards the arterial wound.
Steps involved in the Proglide SMC deployment are positioning the device, needle deployment, suture capture, needle removal, knot advancement and trimming of the excess thread. Each step requires meticulous care and chances of technical failures are high if not properly trained. Studies have documented a “learning curve” phenomenon with vascular closure devices (VCD)3, 4 especially with SMC. Balzer et al.4 showed that the learning curve for technical success with suture-based closure was steeper and longer (>350 patients).
Although there are no contraindications for the use of Perclose Proglide SMC, manufacturer has mentioned certain patient groups in which safety and effectiveness has not been established.2 These includes small femoral arteries (<5 mm), access site above the most inferior border of inferior epigastric artery, Non-CFA access site, fluroscopically visible CFA calcification, anterograde punctures, morbid obesity, access site in vascular graft, posterior wall puncture and multiple punctures.5
Complications6 noted during the arterial closure include infection, bleeding both minor and major, pseudoaneurysm, arterial laceration, arteriovenous fistula, embolisation, limb ischaemia, femoral artery thrombosis or dissection, access site pain, nerve injury and death. Review of literature shows that the rate of major complications varies from 3% to 4.6% with Perclose SMC7, 8 and 1.5–9% with manual compression.9, 10 Our study had a procedure success rate of 99.7% (322/323), major complication rate of 1.5% and minor complication rate of 2% (Table 3). If Puncture size is ≥8F, two or more devices1 are recommended by the manufacturer in a preclose manner. But in our series in one case it was able to close successfully a 11F size arterial puncture with a single Proglide device which was deployed after the percutaneous procedure. “Preclose” technique with two devices was used in wound sizes up to 14F and three devices in >14F puncture wound. “Preclose technique” was successfully used in 6 patients to achieve complete haemostasis without any complication.
The reasons for device failure in the study were that in two cases knot came out along with the device when the device was attempted to be removed and knot could not be repositioned by pulling the thread. In other cases when plunger was retracted, the threads were found to be detached from the plunger and knot could not be made. These problems could be noticed while the device was still inside the arterial lumen. In these cases 0.035 J wire was reintroduced through the wire port and failed device replaced with a second one and successful suture placement accomplished. In one case second device also failed and final hemostasis was achieved with manual compression. Other causes of device failure11 described in the literature include artery getting split at the site of device entry, failure to deploy needle due to vessel calcification,7, 12, 13, 14, 15 failure to approximate the arteriotomy wound or failure to form or deliver the knot. Mechanism of the two access site artery stenosis in our series remain unclear. It may be due to the posterior wall getting caught by the foot plate or an inadequate longitudinal alignment of the device feet inside the arterial lumen due to tilt or rotation of device. Other described causes in literature are intraluminal atheroma being snared causing luminal obstruction16 and acute thrombosis at the access site. Thrombosis17 occurs if device is advanced too far within the artery and the foot plate is dragged against the posterior arterial wall. Infection rate in our study was low even though antibiotic prophylaxis was not used.
Review of literature showed varied results with the use of suture mediated closure system (Table 5, Supplementary Data) and there is no Indian data available regarding Perclose Proglide SMC. Chamberlin et al.18 have showed a device failure rate of 14.3%, retroperitoneal bleed of 1.8% and zero infections with the use of Perclose SMC. Quinn et al.19 showed that Perclose Prostar SMC was associated with device failure rate of 4%, acute femoro popliteal thrombosis in 1%, pseudoaneurysm in 5%, need for additional manual compression in 2%, infection rate of 1% and asymptomatic intimal dissection flap in 1%. Wagner et al.8 in their study with perclose closure SMC showed device failure rate of 7%, minor and major complication rate of 4% and 3% respectively. Duda et al.20 achieved technical success in 97.5% with minor complication of 6% with the use of Techstar and Prostar Plus SMC. Bilge et al.21 reported a procedural success and device failure of 98.8% and 1.2% with single Proglide and 94.2% and 5.8% with double Proglide use respectively. Mackrell et al.22 showed 95% success rate and major complications of 1.4% with the use of Perclose SMC. PEVAR trial,23 in which Perclose Proglide was used in a “preclose” manner for achieving hemostasis after percutaneous endovascular aortic repair showed a major complication rate of 6% and a device failure rate of 6%.
