Abstract
Obesity and its accompanying metabolic syndrome are strongly associated with heightened morbidity and mortality in older adults. In our review of more than 20 epidemiologic studies of major infectious diseases, including tuberculosis, community-acquired pneumonia and sepsis, obesity was associated with better outcomes. A cause-and-effect relationship between over-nutrition and survival with infection is suggested by the results of two preliminary studies of infections in mice, where high-fat feeding for 8–10 wks provided much better outcomes. These better outcomes are reminiscent of many recent studies of “sterile” noninfectious medical and surgical conditions where outcomes for obese patients were better than for their thinner counterparts; this was given the tag “obesity paradox.” Turning to the history of medicine and biological evolution, we hypothesize that metabolic syndrome has ancient origins and is part of a lifelong metabolic program. While that part of the program promotes morbidity and mortality with aging, it helps infants and children as well as adults fight infections and recover from injuries, key events in the centuries before the public health advances of the 20th century. We conclude with speculation on how an understanding of the biological elements that protect obese patients with infections or injuries might be applied advantageously to thin patients with the same medical challenges.
INTRODUCTION
The continuing increase in the prevalence of obesity and the tapering of tobacco abuse lead experts to predict that obesity will shortly become the leading personal health problem worldwide. Obesity and the accompanying metabolic syndrome are typically associated with shortened life expectancy, premature disability and heightened prevalence of cardiovascular disorders, cancer, diabetes and Alzheimer’s disease as well as multiple other disorders linked to advancing age.
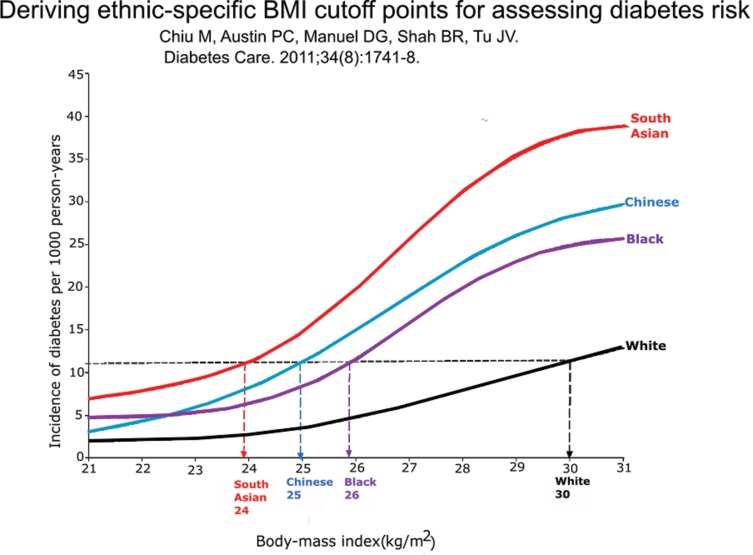
In the jeremiads inspired by obesity, the modest but deeply rooted health advantages of obesity are typically neglected (Table 1). In this paper we add further to the list of advantages of obesity; we review over 20 epidemiology studies of six serious infectious diseases, including tuberculosis, pneumonia and sepsis, where outcomes are inversely related to body mass index. The consistency of the obesity advantage is especially remarkable, because the usual measurements to express adiposity, that is, body mass index (BMI; weight in kilograms divided by the square of height in meters) or waist circumference or neck circumference, are such rough approximations of total body fat or visceral fat or metabolic syndrome. The connection between body mass index and metabolic syndrome in epidemiology studies is further loosened by impressive ethnic differences (Figure 1) (1) and changes with aging. To support the idea that the inverse relationship between adiposity in patients and infection outcomes likely reflects cause and effect (rather than simply an association), we shall review preliminary experiments with two models of short-term infections in mice where high-fat feeding over a few weeks reproduced the advantage of obesity in overcoming infection.
Table 1.
Obesity and its benefits.
| Benefits of obesity in daily life | Surgical conditions showing benefits of obesity (49) | Nonsurgical conditions showing benefits of obesity (49) |
|---|---|---|
|
|
|
Figure 1.
The relationship between body mass index and incidence of diabetes in four ethnic groups in Canada. In Ontario, Canada, approximately 60,000 nondiabetic men and women ages 30 and up were followed for up to 12.8 years for diabetes incidence. The median duration of follow-up was 6 years. The incidence of diabetes increased with increased body mass index (BMI) in each ethnic group, as expected. South Asians became diabetic at the lowest BMI values, followed by Chinese, blacks, and whites. The graph shows the incidence rate of diabetes per 1,000 person-years at each level of BMI (1).
The obesity advantage recounted here with clinical and experimental infectious diseases is concordant with many publications in the last decade citing multiple (infection-free, or “sterile”) medical and surgical situations where obesity was associated with better outcomes (Table 1). That so many of these were tagged as an “obesity paradox” reflects the health community’s puzzlement about obesity.
In the concluding section, based on the history of medicine and biological evolution, we marshal evidence to propose a union of these ideas. We posit that metabolic syndrome does not arise de novo in middle life. Rather, it is part of a continuous lifelong metabolic program. This program in early life (and probably its primary raison d’etre for millions of years) provided an inflammatory defense that was vitally needed to help in the battle against the many infections that decimated youngsters before the 20th-century introduction of clean water, vaccinations, insect control and antibiotics. Those with better nutrition, more body fat and more highly activated immune functions were favored to survive. The same program helped advance reproductive capacity (in females) and physical strength (in males and females) and helped both sexes survive infections and sterile challenges, including wounds and injuries. In middle life, these same beneficial processes were subordinated to the damaging forces we associate with metabolic syndrome and premature death. (With tongue in cheek, we can say that adiposity shortens an individual’s life span but provides better “road service” on the way to the cemetery, for example, by resolving infections and healing fractures.) To close, we initiate a discussion of how future research might yield insights whereby the positive elements of obesity that we catalog here could be applied to thin patients in the short term to better overcome infections and noninfectious challenges.
INFECTIOUS DISEASES
Tuberculosis as an Unconquerable
Tuberculosis is the epitome of what we have designated as an unconquerable infectious disease, one that is not curable without specific pharmacologic therapy (2,3). (Another widespread unconquerable is American trypanosomiasis, ie, Chagas disease). Absent specific modern drugs, tuberculosis infection is lifelong, irrespective of whether the disease (i) is active, (ii) was active and is now in remission or (iii) was never clinically active but latent and is capable of progression to active disease, in the absence and presence of risk factors such as HIV/AIDS or tumor necrosis factor (TNF) blockers (4–6).
