Abstract
Each neuronal subtype is distinct in how it develops, responds to environmental cues, and whether it is capable of mounting a regenerative response following injury. Although the adult central nervous system (CNS) does not regenerate, several experimental interventions have been trialled with successful albeit limited instances of axonal repair. We highlight here some of these approaches including extracellular matrix (ECM) modification, cellular grafting, gene therapy-induced replacement of proteins, as well as application of biomaterials. We also review the recent report demonstrating the failure of axonal localization and transport of growth-promoting receptors within certain classes of mature neurons. More specifically, we discuss an inability of integrin receptors to localize within the axonal compartment of mature motor neurons such as in the corticospinal and rubrospinal tracts, whereas in immature neurons of those pathways and in mature sensory tracts such as in the optic nerve and dorsal column pathways these receptors readily localize within axons. Furthermore we assert that this failure of axonal localization contributes to the intrinsic inability of axonal regeneration. We conclude by highlighting the necessity for both combined therapies as well as a targeted approach specific to both age and neuronal subtype will be required to induce substantial CNS repair.
Keywords: axonal transport, cellular therapies, extracellular matrix, gene therapy, integrin, regeneration, viral vectors
Introduction
As the mammalian central nervous system (CNS) matures, it loses its ability to repair itself. Alongside the inhibitory environment characterized by a glial scar and an upregulation of inhibitory proteins such as chondroitin sulphate proteoglycans (CSPGs) and myelin-associated glycoproteins (MAGs), CNS axons have an intrinsically low capacity for self-repair which continually diminishes with age (Reviewed by Chew et al., 2012). As neurons mature, proteins that were once key regulators of axon guidance and elongation are downregulated resulting in a reduced capacity for axonal repair after injury. By recapitulating neuronal expression of growth-promoting proteins, such as integrins, transmembrane receptors involved in mediating cell-cell and cell-matrix interactions, neurite outgrowth and axon regeneration can be significantly enhanced (Condic, 2001; Andrews et al., 2009; Cheah et al., 2016). The α9β1 integrin heterodimer, for example, is highly expressed during CNS development aiding growth cone formation and axonal elongation but is downregulated in mature CNS axons. It binds the main extracellular matrix (ECM) glycoprotein of the CNS, tenascin-C, which is highly upregulated after injury and has been a recent target of axonal regeneration research (Andrews et al., 2009; Chen et al., 2010; Cheah et al., 2016). For example, increasing expression of the alpha9 subunit in adult dorsal root ganglion (DRG) neurons alongside its activator Kindlin-1, has been shown to promote growth cone formation and regeneration of severed axons after dorsal root injury (Cheah et al., 2016). Despite these promising findings, recent data reveals region-specific and age-specific differences exist resulting in variations in integrin trafficking into the axonal compartment (Franssen et al., 2015; Andrews et al., 2016) creating yet another hurdle for regeneration.
Blockage of Integrin Localization into the Axonal Compartment
In newly published work, Andrews et al. (2016) highlight differences in integrin localization in distinct neuronal subtypes, following viral vector-based expression. Their findings show that in mature corticospinal tract (CST) and rubrospinal tract (RST) axons, exogenously-expressed integrins are not localized or transported into the axonal compartment, remaining instead in the somatodendritic compartment (Andrews et al., 2016). On the other hand, exogenously-expressed integrins in early postnatal CST neurons are readily localized within the axonal compartment of developing CST axons. Furthermore, exogenously-expressed integrins successfully localize in mature optic nerve axons as well as mature dorsal root axons following intravitreal or dorsal root ganglia injections, respectively (Andrews et al., 2016). These data establish a differential ability of transmembrane receptors to localize in distinct areas of the nervous system. Previous research in cultured cortical neurons suggests this is due to the axon initial segment acting as a filter and barrier for integrin entry into the axonal compartment (Franssen et al., 2015). Therefore, region-specific and age-specific axon transport mechanisms are likely to play a role in modulating intrinsic CNS repair. In this article we consider the difficulties of enhancing intrinsic-mediated repair of neurons including the delivery of growth-promoting proteins necessary for regrowth of adult axons in addition to considering extrinsic targets and therapies for enhancing CNS repair.
Current Approaches to CNS Repair
A number of experimental avenues have been pursued with the hope of finding a robust treatment to promote axonal regeneration within the CNS (Figure 1). Treatments include modification of ECM components, such as through the removal of CSPGs, to stimulate neuronal plasticity, and removal of inhibitory proteins, such as Nogo-A, to alleviate degradation and apoptotic pathways (Reviewed by Chew et al., 2012). Furthermore, cellular replacement therapies and application of biomaterials to stabilize lesion architecture have also been utilized as therapies for CNS repair (Reviewed by Assunção-Silva et al., 2015). In addition, a number of signalling pathways involved in maintaining axonal growth, guidance, trafficking, receptor turnover and apoptosis, including neurotrophic factors and other growth-promoting receptors such as integrins, have provided several targets for viral vector-based gene therapy to promote intrinsic regeneration following axonal injury.
