Abstract
The glial regenerative response to central nervous system (CNS) injury, although limited, can be harnessed to promote regeneration and repair. Injury provokes the proliferation of ensheathing glial cells, which can differentiate to remyelinate axons, and partially restore function. This response is evolutionarily conserved, strongly implying an underlying genetic mechanism. In mammals, it is elicited by NG2 glia, but most often newly generated cells fail to differentiate. Thus an important goal had been to find out how to promote glial differentiation following the proliferative response. A gene network involving Notch and prospero (pros) controls the balance between glial proliferation and differentiation in flies and mice, and promotes CNS repair at least in fruit-flies. A key missing link had been how to relate the function of NG2 to this gene network. Recent findings by Losada-Perez et al., published in JCB, demonstrated that the Drosophila NG2 homologue kon-tiki (kon) is functionally linked to Notch and pros in glia. By engaging in two feedback loops with Notch and Pros, in response to injury, Kon can regulate both glial cell number and glial shape homeostasis, essential for repair. Drosophila offers powerful genetics to unravel the control of stem and progenitor cells for regeneration and repair.
Keywords: NG2, kon-tiki, glia, Drosophila, injury, regeneration, repair, CNS
Introduction
Regenerative responses to central nervous system (CNS) injury, although limited, reveal underlying natural mechanisms that could be harnessed to promote regeneration and repair. Conversely, these same mechanisms may promote CNS structural robustness and homeostasis during normal growth and adult life, and their impairment could underlie the disregulation that accompanies ageing, neurodegenerative diseases and brain tumours. Demyelinating diseases, like multiple sclerosis, and traumatic brain and spinal cord injury, provoke a spontaneous, natural regenerative response in ensheathing glial cell progenitors (i.e., in mammals, oligodendrocyte progenitor cells, OPCs) (Franklin and Ffrench-Constant, 2008). In response to damage, ensheathing glia proliferate and re-enwrap axons, leading to recovery of behavior. This response is very limited in humans, partly due to the fact that newly produced cells can fail to differentiate (Franklin and Ffrench-Constant, 2008). Thus a key goal has long been to find out how to promote differentiation of oligodendrocytes (OLs) following the regenerative proliferation of their progenitors. Importantly, the regenerative response of ensheathing glia is evolutionarily conserved, and occurs in cockroach, fruit-flies, fish and rodents too (Smith et al., 1987; Dubois-Dalcq et al., 2008; Franklin and Ffrench-Constant, 2008; Kato et al., 2011), implying there is an underlying genetic mechanism. The discovery of gene networks underlying regenerative responses to injury is key to understand how cells achieve and maintain normal body integrity. Importantly, they will enable the manipulation of stem cells, progenitor cells and neural circuits for therapeutic aims.
The fruit-fly Drosophila as Model Organism for CNS repair
The fruit-fly Drosophila has recurrently proven to be an extremely powerful model organism to discover evolutionarily conserved gene networks with relevance for humans. The Hidalgo lab demonstrated that the glial regenerative response to CNS injury in Drosophila depends on a gene network involving the genes Notch and prospero (pros), whereby Notch promotes glial proliferation and Pros glial differentiation (Griffiths and Hidalgo, 2004; Griffiths et al., 2007; Kato et al., 2011). This was an important finding: in mammals Notch was known to maintain OPCs proliferative, but upon injury, Notch prevents OL differentiation (Wang et al., 1998), and a challenge was to discover genes related to Notch that could promote glial differentiation. We demonstrated that similarly to pros in Drosophila, its homologue prox1 in the mouse is also required for OL differentiation (Kato et al., 2015). A key missing link was the yet unknown relationship of Notch1 and Prox1 to NG2.
In mammals, the glial regenerative response to CNS injury is carried out by NG2+ OPCs, also known as NG2-glia (Zuo and Nishiyama, 2013). The NG2 protein is required for the glial regenerative response: like Notch1, NG2 levels are also up-regulated upon injury, and mice lacking NG2 have reduced OPC proliferation during normal development, and in response to injury (Kucharova and Stallcup, 2010; Kucharova et al., 2011). Moreover, the size of demyelinating lesions decreases over time in wild type animals as the CNS tends to repair naturally, but in NG2 knock-out mice lesions fail to shrink, due to reduced OPC proliferation and resulting depletion in OLs (Kucharova et al., 2011). Thus, NG2 is a crucial factor involved in the regulation of the regenerative response of OPCs. However, finding out what genes related to NG2 might enable the differentiation of ensheathing glial cells following their injury-induced proliferation, was still a crucial missing link.