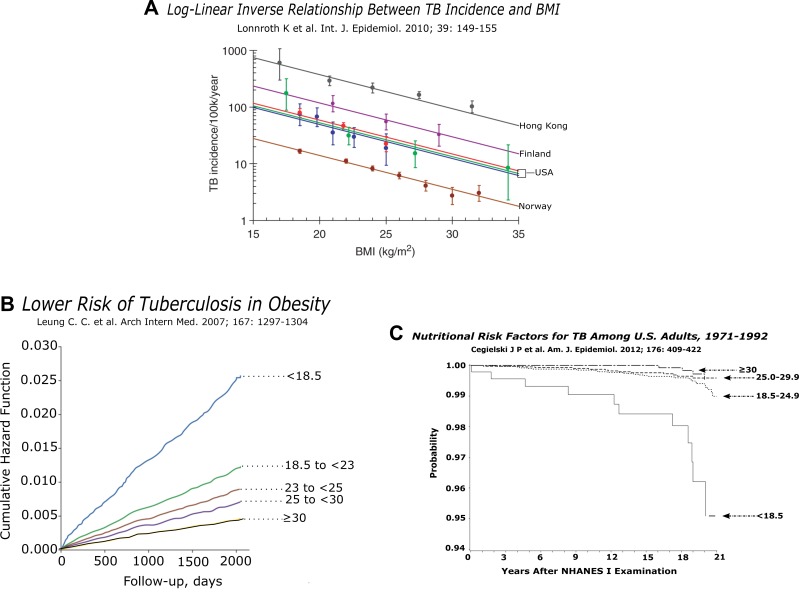
Classically, tuberculosis is associated with an acute or chronic diminution in body weight. The wasting is epitomized by phthisis and consumption, old-fashioned synonyms for active tuberculosis. In Table 2, we present six narratives (of many) where rates of tuberculosis were inversely related to nutrition (7). Several other studies show a similar trend (8,9). In Figure 2A, six modern epidemiologic studies from four countries show a clear inverse relationship between body mass index and incidence of tuberculosis. Not widely appreciated, this relationship is continuous from quite low BMIs through to overweight and obese (10). In the Hong Kong study (Figures 2A and B), more than 40,000 patients over age 65 were followed for 5 years or more. The curve tracking morbidity (“cumulative hazard function”) shows a clear change in slope for each of five BMI categories, from less than 18.5 to more than 30 (Figure 2B) (11). In a later, larger study rooted within the National Health and Nutrition Examination Survey in the USA, recruits who were followed for around a decade showed a similar trend, although the rates of tuberculosis were much lower and the patients were younger (Figure 2C). Underweight patients were clearly at highest risk. Across the range from normal weight to obese, differences appeared to be continuous but less marked than in Hong Kong (12).
Table 2.
Summary of selected reports relating nutrition to tuberculosis (adapted from Cegielski and McMurray, 2003 (7)).
|
Figure 2.
(A) The authors describe “a remarkably consistent inverse logarithmic relationship between TB incidence and BMI, across studies that had been carried out over the past 50 years in diverse study populations with a large variation in average TB incidence, and which had controlled for various sets of confounders” (10). (B) Obesity reduces risk of tuberculosis. Hazard function curves illustrating morbidity with active tuberculosis for different BMI categories. Individuals with a low BMI have an increased risk of active tuberculosis. The opposite is true for individuals with high BMI, illustrating the protective effect of obesity. These data were reformulated for the Hong Kong curve in Figure 2A (11). (C) This figure depicts the probability of individuals in the inaugural NHANES study remaining free of tuberculosis from 1971–1992 (stratified by initial BMI into four groups) (12).
Interestingly, before the introduction of streptomycin and other tuberculosis-targeted drugs in the mid-1950s, a long list of therapies were recommended. William Osler, the leading physician in 19th century North America, promulgated recommendations that were quite representative of these past therapies: “Make patient grow fat and strong.… The importance of rest must be always kept in mind and the amount of exertion allowed carefully ordered.” For patients with fever, “absolute rest” was recommended. In retrospect, Osler et al. exhorted physicians to launch their tuberculosis patients headlong into metabolic syndrome and to sustain it as long as possible (13).
Community-Acquired Pneumonia
With tuberculosis presented as the lead example of unconquerable infections, we turn to community-acquired pneumonia as the model of an acute chance infection (2,3). Community-acquired pneumonia in most adult patients is likely to be the result of a new infection, with Streptococcus pneumoniae as the most frequent bacterium (14,15). In a recent study, S. pneumoniae was found in fewer than 5% of healthy adults (16). Diagnosis of a respiratory infection on the screening day was the only significant risk factor for a positive culture (P < 0.05) (16). (This is in sharp contrast to children, for whom typical carrier rates for S. pneumoniae were 10 times higher.) In the preantibiotic, prevaccine era, a typical patient with pneumonia presented acutely, with a severe febrile course over a few days culminating in either death or rapid recovery. Despite the lack of specific therapies, signs of the illness were markedly better in a few days and largely gone in a few months, leaving the patient with long-term immunity to that subtype of the bacterium.
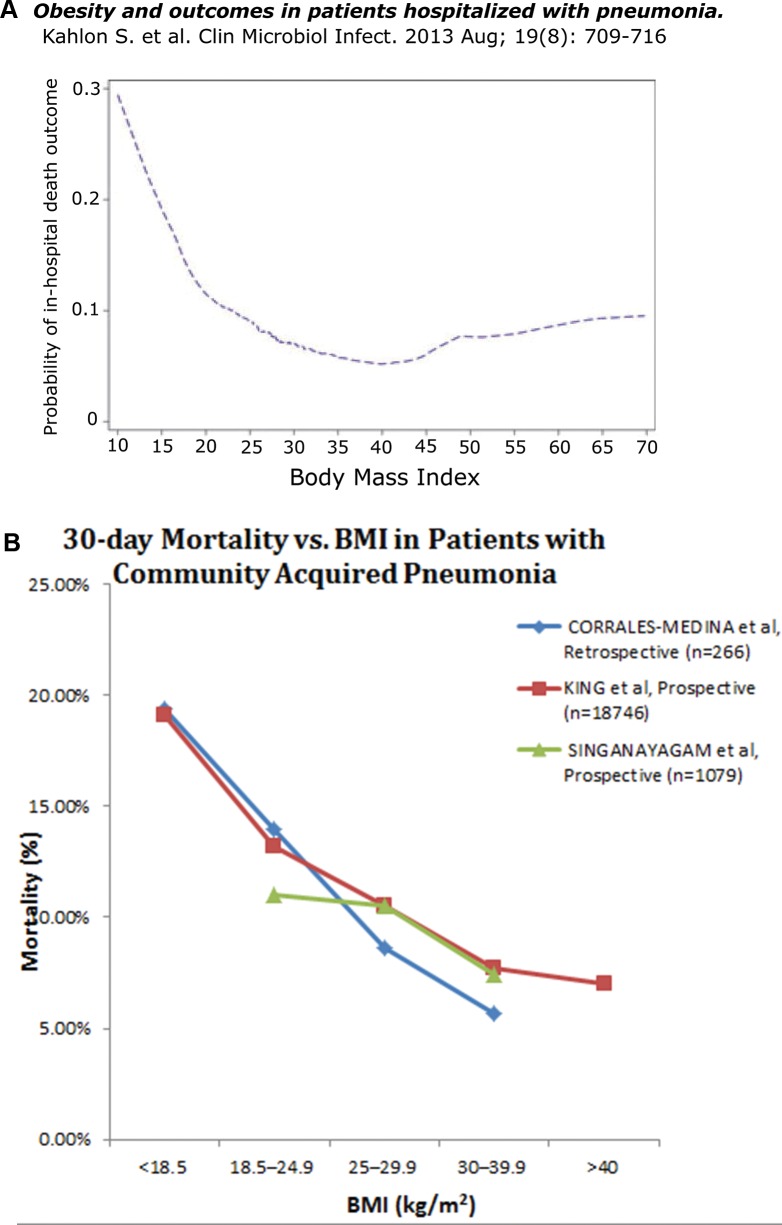
Mortality with pneumonia appears to be inversely related to adiposity (Figure 3A) (17). In another recent study with patients in one veterans hospital, the 30 d mortality from community-acquired bacterial pneumonia was four times higher in underweight patients (BMI < 18.5) than obese patients (BMI > 30) (Figure 3B) (18). Those patients designated as normal weight and overweight showed intermediate values, suggesting that these differences were not overly dependent on an accumulation of susceptibility factors in underweight patients. In Figure 3B, the results of the Corrales-Medina study are compared with results of large studies by King et al. and Singanayagam et al. (19,20). In two other studies (data not shown), one very large study of middle-aged and elderly Japanese and another study of 226 older patients with community-acquired pneumonia in Spain, elevated BMI was associated with better outcomes and reduced BMI was linked to poorer outcomes (21,22). The better outcomes among the obese patients occurred in the face of a higher prevalence of comorbidities that can affect susceptibility and outcomes with pneumonia (eg, diabetes, gastroesophageal reflux, reduced respiratory excursions and less effective coughing).
