It is generally accepted that a severed spinal cord is associated with permanent paralysis. Recently, a spinal cord fusion protocol (GEMINI) has been proposed, whereby an acutely controlled, sharp, operative transection of the spinal cord is carried out. This scenario is not comparable (even in principle) to the clinical situation of a traumatic spinal cord injury, in which major tissue disruption (mechanical, hemorrhagic, scar- and cyst-associated) occurs (Canavero, 2015). During 1950s–1960s, neurosurgeon Dr. Freeman made extensive observations of what happens when a spinal cord is sharply transected. He reported slow recovery of behavioral motor function in several animals over months (reviewed in Canavero et al., 2016), with clear signs of electrophysiological conduction. Most importantly, silver stained histologic sections showed numerous growing axons connecting the divided ends (Freeman, 1963). This recovery can be accelerated by treating the severed cord with a fusogen (e.g., polyethylene glycol) (see for full discussion and rationale from Canavero, 2013; Kim, 2016). Here, we clearly prove that axonal regrowth is possible across the severance interface using immunohistochemistry and electron microscopy.
The experiment was approved by the Institutional Animal Care and Use Committee of the Konkuk University (Seoul, Korea). Our previous experiment reported a mild recovery of the limb to the level of a sweeping behavior without weight support at the 4th week after C5 laminectomy, equal to a Basso, Beattie and Bresnahan (BBB) locomotor scale score of 6–8 (Kim et al., 2016), and aiming at confirming the possibility of direct reconnection of a fully severed spinal cord. In this model, reproducing traumatic severance with a blade as happens after clinical stab wounds, no gap exists between the severed stumps. In this study, to confirm neuroregeneration across the contact interface, C5 laminectomy was performed in female ICR mice as described previously (Kim et al., 2016). After gently raising the cervical cord with a hook, complete severance was performed with surgical sharp blades #11. Polyethylene glycol (PEG MW 400, Sigma-Aldrich, St. Louis, MO, USA), a cell membrane fusogen (Ye et al., 2016)) was dripped onto the cut area. After the muscle and fascia were sutured and the skin closed, normal saline solution was provided with total parenteral nutrition (TPN, Chong Kun Dang, Korea) through the tail vein four times a day. All mice sacrificed at 4 weeks after operation showed partial behavior recovery as reported in a previous study (Kim et al., 2016). The spinal cervical cords were removed, frozen on dry ice and sectioned into longitudinal and transverse slices. Sections were fixed with 4% paraformaldehyde solution. Fixed sections were immunohistochemically stained for anti-neurofilament 200 (NF200; Sigma, 1:50), and mounted with 4′,6-diamidino-2-phenyindole (DAPI). Fluorescence was visualized using fluorescence or confocal microscopy. The transverse slices of fixed tissue were examined by transmission electron microscopy (TEM) after being postfixed in osmium tetroxide and processed conventionally.
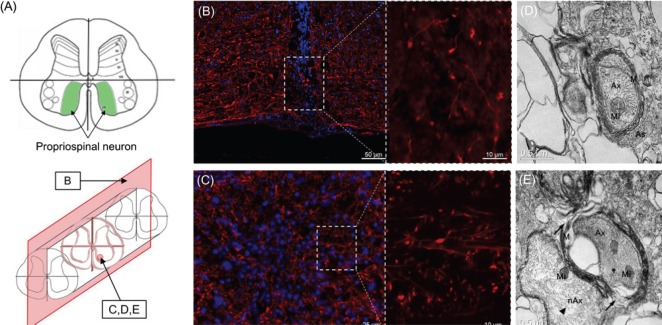
This report focuses on the ventral compartment of the cord where the motor system is located. Histologically, clear signs of axonal sprouting were detected across the gap at both low and high magnifications in longitudinal sections (Figure 1B). As expected, given the sharp severance, cyst formation normally observed in spinal cord injury was absent, and the gap between cuts was filled by the DAPI stained migrated cells. In line with another study showing regeneration of rat spinal cord fibers across the gap using graphene nano-ribbons in a hemi-transection model (Palejwala et al., 2016), our data provide further histological evidence that cervical spinal cord regeneration is possible. In transverse sections, a large amount of sprouting axons are shown in the reconnected area (Figure 1C), in a region especially rich in propriospinal neurons (Figure 1A). Ultrastructurally, both myelinated axons with the axoplasm containing intact mitochondria, adjacent astrocytes (Figure 1D), disorganized splitting myelination (Figure 1E) and non-myelinating axons (Figure 1E) were observed.
Figure 1.
Schematic view and image analysis of spinal cervical cord.