Figure 1.
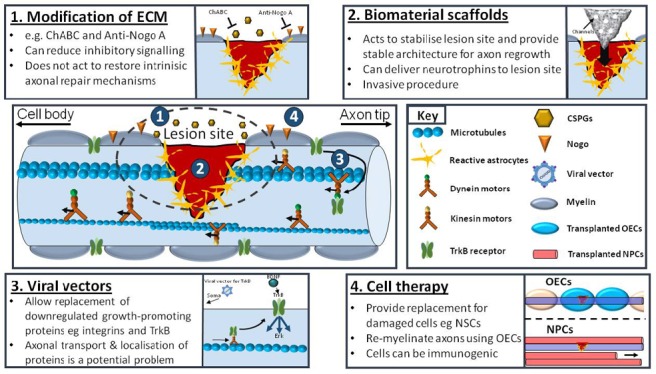
Current approaches for promoting axonal repair following central nervous system (CNS) injury.
Schematic diagram highlighting an overview of axonal injury and degradation at the lesion site along with current approaches to enhance repair. After CNS injury, an inhibitory lesion site is created surrounded by reactive astrocytes and a number of growth-inhibiting proteins including CSPGs and Nogo. Transport within axons is mediated by both kinesin and dynein motors and allows cargo-carrying vesicles to travel in both anterograde and retrograde directions. However, within the mature CNS many growth-promoting proteins, such as TrkB, are trafficked back to the cell body in the retrograde direction resulting in a reduced ability of axons to regenerate after injury. (1) Modification of the ECM using ChABC enzyme to degrade CSPGs or anti-Nogo A antibodies to inhibit Nogo activity can reduce the action and signalling of inhibitory components in the injury site to promote axonal regeneration after injury. (2) Biomaterials can be used to stabilize the lesion site as well as provide guidance channels for axon regrowth. Modified scaffolds have been used to deliver multiple neurotrophic factors to lesion site to aid axon repair. (3) Alternatively, delivery of viral vectors that are tissue or cell-specific can carry DNA encoding for growth-promoting proteins e.g., TrkB to the lesion site. Using gene therapy to reinstate proteins involved in axon growth and guidance can prompt growth-promoting signalling pathways. (4) Cell therapy involves replacing lost or damaged cells at the lesion site. These can include OECs to promote remyelination of injured axons or transplantation of NSCs to replace injured neurons/axons. ChABC: Chondroitinase ABC; CSPGs: chondroitin sulphate proteoglycans; ECM: extracellular matrix; NPC: neural progenitor cell; NSC: neural stem cell; OECs: olfactory ensheathing cells; TrkB: tropomyosin receptor kinase B.
Failed Axonal Transport of Signalling Molecules in CNS Repair
Current research has focused on targeting and reinstating regenerative signalling pathways associated with neurite outgrowth and survival, such as tropomyosin-related kinase (Trk) receptors, insulin-like growth factor 1 (IGF-I) receptors and integrin receptors following injury. Advancements in viral vector research have made gene therapy a commonly used experimental tool to study regenerative signalling pathways together with axonal repair. Research suggests expression levels of endogenous TrkB in injured CST axons are not sufficient to promote neurite outgrowth and repair (Lu et al., 2001) however nowever forced expression of TrkB using lentiviral vectors following CST lesioning resulted in enhanced regeneration (Hollis et al., 2009). In that study, however, Hollis et al. (2009) demonstrated that exogenously expressed TrkB was only localized as far as the proximal part of CST axons (subcortical white matter) suggesting that intrinsic transport mechanisms within these axons are compromised in the adult nervous system. Axonal transport and localization of growth-promoting molecules involved in neuronal signalling are necessary and essential for the development of the nervous system as well as for axon growth and homeostasis. Long-distance transport is driven by kinesin and dynein motors which are regulated by Rab GTPases, kinases and a number of scaffolding proteins (Maday et al., 2014). Failure of axon transport can have a detrimental effect on axon growth and function and has been linked to disease and injury, such as in motor neuron disease, Alzheimer's disease, and spinal cord injury (SCI). Research now suggests that axon transport and localization in certain neuronal regions is inherently downregulated as the normal uninjured CNS matures (Andrews et al., 2016). Furthermore, evidence suggests growth-promoting receptors are excluded from mature CST axons, including TrkB, the receptor for brain-derived neurotrophic factor (BDNF) (Lu et al., 2001) and integrins (Franssen at al., 2015; Andrews et al., 2016).