Figure 3.
(A) Among the 907 patients with community-acquired pneumonia in this prospective study admitted to six hospitals in Edmonton, Canada, the in-hospital mortality rate was inversely related to BMI (P < 0.001). The nadir in the mortality rate was at BMI = 40, with only a shallow slope upward at BMI levels over 40 that we associate with extreme obesity (17). (B) Three additional studies. 1) In this retrospective study of 266 patients with proven community-acquired pneumococcal (or Hemophilus) pneumonia, mortality at d 30 of admission is plotted as a function of BMI. The inverse relationship between mortality and BMI is significant at P < 0.01 in univariate and multivariate logistic regression analyses (18). 2) From this retrospective study of 18,746 patients with a discharge diagnosis of pneumonia (using data from the Department of Veterans Affairs), 30 d post-admission mortality is plotted as a function of BMI. In the univariate model, there is an inverse relationship between mortality and BMI (P < 0.0001). This relationship persists after adjusting for potential confounders in regression models, i.e., obesity is associated with decreased mortality (OR 0.86, 95% CI 0.74-0.99) and underweight patients had increased mortality (OR 1.40, 95% CI 1.14-1.73) (19). 3) The results of a prospective observational study from Edinburgh. The 30 d mortality is plotted against BMI for 1,079 patients diagnosed with community-acquired pneumonia. After Cox proportional hazards regression analysis, obesity is independently associated with decreased 30 d mortality (HR 0.53, 95% CI 0.29–.98) (20).
Sepsis
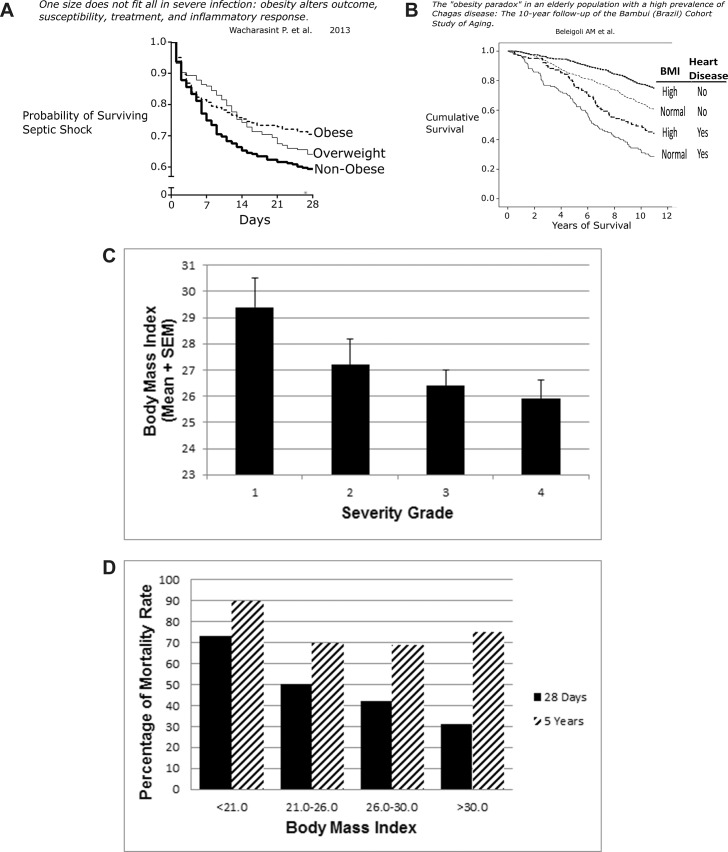
For patients with severe sepsis (including those with septic shock), mortality was reduced as a function of increased BMI. In a retrospective study of 1,404 adults over 65 years of age with severe sepsis, there was a dose-response relationship between one-year mortality and BMI for normal weight through severe obesity (23). Underweight patients were excluded. Wacharasint et al., analyzing a drug study cohort retrospectively, found in 713 patients with septic shock that 28 d mortality was inversely related to BMI; every unit increase in BMI was associated with an adjusted decrease of 2% mortality (Figure 4A) (24). Using a multivariate logistic regression model on 301 patients, Wurzinger et al. found that overweight and obese patients with septic shock in intensive care units had better survival rates than those with normal or reduced BMI (25).
Figure 4.
(A) Survival with septic shock. Among 730 patients in hospital with septic shock, mortality recorded in the initial 28 d was inversely related to BMI (P < 0.02) (24). (B) This longitudinal study is from a small city in Brazil where longstanding endemic Chagas was stopped by 1970 through the use of insecticides. Residents over 60 years of age who acquired Chagas in their youth (n = 1271) were followed from (1997–2007) with normal BMI = 18.(5–24).9 and high BMI > 25. Heart disease was defined by EKG and B-type natriuretic peptide measurements. The Kaplan-Meier survival curves show that among patients without heart disease, 10-year survival of obese patients was better than their thinner, normal-weight counterparts. Patients with heart disease showed much lower survival than those free of heart disease, but those with heart disease and elevated BMI did better than patients with heart disease and normal weight (28). (C) Biliary tract infection. All patients in this group had biliary bacteria. Association of BMI and illness severity was significant (P < 0.02). Group 1: jaundice but no evidence of infection or inflammation. Group 2: fever, leukocytosis, tachypnea and tachycardia. Group 3: cholangitis, empyema of gallbladder, gangrenous cholecystitis or abscess. Group 4: bacteremia, hypotension, organ dysfunction (29). (D) Peritonitis with sepsis. The graph depicts the short-term mortality (28 d, black bar) and long-term mortality (5 years, striped bar) in 253 consecutive patients with peritonitis and sepsis who needed intensive care for more than 2 d postoperatively. Patients were severely ill, with high APACHE II scores, which correlated well with the mortality rate. Excluded were patients with acute pancreatitis or primary spontaneous bacterial peritonitis (31).