(A) Schematic view of mouse spinal cervical cord segment C5 cut in transection (top), and the position of the section (bottom). (B, C) Immunohistological analysis (neurofilament-200: red; 4′,6-diamidino-2-phenylindole: blue) of longitudinal and transverse sections, respectively. Sprouting axons across the severed planes are observed in confocal image (dotted line). (D, E) Transmission electron microscopy analysis of a transverse section. Splitting myelination (E, arrow) and non-myelinating axon (E; arrowhead) are observed. Ax: Axon; Mi: mitochondria; M: myelin; As: astrocyte; nAx: non-myelinating axons.
Contrary to neurological dogma, the true engine of motor function in mammals, including man, is the so-called cortico-trunco-reticulo-propriospinal pathway (Canavero and Ren, 2016; Canavero et al., 2016), a network of propriospinal neurons that spans the whole length of the spinal cord and channels impulses from the brainstem reticular formation to the motor neurons. For instance, primates can perform arm and hand movements (including the dexterous movements of the fingers and precision grip) without a pyramidal tract because of the neural circuits of the propriospinal system alone (Canavero and Ren, 2016). After sharp severance, it is the sprouting of the propriospinal neurons across the gap that sustains motor recovery (Canavero and Ren, 2016). Recent data proved that propriospinal neurons, unlike supraspinal axons, are capable of extending axons through a spinal lesion, which is rich in neuroactive substances that inhibit growth, i.e., can penetrate the “hostile” micro-environment of a traumatic injury as in patients with spinal cord injury (Fenrich and Rose, 2009), but no correlative behavioral study was done, making that finding clinically dubious.
We previously showed that a fusogen-assisted sharp section of the cervical (Kim et al., 2016) and dorsal (Ye et al., 2016) cord is followed by behaviorally relevant motor recovery in experimental animals. Here, we correlate the recovery to evidence of sprouting across the polyethylene glycol-treated plane of fusion. Sprouting neurons were especially located in lamina VIII, where propriospinal neurons are particularly concentrated in the mouse cervical cord.
The data reported above clearly show that a sharply severed spinal cord can be reconnected by sprouting neurons across the severance interface, disproving a decades-old dogma.
The work was partially supported by the National Research Foundation of Korea (NRF), No. 2015R1C1A1A02037047.
References
- Canavero S. HEAVEN: The head anastomosis venture project outline for the first human head transplantation with spinal linkage (GEMINI) Surg Neurol Int. 2013;4:S335–342. doi: 10.4103/2152-7806.113444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavero S. The “Gemini” spinal cord fusion protocol: Reloaded. Surg Neurol Int. 2015;6:18. doi: 10.4103/2152-7806.150674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavero S, Ren XP. The spark of life: engaging the cortico-truncoreticulo-propriospinal pathway by electrical stimulation. CNS Neurosci Ther. 2016;22:260–261. doi: 10.1111/cns.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavero S, Ren X, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI) Surgery. 2016;160:11–19. doi: 10.1016/j.surg.2016.01.027. [DOI] [PubMed] [Google Scholar]
- Fenrich KK, Rose PK. Spinal interneuron axons spontaneously regenerate after spinal cord injury in the adult feline. J Neurosci. 2009;29(39):12145–12158. doi: 10.1523/JNEUROSCI.0897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L. Observation on the Regeneration of Spinal Axons in Mammals. Proceedings, X Congreso Latinoamericano de Neurochirurgia Editorial Don Bosco. 1963:135–144. [Google Scholar]
- Kim CY. PEG-assisted reconstruction of the cervical spinal cord in rats: effects on motor conduction at 1 h. Spinal cord. 2016;54:910–912. doi: 10.1038/sc.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CY, Oh H, Hwang IK, Hong KS. GEMINI: Initial behavioral results after full severance of the cervical spinal cord in mice. Surg Neurol Int. 2016;7(Suppl 24):S629–631. doi: 10.4103/2152-7806.190474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palejwala AH, Fridley JS, Mata JA, Samuel EL, Luerssen TG, Perlaky L, Kent TA, Tour JM, Jea A. Biocompatibility of reduced graphene oxide nanoscaffolds following acute spinal cord injury in rats. Surg Neurol Int. 2016;7:75. doi: 10.4103/2152-7806.188905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Kim CY, Miao Q, Ren X. Fusogen-assisted rapid reconstitution of anatomophysiologic continuity of the transected spinal cord. Surgery. 2016;160:20–25. doi: 10.1016/j.surg.2016.03.023. [DOI] [PubMed] [Google Scholar]