Use of Biomaterials to Bridge CNS Lesion Sites
Not only is it necessary to consider localization of virally-expressed proteins in neurons, viral vectors themselves may have limited temporal expression, although more treatments currently utilize standard adeno-associated virus or lentivirus which have been shown to have long-term sustained expression. The literature suggests however that by combining gene therapy with biomaterials, better long-term expression may result (Thomas et al., 2014). Scaffold bridges made from poly(lactide-co-glycolide) (PLG) have been used to deliver lentiviral vectors carrying sonic hedgehog (Shh) and neurotrophin-3 to T9/T10 region of spinal cords of mice following lateral hemisection (Thomas et al., 2014). Results demonstrate that expression of both proteins was sustained at the injury site, promoting axonal regrowth through the bridge architecture as well as facilitating recruitment of endogenous oligodendrocyte precursors and Schwann cells. This also resulted in increased axonal myelination 8 weeks post-transplant. Use of biodegradable bridges that contain a channel network for axonal guidance allows for localized delivery of multiple transgenes to the injury site, however, delivery of scaffolds can be invasive and does not overcome the inhibitory milieu created after injury. Likewise, there are also cases where sustained expression of virally-expressed proteins is unnecessary. In these cases, tamoxifen-inducible systems would overcome issues in regulating expression.
Modification of CNS Extracellular Matrix to Promote Axonal Growth
Restoring intracellular signalling pathways is one approach to tackle the failure in axon repair however we can also target removal of inhibitory proteins. The lesion environment consists of proteins that are known to prevent axonal regeneration including CSPGs and myelin-derived inhibitory proteins, such as Nogo, MAG and oligodendrocyte-myelin glycoprotein (OMgp). Removing or inactivating inhibitory molecules by blocking their action has shown efficacy in promoting regeneration. These strategies include application of the bacterial enzyme, chondroitinase ABC (ChABC) to digest glycosaminoglycan side chains of CSPGs and monoclonal antibodies against Nogo-A to inhibit Nogo activity. Research indicates a combination of treatments may provide the best recovery after axonal injury. Indeed, combined administration of anti-Nogo-A antibodies by intrathecal infusion, and ChABC by intraspinal injections and intrathecal infusion, alongside behavioral rehabilitation was shown to promote axonal regeneration and functional recovery following SCI, more than either treatment alone (Zhao et al., 2013). Unfortunately, administration of both molecules is invasive with ChABC requiring multiple applications to maintain the level of enzymatic activity required for axonal regeneration and sprouting.
Cellular Grafting to Reconnect Damaged Pathways
Bridging SCI lesion sites with cellular replacement therapies including oligodendrocytes and Schwann cells to encourage remyelination, as well as astrocytes and neural stem cells to replace lost cells is another avenue that has resulted in successful regeneration following damage. Research suggests however that a lesion environment can affect transplanted cell survival, engraftment, migration, proliferation and the availability of differentiation and growth promoting cues (Sontag et al., 2014). This study suggests that cell transplant treatments need to be combined with tandem therapies to utilize ECM environment to support and promote the grafted cells in vivo. Indeed, combining a neural stem cell (NSC) graft with ChABC enzyme to degrade CSPGs can promote graft survival, regeneration, axonal plasticity and functional recovery in a chronic animal model of SCI (Karimi-Abdolrezaee et al., 2010).
Age and Neuronal Type Dictates Axonal Transport and Localization
Research indicates that there are age-associated changes within CNS axonal transport, including a decrease in anterograde trafficking (Milde et al., 2015). Interestingly this decrease in transport in mature neurons can be partially reversed within peripheral nerves (Milde et al., 2015). Notably age-associated changes in axonal transport are also region specific, with differing transport rates in different neuronal areas, such as optic nerve, sciatic nerve and areas of the hippocampus (Milde et al., 2015). This brings us back to the current study. Andrews et al. (2016) showed developing motor and sensory neurons have the ability to traffic growth-promoting integrins within the axonal compartment but only sensory neurons of the PNS, specifically neurons of the DRG and retinal ganglion cells (RGCs), retain this ability as they mature. In the case of mature CST and RST axons, integrins are excluded and instead are retained within the somatodendritic compartment. This work confirms observations of Milde et al. (2015) that there are region-specific differences in axonal transport, but also indicates there are age-related changes. This suggests that treatment for CNS damage would not be the same for every injury and would likely have to be modified to target injuries in different neuronal regions.