Trivedi et al., in their comprehensive systematic review, selected seven studies for detailed analysis (out of 183 published studies of obesity and sepsis mortality) (26). They agreed with the validity of the conclusions in the three studies we reviewed in the previous paragraph, i.e., better outcomes in obese patients with sepsis. Of the four other studies that met their validation criteria, three studies failed to show a significant correlation after multivariate analysis. In the seventh study, obesity with sepsis was associated with poorer outcomes. We cautiously agree that overweight and obese patients do better than patients with normal or reduced weight, but the extreme heterogeneity of patients does not allow for unambiguous conclusions at the time of this writing.
American Trypanosomiasis
Trypanosoma cruzi, an insect-borne protozoan, causes American trypanosomiasis, or Chagas disease, a chronic unconquerable infection widespread in Latin America that results in debilitating cardiac disease, mega-syndromes of the viscera, disability and shortened life span (27). Successes in prevention and therapy of Chagas disease are improving. Among Chagas patients with heart disease followed for 10 years, obese patients survived longer, despite the burdens that obesity places on cardiovascular function. Also, in patients who were free of heart disease, the long-term outcome with obesity was also clearly superior (Figure 4B) (28).
Gallbladder Disease
Among 427 patients with acute cholecystitis, the obese patients had milder disease. Most infected patients had a BMI within the normal range. BMI inversely correlated with the presence of biliary bacteria, bacteremia, pigmented (more bacteria-friendly) stones and severity of illness (Figure 4C) (29). In another retrospective study of 139 patients post-cholecystectomy, BMI was also inversely related to bacteremia and bactibilia (30).
Surgical Peritonitis
Among 253 consecutive patients with severe secondary surgical peritonitis with sepsis who needed intensive care for more than 2 d, the mortality rate at 28 d fell progressively from 73% to 31% in those with BMI < 21 versus those with BMI > 30 (Figure 4D) (31). This obesity advantage held despite the finding that those in the obese quartile had modestly more diabetes, coronary artery disease and heart failure than observed in the other three groups. The mortality advantage observed in the obese patients with infection was in the short term. By five years, the negative consequences of obesity were manifest (31). These findings suggest that with serious infection, obesity provides an advantage over the short term. Absent infection, over the longer term, the well-recognized detrimental effects of obesity dominate.
Evidence from Animal Studies
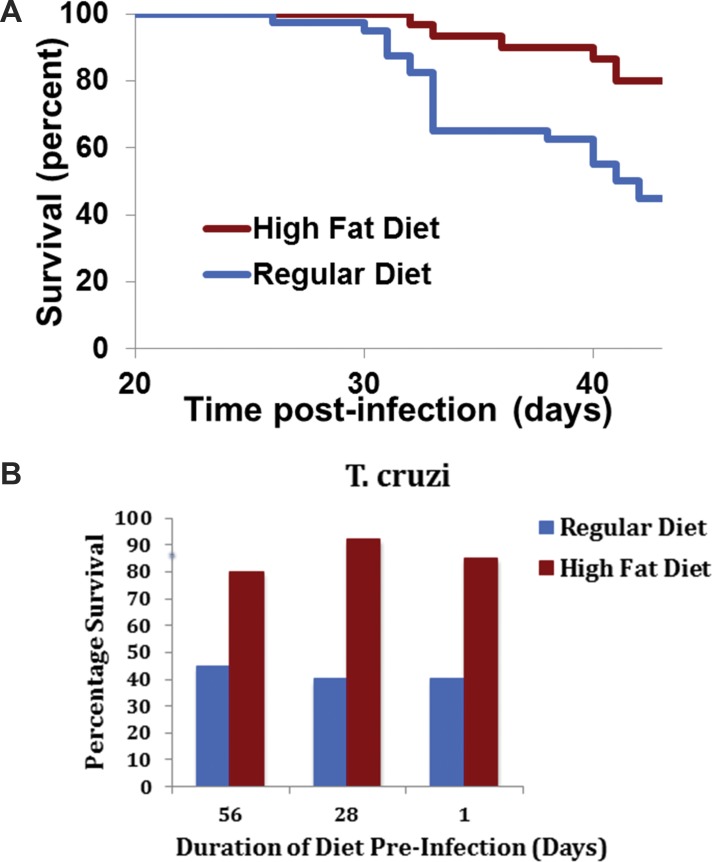
To distinguish an association from a cause-and-effect relationship, we turned to experiments in mice. With T. cruzi infections, we and our colleagues raised survival from 40% to 80% by administering a standard high-fat diet (Figures 5A and B) (32). That diet, initiated weeks or as few as 1 d before infection and carried through several weeks of the acute infectious process, rapidly promoted obesity and metabolic syndrome, along with increased survival. In addition, the high-fat diet protected mice from T. cruzi infection–induced myocardial damage (33).
Figure 5.
Effect of diet on survival post infection with Trypanosomiasis cruzi (A) Mice were fed a regular diet until d 56 preinfection, when half were switched to a high-fat diet for the duration of the experiment. At d 0, half the mice in each of the two diet groups were infected with T. cruzi. With the high-fat diet, about 80% of the mice survived, while around 40% of the regular-diet mice survived (32). (B) Similar results were observed when the high-fat diet was started 56, 28 and 1 d before infection and continued for 42 d post infection (33).
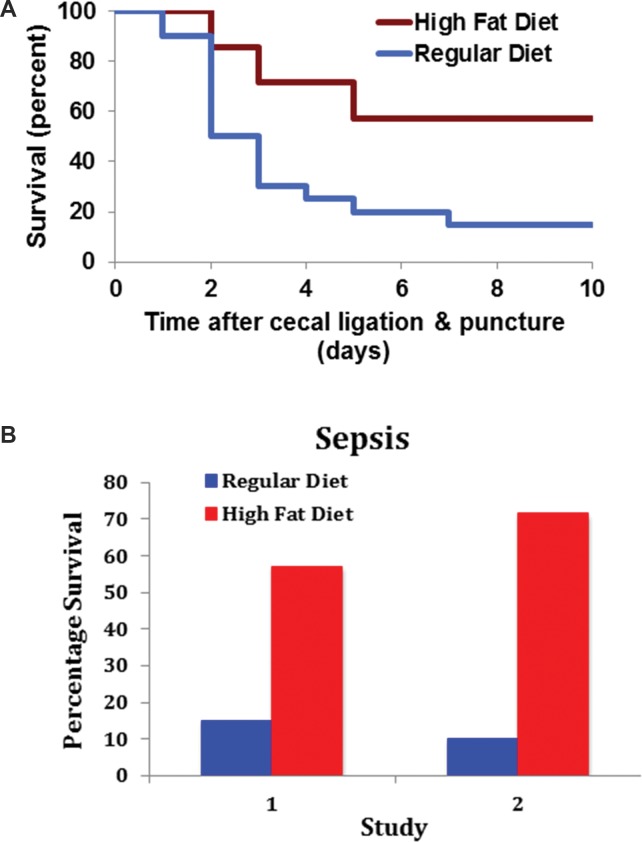
With a standard model of severe sepsis in mice (polymicrobial sepsis following perforated appendix; i.e., cecal ligation and puncture), animals prefed a high-fat diet to produce obesity showed markedly enhanced survival (Figures 6A and B) (34). In another study, a high-fat diet inaugurated 12 wks before surgery raised survival from sepsis with approximately 10% to 65% (Figure 6B) (35).