We approached this question by investigating whether an NG2 homologue might operate in Drosophila glia, in response to injury. NG2 is an evolutionarily conserved, extracellular protein, with two N-terminal Laminin Neurexin Sex-hormone Globulin (LNS) motifs, a single transmembrane domain and a small intracellular PDZ domain (Trotter et al., 2010). Cleavage can release four protein products, including a large secreted ectodomain, and an intracellular domain, which functions as a transcription factor (Trotter et al., 2010). Drosophila has an NG2 homologue, called kon-tiki (kon) or perdido (Estrada et al., 2007; Schnorrer et al., 2007). Both the extracellular domain and intracellular PDZ motif of NG2 and Kon are highly conserved (Estrada et al., 2007; Schnorrer et al., 2007; Trotter et al., 2010). Previous work on Kon had centered on its role in muscle (Stegmuller et al., 2003; Estrada et al., 2007; Schnorrer et al., 2007), but whether it had functions in the CNS was unknown.
To investigate a potential link between the Notch-Pros glial gene network and Kon, we first developed a new crush injury paradigm in the Drosophila ventral nerve cord (VNC, equivalent to the vertebrate spinal cord) (Losada-Perez et al., 2016). We had carried out stabbing injury before (Kato et al., 2011; Kato and Hidalgo, 2013), but we reasoned crush injury might mimic more closely natural accidental injury. Most importantly, we found that both methods induced an equivalent regenerative response in glial cells. Firstly, neuropile associated glial cells (which normally enwrap CNS axons) proliferate upon both types of injury. Secondly, these glial cells are also involved in phagocytosis and clearance of cell debris resulting from the injury. This function is normally accomplished by microglia/macrophages in mammals, which can also express NG2. And third, in both injury types, we could increase or prevent repair by manipulating gene expression in neuropile associated glial cells. This indicated that the regenerative response to injury is robustly induced in Drosophila, opening the opportunity to develop further types of CNS injury, which could be even more amenable to genetic analysis.
A Gene Network for kon/NG2 to Promote CNS Repair
Using this novel crush injury method in the VNC of the fruit-fly larval CNS, we asked whether Kon was involved in the glial regenerative response (Losada-Perez et al., 2016) (Figure 1). We found that in the normal CNS, kon is hardly expressed, but injury induces its up-regulation in neuropile glia. This is analogous to the injury-induced up-regulation of NG2 levels in OPCs in vertebrates (Kucharova et al., 2011). Injury also provokes the activation of Notch1 signalling, and of the pro-inflammatory tumor necrosis factor (TNF)/TNF receptor (TNFR)/nuclear factor-κB (NF-κB) pathway in mammals to trigger OPC proliferation (Figure 1B). We had previously shown that, similarly, in Drosophila, the TNF homologue Egr through its TNFR receptor Wengen induced the translocation of Dorsal/NF-κB in response to injury, and together with Notch, promoted glial proliferation (Kato et al., 2011). Blocking NF-κB translocation (by over-expressing cactus, a known inhibitor of NF-κB), Kon levels did not rise after injury, meaning that NF-κB is linked to the up-regulation of Kon (Losada-Perez et al., 2016) (Figure 1B). Kon activation also depends on Notch, since levels of kon also failed to rise after injury in Notch mutants (Figure 1B). Furthermore, Kon is sufficient to promote glial proliferation in Pros+ neuropile glia cells, indicating that ultimately the increase in Kon levels upon injury is the trigger for glial cell division. Importantly, Kon cannot induce proliferation of neuropile glia that do not express both Notch and Pros. Notch and Pros together maintain glial progenitors quiescent in G1, with proliferative potential (Griffiths and Hidalgo, 2004; Kato et al., 2011). Neuropile glia lacking Notch and Pros fully exit the cell cycle, and cannot divide, even in the presence of Kon. Together, this further meant that Kon function is linked to those of Pros and Notch (Losada-Perez et al., 2016). Notch and Kon act together as cell cycle activators breaking the Notch-Pros balance and pushing the Pros+ glia to divide (Figure 1B).
Figure 1.