8.1. Limitations
This was a retrospective observational study. A control group was not employed. It was conducted in a single centre and by a single operator. Results may vary with the experience of the centre and operator, as a learning curve phenomenon has been reported by some authors. Real world analysis of cost involved could not be done as it was conducted in a Government institution where services both medical and nursing are offered without a price.
9. Conclusion
Perclose Proglide SMC is a safe and effective option for closure of up to 22F puncture size arteriotomy wound in Indian population. The operator was successful in closing an arteriotomy wound of 11F size with single Proglide deployed after the procedure, but this cannot be recommended as a standard practice until validated in bigger samples. “Preclose” technique with two devices were used successfully in arteriotomy size of 14F and with three devices if arteriotomy wound is >14F and was found to be a safe and effective option for Indian population as well. Our study showed low complication rates with Perclose Proglide SMC if properly deployed. As compared with the major complication rates of manual compression7, 8 mentioned in the literature, our results also showed comparably low rates. However large scale RCTs comparing manual compression and Perclose Proglide SMC may be needed to assess the actual advantage of one method over the other. Major limiting factor for the use of Perclose Proglide SMC in Indian scenario is cost of the device. But this can be negated by a shorter hospital stay and reduced man power utilisation as compared with manual compression. Cost analysis also needs a large scale RCT. Successful closure of arteriotomy wounds of up to 22F indicate the feasibility of percutaneous closure in large arteriotomy wounds as required in EVAR and TAVI. This clearly helps to reduce the need for open arteriotomy and the ensuing morbidity and additional cost and offers a distinct advantage over open surgical arteriotomy.
Conflicts of interest
The authors have none to declare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ihj.2016.06.008.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.PercloseProGlide® 6F Suture-Mediated Closure (SMC) System Instructions for Use: IFU (Full Version). http://www.abbottvascular.com/us/products/vessel-closure/perclose-proglide.html.
- 2.van den Berg J.C. A close look at closure devices. J Cardiovasc Surg. 2006;47(3):285–295. [PubMed] [Google Scholar]
- 3.Warren B.S., Warren S.G., Miller S.D. Predictors of complications and learning curve using the Angio-Seal closure device following interventional and diagnostic catheterization. Catheter Cardiovasc Interv. 1999;48:162–166. doi: 10.1002/(sici)1522-726x(199910)48:2<162::aid-ccd8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Balzer J.O., Scheinert D., Diebold T. Postinterventional transcutaneous suture of femoral artery access sites in patients with peripheral arterial occlusive disease: a study of 930 patients. Catheter Cardiovasc Interv. 2001;53:174–181. doi: 10.1002/ccd.1144. [DOI] [PubMed] [Google Scholar]
- 5.http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-Approved Devices/ucm351099.htm
- 6.Bechara C.F., Annambhotla S., Lin P.H. Access site management with vascular closure devices for percutaneous transarterial procedures. J Vasc Surg. 2010;52(December (6)):1682–1696. doi: 10.1016/j.jvs.2010.04.079. [DOI] [PubMed] [Google Scholar]
- 7.Lee W.A., Brown M.P., Nelson P.R. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg. 2008;47:919–923. doi: 10.1016/j.jvs.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Wagner S.C., Gonsalves C.F., Eschelman D.J. Complications of a percutaneous suture-mediated closure device versus manual compression for arteriotomy closure: a case–controlled study. J Vasc Interv Radiol. 2003;14(6):735–741. doi: 10.1097/01.rvi.0000079982.80153.d9. [DOI] [PubMed] [Google Scholar]