Figure 6.
Experimental peritonitis with sepsis. (A) Peritonitis and sepsis were produced in mice by cecal ligation and puncture. Mice fed a high-fat diet (upper curve) displayed enhanced survival (34). In (B), the data from (A) are presented again as Study 1. Survival after cecal ligation and puncture of C57BL/6 mice that were fed a high-fat diet for 12 wks were compared with those on a regular diet. Survival of controls without the operation was 100% (35).
Potential Sources of Confusion
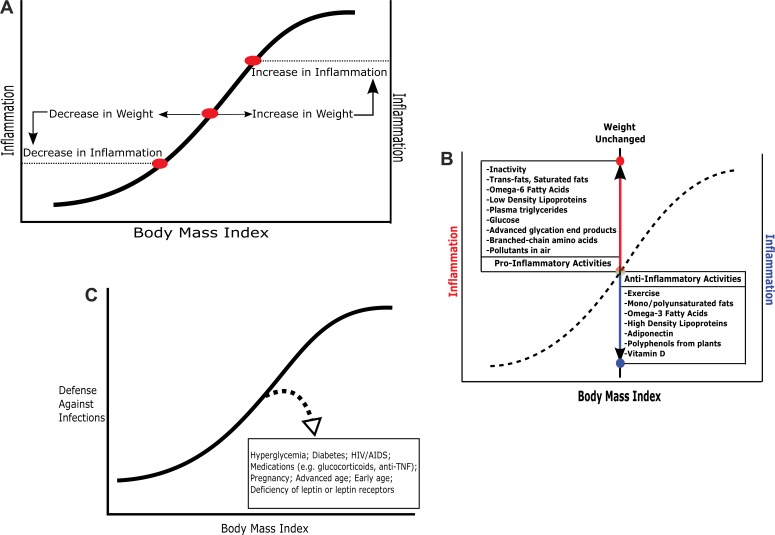
Overfed animals with obesity have elevated levels of circulating leptin. Leptin is a robust, broadly acting promoter of immune activation, as, for example, with metabolic syndrome. Humans and rodents lacking leptin (ob/ob, Zucker, other) or lacking leptin receptors (db/db) are very obese but have immune deficiencies (36,37). When interpreting experiments, it is vital that hyperleptinemic obesity, which is similar to the common condition in obese humans, is distinguished from hypoleptinemic obesity, an uncommon condition in humans and rodents but widely used in laboratory experiments. Hyperglycemia and diabetes are also sources of confusion (Figure 7). As adiposity increases, metabolic syndrome intensifies, raising the level of immune protection (Figures 7A and B). Simultaneously, the groundwork is laid for dysglycemia and diabetes, with serious undermining of multiple components of immune protection (Figure 7C).
Figure 7.
(A) The link between body mass index and inflammation. The curved line is a schematic representation of the baseline relationship between inflammatory processes and adiposity in one hypothetical individual. As the body mass index in an individual increases or decreases, inflammation typically follows. (B) The dashed-curved line represents the relationship between body mass index and inflammation in a hypothetical individual (equivalent to the solid line in the previous graph). When body weight does not change, changes in inflammation can still occur. The box at lower right catalogs antiinflammatory activities. The box at the upper left shows proinflammatory activities. (C) Typically with body weight increases, the defenses against infection increase. The dashed line with an arrow points to conditions that diminish the immune response below the level expected for that body mass index.
Exceptions
We have suggested that infections in general are associated with better survival in overweight patients, but also recognize that outcomes in individual infections can be worse with obesity (38,39). These infections include H1N1 influenza A (40–43) and hepatitis B and C (44–47). The influence of obesity on additional individual infectious diseases warrants further study.
STERILE (NON-INFECTIOUS) DISEASES
Infectious Processes and Sterile Injury
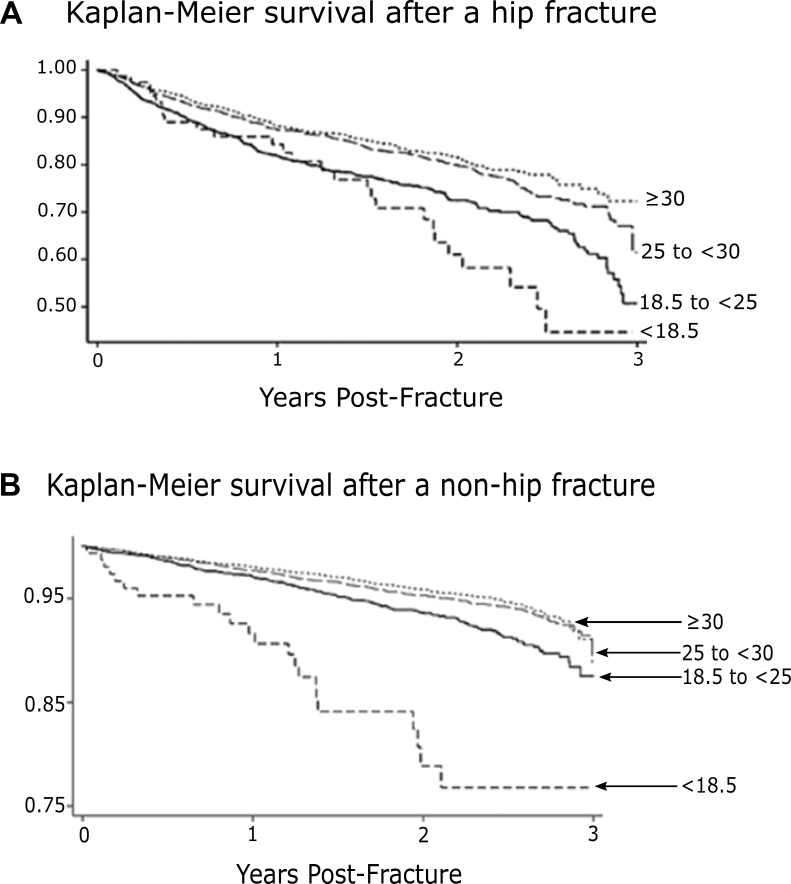
More than 20 epidemiology studies of six major infectious diseases, including leaders like tuberculosis, community-acquired pneumonia, sepsis and American trypanosomiasis, show that elevated adiposity is associated with a significant survival advantage. A similar advantage with adiposity has been widely noted among patients with noninfectious disorders, including those designated as “sterile injury” (Table 1). Because obese and overweight patients often fare better than their normal-weight counterparts, the tag “obesity paradox” has often been applied. As shown in Figures 8A and B, obese patients who have uninfected fractures of the hip or other bones show better outcomes that are reminiscent of those observed with so-called unconquerable infections (48). Valentijn et al. compiled a collection of published reports of surgical entities and a similar collection of nonsurgical entities where patients with above-normal adiposity show better outcomes (49,50). Finally, we were unable to measure the effects of publication bias; publications with results that are statistically significant are much more likely to overcome the multiple barriers that line the road to publication.