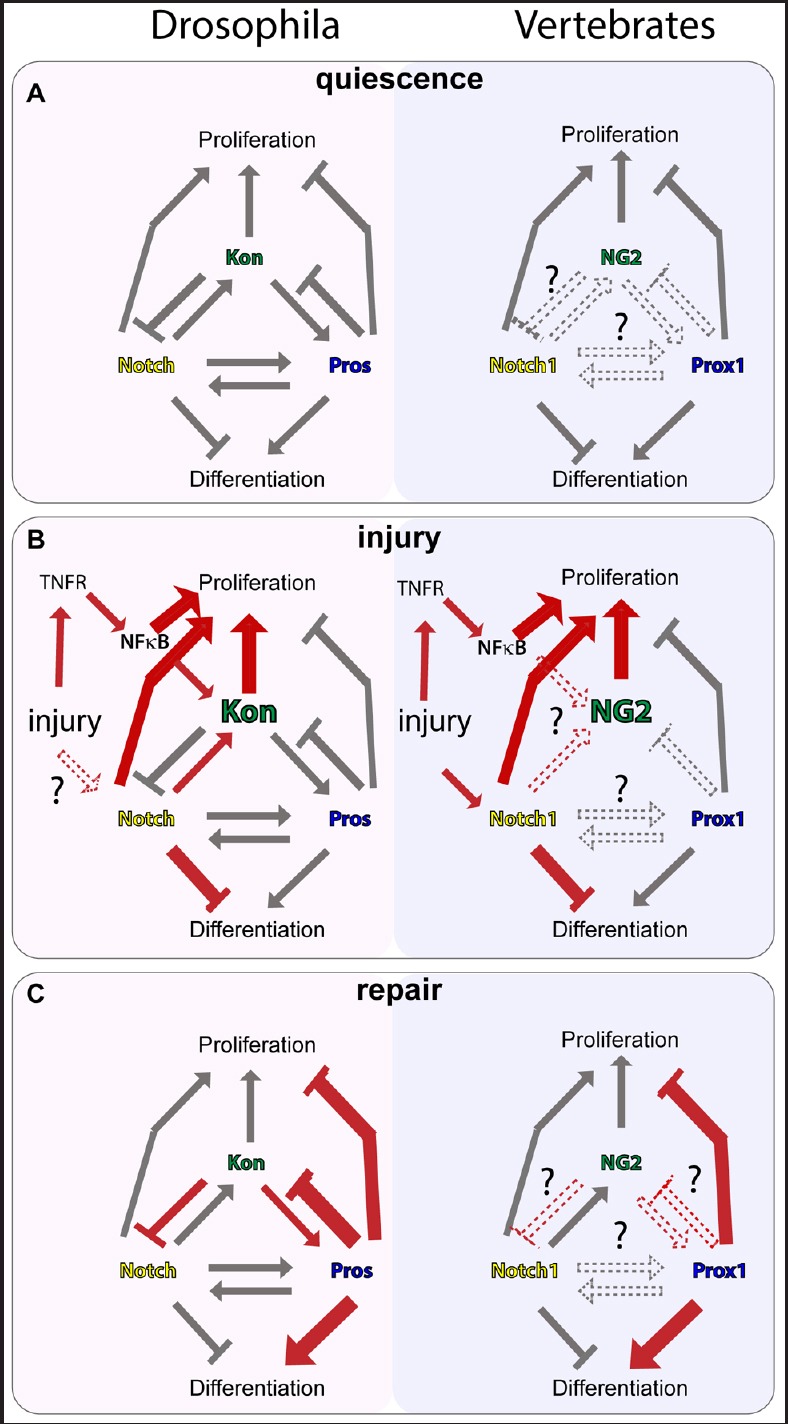
Glial kon/NG2 gene network for central nervous system (CNS) repair.
Illustration showing the gene network operating in the glial regenerative response to CNS injury in neuropile glia of the Drosophila larva, compared to oligodendrocyte progenitor cells and oligodendrocytes in mammals. (A) Quiescence state of the normal CNS. (B) The glial response to CNS injury starts with the induction of glial proliferation. (C) During repair, cell proliferation is switched off, and glial differentiation is activated, re-establishing axonal enwrapment and CNS integrity. Arrows indicate ‘gene activation’; block lines indicate ‘gene repression’; their ‘grey’ colouring indicates known genetic interactions; ‘red’ indicates active during injury or during repair; ‘dashed lines’ indicate unknown genetic relationships.