- 9.Popma J.J., Satler L.F., Pichard A.D. Vascular complications after balloon and new device angioplasty. Circulation. 1993;88:1569–1578. doi: 10.1161/01.cir.88.4.1569. [DOI] [PubMed] [Google Scholar]
- 10.Nasser T.K., Mohler E.R., Wilensky R.L. Peripheral vascular complications following coronary interventional procedures. Clin Cardiol. 1995;18(November (11)):609–614. doi: 10.1002/clc.4960181105. [DOI] [PubMed] [Google Scholar]
- 11.Vercauteren S.R.W., De Roover D. Preclosure for PEVAR: this technique can be applied in a majority of AAA patients. Endovasc Today. 2014:74–77. http://evtoday.com/2014/03/preclosure-for-pevar/ [Google Scholar]
- 12.Lee W.A., Brown M.P., Nelson P.R. Total percutaneous access for endovascular aortic aneurysm repair (“Preclose” technique) J Vasc Surg. 2007;45:1095–1101. doi: 10.1016/j.jvs.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 13.Eisenack M., Umscheid T., Tessarek J. Percutaneous endovascular aortic aneurysm repair: a prospective evaluation of safety, efficiency, and risk. J Endovasc Ther. 2009;16:708–713. doi: 10.1583/08-2622.1. [DOI] [PubMed] [Google Scholar]
- 14.Manunga J.M., Gloviczki P., Oderich G.S. Femoral artery calcification as a determinant of success for percutaneous access for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2013;58:1208–1212. doi: 10.1016/j.jvs.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Mousa A.Y., Campbell J.E., Broce M. Predictors of percutaneous access failure requiring open femoral surgical conversion during endovascular aortic aneurysm repair. J Vasc Surg. 2013;58:1213–1219. doi: 10.1016/j.jvs.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 16.Park J.Y., Choe Y.M., Shin W.Y. Intraluminal snared-atheroma causing common femoral artery stenosis after using perclose suture-mediated closure system. Korean J Vasc Endovasc Surg. 2013;29(3):103–108. [Google Scholar]
- 17.Nehler M.R., Lawrence W.A., Whitehill T.A. Iatrogenic vascular injuries from percutaneous vascular suturing devices. J Vasc Surg. 2001;33(May (5)):943–947. doi: 10.1067/mva.2001.115002. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlin J.R., Lardi A.B., McKeever L.S. Use of vascular sealing devices (VasoSeal and Perclose) versus assisted manual compression (Femostop) in transcatheter coronary interventions requiring abciximab (ReoPro) Catheter Cardiovasc Interv. 1999;47(June (2)):143–147. doi: 10.1002/(SICI)1522-726X(199906)47:2<143::AID-CCD1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Quinn S.F., Kim J. Percutaneous femoral closure following stent-graft placement: use of the perclose device. Cardiovasc Intervent Radiol. 2004;27:231–236. doi: 10.1007/s00270-003-2713-y. [DOI] [PubMed] [Google Scholar]
- 20.Duda S.H., Wiskirchen J., Erb M. Suture-mediated percutaneous closure of antegrade femoral arterial access sites inpatients who have received full anticoagulation therapy. Radiology. 1999;210:47–52. doi: 10.1148/radiology.210.1.r99ja3047. [DOI] [PubMed] [Google Scholar]
- 21.Bilge M., Alemdar R., Ali S. Efficacy and safety of percutaneous suture-mediated closure devices in interventional cardiology. Outcomes of the largest series of percutaneous vascular closure in Turkey. J Am Coll Cardiol. 2013;62(18) [Google Scholar]
- 22.Mackrell P.J., Kalbaugh C.A., Langan E.M., III Can the perclose suture-mediated closure system be used safely in patients undergoing diagnostic and therapeutic angiography to treat chronic lower extremity ischemia? J Vasc Surg. 2003;38(6):1305–1308. doi: 10.1016/s0741-5214(03)00948-0. [DOI] [PubMed] [Google Scholar]
- 23.Nelson P.R., Kracjer Z., Kansal N. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair: the PEVAR trial. J Vasc Surg. 2014;59(May (5)):1181–1193. doi: 10.1016/j.jvs.2013.10.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.