Figure 8.
Survival following hip (A) and non-hip fractures (B). This figure records the association of adiposity with mortality after hip fractures (upper) and non-hip fractures (lower) in men and women over 40 years of age who were registered in a nationwide electronic database in Spain. The numbers at the right of each curve represents body mass index obtained at time zero. Among the 6,988 hip fracture patients, early survival (6 months) and long-term survival (3 years) were highest in the top two body weight categories (BMI 25 and above). In 3,768 patients with non-hip fractures, survival was progressively better in the heavier weight categories, especially notable by the three-year follow-up (48).
Overlaps of Body Responses to Sterile and Infectious Processes
Local and systemic responses to sterile injury, including ischemia and inflammation, in many ways resemble the body’s responses to infection. The old, no longer favored, idea that the response to infection involves two sets of sensing pathways, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), while sterile inflammation involves only the DAMPs, has been replaced by the idea that both conditions involve both clusters of endogenous response elements (51). As research advances, more overlaps between these two pathways are being recognized. Recent data suggest that products of commensal organisms from the gut microbiota supply PAMP activators during so-called sterile events, heightening our appreciation of the overlap of the two pathways. That would be consistent with the widespread view that death with most infections is not due directly to the microbe but to endogenous processes, immunologic and other, inaugurated by the infection but with a sustaining momentum of their own, as occurs with so-called sterile events. Given the similarities, the favorable therapeutic response in mice from activating elements of metabolic syndrome observed in preliminary experiments with trypanosomiasis and polymicrobial sepsis raises our optimism that similar tools might also be effective in many so-called sterile conditions (52–57).
HISTORY AND EVOLUTION
History May Provide Intellectual Unity
Evolutionary history rationalizes the coexistence of obesity’s positive and negative contributions. Metabolic syndrome, with its continually growing list of negative consequences, has made the biomedical community resistant to recognize benefits to patients that might accrue from obesity. This reticence is reflected in the growing use of the term “obesity paradox” when benefits are uncovered, especially those associated with sterile injury. Our reports here of benefits from obesity with infectious diseases join this list. Reflections on possible evolutionary origins lead us to suggest that this help with infections appeared very early in human evolution and provides benefits very early in the lives of individuals. We hypothesize that the benefits with sterile injury arose later in human evolution and typically have more influence later in life. Not only does the metabolic syndrome become prominent late in the life of an individual, but historically it rose to prominence only in recent times, as adiposity and longevity increased.
Evolution
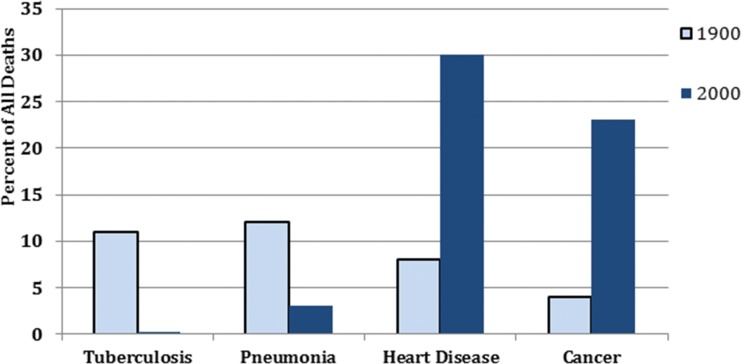
In contrast to deaths in 2000, the causes and demographics of mortality in 1900 were probably much closer to what was seen in the first 150,000 years of Homo sapiens on the planet, or in the millions of years for our hominid ancestors (Figure 9). Infections, along with trauma, were dominant, while the degenerative diseases of old age were uncommon causes of death. From an evolutionary point of view, it is remarkable that the metabolic syndromes in mice and rats are so similar to each other and to the syndrome in humans, although the two rodent lines separated evolutionarily more than one million years ago and we share a common ancestor with rodents from more than 10 million years ago (2,3). The durability of the program over time across species supports our proposal of its vital role, particularly in youngsters.
Figure 9.
Comparison of causes of death in the USA, 1900 versus 2000. The percentage of all deaths due to four classes of diseases are shown for the years 1900 (light blue bars) and 2000 (dark blue bars). The dark blue bar for tuberculosis in 2000 in actuality should have been invisible. However, for this figure, we falsely increased the height of the bar to make it just visible (59).
“One of the Most Remarkable Transformations in the History of Mankind”
Rothstein reminds us that “the twentieth century has witnessed the greatest and most rapid changes in both death rates and causes of death in recorded history. At the beginning of the century, infectious diseases were the paramount cause of death in all societies, killing millions of infants, children, and young adults. After 1900, death rates from these diseases declined rapidly in advanced countries, enabling many more people to live to old age” (3,58) (Figure 9). In 1900, only 2% of people in the USA were over the age of 65. By 2000, that percentage had increased six-fold. In contemporary Japan, Germany and Italy, people over 65 constitute 20% of the population. The demographic shift is mirrored in the causes of death. In 1900, pneumonia was a leader and affected all ages. Tuberculosis, a close second, accounted for more than 10% of all deaths in the USA (Figure 9). Tuberculosis affected all ages, “from the newborn to the octogenarian but ages (18–35) were hardest hit” (13). The ferocity of tuberculosis was reflected in its nicknames, “the captain of the men of death” and “the white plague.”
At present, with the decline in infectious diseases and 12% of the US population older than 65, heart disease and cancer are the leading causes of death, and most of those affected are well along in years (Figure 9). Deaths from pneumonia are in the single digits and typically affect those who are very old, very young or already ill). Tuberculosis deaths are so few that we artificially inflated the figure in the column for tuberculosis 2000 so it could be visible. Worldwide, tuberculosis is still a major infectious disease killer, ranking first or second, depending on the criteria for calculation. We estimate that in the time it takes to read this article, 50 to 100 humans will die of tuberculosis (59).