Conclusion
Together this work emphasizes promoting transport and expression of growth-promoting proteins can prompt axon repair however, a better awareness of the mechanisms required for targeting delivery into CNS axons remains. The answer to this problem may include a combination of several approaches such as biomaterials and nanoparticles, cell replacement, modification of ECM, and gene therapy. Viral vectors allow for long-term gene expression however there is a small risk of insertional mutagenesis which keeps many of these techniques currently out of clinical trials. As researchers we need to consider how these age-related and site-specific changes in axon transport can tailor our approaches for repair. This includes CNS delivery methods as research indicates different viral vectors transduce DRGs differently (Mason et al., 2010). The question is not, do we need to re-establish axon transport, but rather how can it be done in a controlled site-specific, age-specific manner. Within this review we have discussed how reinstating age-associated changes in signalling pathways can lead to enhanced repair after injury, but when it comes to CNS injury this is only half the battle. A combination of treatments that target the milieu of injury-induced proteins to alleviate inhibitory signalling alongside region-specific modification of target signalling pathways together with physical rehabilitation is likely to offer the best hope for robust functional recovery after CNS injury.
Footnotes
Funding: MRA acknowledges support from the Morton Cure Paralysis Fund and Royal Society Research grant.
Conflicts of interest: None declared.
References
- Andrews MR, Soleman S, Cheah M, Tumbarello DA, Mason MR, Moloney E, Verhaagen J, Bensadoun JC, Schneider B, Aebischer P, Fawcett JW. Axonal localization of integrins in the CNS is neuronal type and age dependent. eNeuro. 2016;3:0029–16. doi: 10.1523/ENEURO.0029-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, Gage FH, ffrench-Constant C, Fawcett JW. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci. 2009;29:5546–5557. doi: 10.1523/JNEUROSCI.0759-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção-Silva RC, Gomes ED, Sousa N, Silva NA, Salgado AJ. Hydrogels and cell based therapies in spinal cord injury regeneration. Stem Cells Int. 2015;2015:948040. doi: 10.1155/2015/948040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M, Andrews MR, Chew DJ, Moloney EB, Verhaagen J, Fässler R, Fawcett JW. Expression of an activated integrin promotes long-distance sensory axon regeneration in the spinal cord. J Neurosci. 2016;36:7283–7297. doi: 10.1523/JNEUROSCI.0901-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Joon Lee H, Jakovcevski I, Shah R, Bhagat N, Loers G, Liu HY, Meiners S, Taschenberger G, Kügler S, Irintchev A, Schachner M. The extracellular matrix glycoprotein tenascin-C is beneficial for spinal cord regeneration. Mol Ther. 2010;18:1769–1777. doi: 10.1038/mt.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew DJ, Fawcett JW, Andrews MR. The challenges of long-distance axon regeneration in the injured CNS. Prog Brain Res. 2012;201:253–294. doi: 10.1016/B978-0-444-59544-7.00013-5. [DOI] [PubMed] [Google Scholar]
- Condic ML. Adult neuronal regeneration induced by transgenic integrin expression. J Neurosci. 2001;21:4782–4788. doi: 10.1523/JNEUROSCI.21-13-04782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen EH, Zhao RR, Koseki H, Kanamarlapudi V, Hoogenraad CC, Eva R, Fawcett JW. Exclusion of integrins from CNS axons is regulated by Arf6 activation and the AIS. J Neurosci. 2015;35:8359–8375. doi: 10.1523/JNEUROSCI.2850-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Jamshidi P, Löw K, Blesch A, Tuszynski MH. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc Natl Acad Sci U S A. 2009;106:7215–7220. doi: 10.1073/pnas.0810624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Blesch A, Tuszynski MH. Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons. J Comp Neurol. 2001;436:456–470. doi: 10.1002/cne.1080. [DOI] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MR, Ehlert EM, Eggers R, Pool CW, Hermening S, Huseinovic A, Timmermans E, Blits B, Verhaagen J. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther. 2010;18:715–724. doi: 10.1038/mt.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde S, Adalbert R, Elaman MH, Coleman MP. Axonal transport declines with age in two distinct phases separated by a period of relative stability. Neurobiol Aging. 2015;36:971–981. doi: 10.1016/j.neurobiolaging.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag CJ, Uchida N, Cummings BJ, Anderson AJ. Injury to the spinal cord niche alters the engraftment dynamics of human neural stem cells. Stem Cell Rep. 2014;2:620–632. doi: 10.1016/j.stemcr.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Seidlits SK, Goodman AG, Kukushliev TV, Hassani DM, Cummings BJ, Anderson AJ, Shea LD. Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integr Biol (Camb) 2014;6:694–705. doi: 10.1039/c4ib00009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RR, Andrews MR, Wang D, Warren P, Gullo M, Schnell L, Schwab ME, Fawcett JW. Combination treatment with anti-Nogo-A and chondroitinase ABC is more effective than single treatments at enhancing functional recovery after spinal cord injury. Eur J Neurosci. 2013;38:2946–2961. doi: 10.1111/ejn.12276. [DOI] [PubMed] [Google Scholar]