The standard 21st-century view of metabolic syndrome as a herald and bearer of the major pathology associated with diseases of aging derives from the brilliant pioneer work of Reaven et al., brought to full flower by him and his successors (60). One major weakness of the current definition that has emerged is the use of a final declaration of yes or no: the individual has or does not have metabolic syndrome. It is treated as a diagnostic entity (yes/no) rather than as a continuous pathophysiologic process. We envision metabolic syndrome to be a continuous process, controlled in a rheostat-like fashion (like the volume dial on a car radio) and affecting all of us all the time. More important, each of the myriad components that characterize excess adiposity and metabolic syndrome represents an individual process, each with its own rheostat-like continuum. A future hypothetical desirable goal would be to harness one or more of these processes for magnification in normal-weight or underweight patients with infectious diseases or sterile injuries to recreate the therapeutic benefits of obesity without the adiposity.
SCOPING THE FUTURE: RESEARCH PROSPECTUS
How can the better outcomes in obese patients be reproduced in thin patients, especially the vulnerable elderly, with these conditions (e.g., tuberculosis, pneumonia, sepsis or hip fracture)? Weight gain for thin patients (1) can be difficult, (2) is likely to bring both desirable and undesirable elements, and (3) is hard to undo once the need is met.
The medical profession’s longstanding fight against obesity may have a useful lesson; efforts to control body weight (short of bypass surgery) continue to have limited success. Greater health benefits have been derived from identifying and treating individual health-harming components of obesity, eg, hypercholesterolemia, hypertension, hyperglycemia and sleep apnea. We can envision researchers identifying elements that provide benefit to obese patients showing the obesity paradox and then recreating in thin patients one or a few of these elements over the short term. Possibly one menu will fit all conditions that manifest the obesity paradox, or, more likely, multiple menus will be devised. Our optimism is fueled by (1) therapeutic successes achieved (in the opposite direction) by altering single elements, eg, TNF or IL-1, in complex inflammatory disorders; (2) deep knowledge and availability of hormone-like agents, eg, leptin, resistin and adiponectin, whose circulating levels differ in thin and obese subjects; and (3) animal models (see earlier) that promise to be in the vanguard of this search.
CONCLUSION
Excess adiposity and its companion, metabolic syndrome, have well-deserved reputations as major detriments to health and longevity. What was unexpected was the inverse relationship between adiposity and mortality that we derived from more than 20 epidemiology studies of six infectious diseases with broadly different etiologies (and a score of sterile medical and surgical obesity paradoxes from other published literature). Recall that body mass index and waist circumference are very inexact measures of visceral fat deposits and the associated metabolic syndrome. Further smudging of the relationship between BMI and metabolic syndrome comes from the sizeable ethnic variations and from distortions from aging, other diseases and medications. It is remarkable that the tightness of the linkage survives the many uncontrollable variables.
ACKNOWLEDGMENTS
We would like to thank Professor Ulf Smith (University of Gothenburg) for his support, encouragement and excellent critique of the paper. We would also like to thank Dr. David B Allison (University of Alabama at Birmingham) for his helpful suggestions. We thank Dr. Fnu Nagajyothi, Dr. Louis M Weiss, and Dr. Herbert B Tanowitz (Albert Einstein School of Medicine) for support of our studies with Trypanosoma cruzi (32).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Roth J, et al. (2016) Obesity paradox, obesity orthodox, and the metabolic syndrome: an approach to unity. Mol. Med. 22:(873–85).
REFERENCES
- 1.Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34(8):1741–48. doi: 10.2337/dc10-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth J. Evolutionary speculation about tuberculosis and the metabolic and inflammatory processes of obesity. JAMA. 2009;301(24):2586–88. doi: 10.1001/jama.2009.930. [DOI] [PubMed] [Google Scholar]
- 3.Roth J, Szulc AL, Danoff A. Energy, evolution, and human diseases: an overview. Am J Clin Nutr. 2011;93(4):S-875–83. doi: 10.3945/ajcn.110.001909. [DOI] [PubMed] [Google Scholar]
- 4.Schneeweiss S, Setoguchi S, Weinblatt ME, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(6):1754–64. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 5.Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology (Oxford) 2007;46(7):1157–60. doi: 10.1093/rheumatology/kem076. [DOI] [PubMed] [Google Scholar]
- 6.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 7.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–98. [PubMed] [Google Scholar]
- 8.Tverdal A. Body mass index and incidence of tuberculosis. Eur J Respir Dis. 1986;69(5):355–62. [PubMed] [Google Scholar]
- 9.Edwards LB, Livesay VT, Acquaviva FA, Palmer CE. Height, weight, tuberculous infection, and tuberculous disease. Arch Environ Health. 1971;22(1):106–12. doi: 10.1080/00039896.1971.10665820. [DOI] [PubMed] [Google Scholar]
- 10.Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39(1):149–55. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 11.Leung CC, Lam TH, Chan WM, et al. Lower risk of tuberculosis in obesity. Arch Intern Med. 2007;167(12):1297–1304. doi: 10.1001/archinte.167.12.1297. [DOI] [PubMed] [Google Scholar]
- 12.Cegielski JP, Arab L, Cornoni-huntley J. Nutritional risk factors for tuberculosis among adults in the United States 1971-1992. Am J Epidemiol. 2012;176(5):409–22. doi: 10.1093/aje/kws007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osler W, McCrae T. 9th Rev Ed. New York, London: D Appleton and Company; 1920. The Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine; p. pp 1168. [Google Scholar]
- 14.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373(5):415–27. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.José RJ, Periselneris JN, Brown JS. Community-acquired pneumonia. Curr Opin Pulm Med. 2015;21(3):212–18. doi: 10.1097/MCP.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 16.Regev-Yochay G, Raz M, Dagan R, et al. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin Infect Dis. 2004;38(5):632–39. doi: 10.1086/381547. [DOI] [PubMed] [Google Scholar]
- 17.Kahlon S, Eurich DT, Padwal RS, et al. Obesity and outcomes in patients hospitalized with pneumonia. Clin Microbiol Infect. 2013;19(8):709–16. doi: 10.1111/j.1469-0691.2012.04003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corrales-Medina VF, Valayam J, Serpa JA, Rueda AM, Musher DM. The obesity paradox in community-acquired bacterial pneumonia. Int J Infect Dis. 2011;15(1):e54–57. doi: 10.1016/j.ijid.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 19.King P, Mortensen EM, Bollinger M, et al. Impact of obesity on outcomes for patients hospitalised with pneumonia. Eur Respir J. 2013;41(4):929–34. doi: 10.1183/09031936.00185211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singanayagam A, Singanayagam A, Chalmers JD. Obesity is associated with improved survival in community-acquired pneumonia. Eur Respir J. 2013;42(1):180–87. doi: 10.1183/09031936.00115312. [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Koizumi A, Wada Y, Hiroyasu I, et al. Risk and protective factors related to mortality from pneumonia among middle-aged and elderly community residents: the JACC study. J Epidemiol. 2007;17(6):194–202. doi: 10.2188/jea.17.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tejera A, Santolaria F, Diez ML, et al. Prognosis of community acquired pneumonia (CAP): value of triggering receptor expressed on myeloid cells-1 (TREM-1) and other mediators of the inflammatory response. Cytokine. 2007;38(3):117–23. doi: 10.1016/j.cyto.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Prescott HC, Chang VW, O'Brien JM, Langa KM, Iwashyna TJ. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit Care Med. 2014;42(8):1766–74. doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wacharasint P, Boyd JH, Russell JA, Walley KR. One size does not fit all in severe infection: obesity alters outcome, susceptibility, treatment, and inflammatory response. Crit Care. 2013;17(3):R122. doi: 10.1186/cc12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurzinger B, Dünser MW, Wohlmuth C, et al. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Wien Klin Wochenschr. 2010;122(1-2):31–36. doi: 10.1007/s00508-009-1241-4. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: A systematic review. J Crit Care. 2015;30(3):518–24. doi: 10.1016/j.jcrc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Machado FS, Dutra WO, Esper L, Tanowitz HB, et al. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol. 2012;34(6):753–70. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beleigoli AM, Ribeiro AL, Diniz Mde F, Lima-costa MF, Boersma E. The “obesity paradox” in an elderly population with a high prevalence of Chagas disease: the 10-year follow-up of the Bambuí (Brazil) Cohort Study of Aging. Int J Cardiol. 2013;166(2):523–26. doi: 10.1016/j.ijcard.2012.09.126. [DOI] [PubMed] [Google Scholar]
- 29.Stewart L, Griffiss JM, Jarvis GA, Way LW. The association between body mass index and severe biliary infections: a multivariate analysis. Am J Surg. 2012;204(5):574–79. doi: 10.1016/j.amjsurg.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Bang CS, Yoon JH, Kim YJ, et al. Clinical impact of body mass index on bactibilia and bacteremia. BMC Gastroenterol. 2014;14:104. doi: 10.1186/1471-230X-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utzolino S, Ditzel CM, Baier PK, Hopt UT, Kaffarnik MF. The obesity paradox in surgical intensive care patients with peritonitis. J Crit Care. 2014;29(5):e1–5. doi: 10.1016/j.jcrc.2014.05.026. 887. [DOI] [PubMed] [Google Scholar]
- 32.Brima W, Eden DJ, Mehdi SF, et al. The brighter (and evolutionarily older) face of the metabolic syndrome: evidence from Trypanosoma cruzi infection in CD-1 mice. Diabetes Metab Res Rev. 2015;31(4):346–59. doi: 10.1002/dmrr.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagajyothi F, Weiss LM, Zhao D, et al. High fat diet modulates Trypanosoma cruzi infection associated myocarditis. PLoS Negl Trop Dis. 2014;8(10):e3118. doi: 10.1371/journal.pntd.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tschöp J, Nogueiras R, Haas-lockie S, et al. CNS leptin action modulates immune response and survival in sepsis. J Neurosci. 2010;30(17):6036–47. doi: 10.1523/JNEUROSCI.4875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegl D, Annecke T, Johnson BL, et al. Obesity-induced hyperleptinemia improves survival and immune response in a murine model of sepsis. Anesthesiology. 2014;121(1):98–114. doi: 10.1097/ALN.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 36.Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15(14):2565–71. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 37.Allison MB, Myers MG. Twenty years of leptin: connecting leptin signaling to biological function. J Endocrinol. 2014;223(1):T25–35. doi: 10.1530/JOE-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37(3):333–40. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 39.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fezeu L, Julia C, Henegar A, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12(8):653–59. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 41.Cui W, Zhao H, Lu X, et al. Factors associated with death in hospitalized pneumonia patients with 2009 H1N1 influenza in Shenyang, China. BMC Infect Dis. 2010;10:145. doi: 10.1186/1471-2334-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Chan KP, Lee RS, et al. Obesity and influenza associated mortality: evidence from an elderly cohort in Hong Kong. Prev Med. 2013;56(2):118–23. doi: 10.1016/j.ypmed.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Kok J, Blyth CC, Foo H, et al. Viral pneumonitis is increased in obese patients during the first wave of pandemic A(H1N1) 2009 virus. PLoS ONE. 2013;8(2):e55631. doi: 10.1371/journal.pone.0055631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarcˇuška P, Janicˇko M, Kružliak P, et al. Hepatitis B virus infection in patients with metabolic syndrome: a complicated relationship. Results of a population based study. Eur J Intern Med. 2014;25(3):286–91. doi: 10.1016/j.ejim.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Wong GL, Chan HL, Yu Z, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B: a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39(8):883–93. doi: 10.1111/apt.12658. [DOI] [PubMed] [Google Scholar]
- 46.Charlton MR, Pockros PJ, Harrison SA. Impact of obesity on treatment of chronic hepatitis C. Hepatology. 2006;43(6):1177–86. doi: 10.1002/hep.21239. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Cui Y, Deng H, Yu J. Association between hepatitis B virus infection and metabolic syndrome: a retrospective cohort study in Shanghai, China. BMC Public Health. 2014;14:516. doi: 10.1186/1471-2458-14-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prieto-Alhambra D, Premaor MO, Aviles FF, et al. Relationship between mortality and BMI after fracture: a population-based study of men and women aged ≥ 40 years. J Bone Miner Res. 2014;29(8):1727–44. doi: 10.1002/jbmr.2209. [DOI] [PubMed] [Google Scholar]
- 49.Valentijn TM, Galal W, Tjeertes EK, Hoeks SE, Verhagen HJ, Stolker RJ. The obesity paradox in the surgical population. Surgeon. 2013;11(3):169–76. doi: 10.1016/j.surge.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Valentijn TM, Galal W, Hoeks SE, Van Gestel YR, Verhagen HJ, Stolker RJ. Impact of obesity on postoperative and long-term outcomes in a general surgery population: a retrospective cohort study. World J Surg. 2013;37(11):2561–68. doi: 10.1007/s00268-013-2162-y. [DOI] [PubMed] [Google Scholar]
- 51.Zhu S, Ashok M, Li J, et al. Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med. 2009;15(7-8):275–82. doi: 10.2119/molmed.2009.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuda A, Jacob A, Wu R, et al. Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol Med. 2011;17(1-2):126–33. doi: 10.2119/molmed.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoque R, Farooq A, Mehal WZ. Sterile inflammation in the liver and pancreas. J Gastroenterol Hepatol. 2013;1(28 Suppl):61–67. doi: 10.1111/jgh.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fleshner M. Stress-evoked sterile inflammation danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav Immun. 2013;27(1):1–7. doi: 10.1016/j.bbi.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 55.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143(5):1158–72. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Land WG. The Role of Damage-Associated Molecular Patterns (DAMPs) in Human Diseases Part II: DAMPs as diagnostics prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J. 2015;15(2):e157–70. [PMC free article] [PubMed] [Google Scholar]
- 58.Rothstein WG. Readings in American Health Care: Current Issues in Socio-Historical Perspective. Madison: University of Wisconsin Press; 1995. Trends in mortality in the twentieth century; p. p. 72. [Google Scholar]
- 59.2016. Data available at: http://www.cdc.gov/nchs/data/nvsr/nvsr50/nvsr50_15. Accessed July 21.
- 60.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–23. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]