Using in vivo functional genetic analysis, we uncovered the relationship between Kon, Notch and Pros (Losada-Perez et al., 2016)(Figure 1B, C). Kon functions in two feedback loops. In the first one, Notch activates the expression of kon, while Kon feeds back and represses Notch signaling or expression. This Notch–Kon feedback loop enables and limits glial proliferation (Figure 1B). In a second feedback loop, Kon activates pros, and pros represses kon expression. This feedback loop constrains the lifetime of kon expression, and restores cell number homeostasis (Figure 1C). This constraint is important as after injury cell division enables repair, but uncontrolled cell division would lead to tumours. In fact, NG2 is a key glioma marker in humans. By activating pros expression, Kon enables the onset of glial differentiation. Kon also regulates the expression of the glial differentiation markers ebony and GS2, involved in the recycling of neurotransmitters. Interfering with Kon function also alters the shape Pros+ glia (Losada-Perez et al., 2016). Thus, Kon is required for glial activation and the onset of glial differentiation, which depends on Pros (Griffiths and Hidalgo, 2004; Kato et al., 2011; Losada-Perez et al., 2016). The Kon–Pros feedback loop enables the transition from glial proliferation to differentiation and restores cell shape homeostasis (Losada-Perez et al., 2016). Most importantly, both feedback loops are homeostatic: they enable change, but within constraints, restoring structural integrity whilst avoiding tumours. Finally, Kon promotes repair, since manipulating Kon levels alters injury size progression. Crush injury induces a typical progression of wound expansion followed by shrinkage (Kato et al., 2011; Losada-Perez et al., 2016). Upon kon knock-down, the wound fails to shrink, and when kon is over-expressed, there is a significant reduction in wound size (Losada-Perez et al., 2016).
Our work has revealed a key functional link between Notch, Kon and Pros for CNS repair, which could be evolutionarily conserved (Losada-Perez et al., 2016) (Figure 1). Importantly, our findings showed evolutionary conservation in the functions of NG2 and Kon in the glial regenerative response to CNS injury: both are up-regulated upon injury, both are required for glial proliferation and for the regenerative reduction in lesion size - in mammals and fruit-flies (Kucharova et al., 2011; Losada-Perez et al., 2016). In mammals, Prox1 is also co-distributed together with Notch1 in NG2+ OPCs, Prox1 levels increase as OPCs differentiate into OLs, and Prox1 is required for OL differentiation (Cahoy et al., 2008; Kato et al., 2015). We have shown that Kon drives the onset of glial differentiation by activating - as well as other glial markers - pros expression. We had also previously shown that Pros in flies, and Prox1 in the mouse, are required for neuropile glia and oligodendrocyte differentiation, respectively (Griffiths and Hidalgo, 2004; Kato et al., 2011, 2015). Thus our findings strongly suggest that Prox1 may be the key target of NG2 to manipulate in glioma, progenitors and stem cells to modulate and exploit the pro-regenerative potential of NG2-glia.
To conclude, Drosophila genetics offers a powerful means to investigate in vivo fundamental biology, regeneration and repair, and discover gene networks as they function in vivo. Our discovery of the gene network underlying the regulation of glial proliferation vs. glial differentiation in response to injury may not only be relevant in the context of the glial regenerative response. It could also provide important insights for the understanding of glioma, and of how to manipulate glial progenitors and stem cells for CNS regeneration and repair.
Footnotes
Conflicts of interest: None declared.
References
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Williams A, Stadelmann C, Stankoff B, Zalc B, Lubetzki C. From fish to man: understanding endogenous remyelination in central nervous system demyelinating diseases. Brain. 2008;131:1686–1700. doi: 10.1093/brain/awn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada B, Gisselbrecht SS, Michelson AM. The transmembrane protein Perdido interacts with Grip and integrins to mediate myotube projection and attachment in the Drosophila embryo. Development. 2007;134:4469–4478. doi: 10.1242/dev.014027. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Griffiths RL, Hidalgo A. Prospero maintains the mitotic potential of glial precursors enabling them to respond to neurons. EMBO J. 2004;23:2440–2450. doi: 10.1038/sj.emboj.7600258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Hidalgo A. An injury paradigm to investigate central nerovus system repair in Drosophila. JoVE. 2013;73:e50306. doi: 10.3791/50306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Forero MG, Fenton JC, Hidalgo A. The glial regenerative response to central nervous system injury is enabled by pros-notch and pros-NFkappaB feedback. PLoS Biol. 2011;9:e1001133. doi: 10.1371/journal.pbio.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Konno D, Berry M, Matsuzaki F, Logan A, Hidalgo A. Prox1 inhibits proliferation and is required for differentiation of the oligodendrocyte cell lineage in the mouse. PLoS One. 2015;10:e0145334. doi: 10.1371/journal.pone.0145334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharova K, Stallcup WB. The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience. 2010;166:185–194. doi: 10.1016/j.neuroscience.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharova K, Chang Y, Boor A, Yong VW, Stallcup WB. Reduced inflammation accompanies diminished myelin damage and repair in the NG2 null mouse spinal cord. J Neuroinflammation. 2011;8:158. doi: 10.1186/1742-2094-8-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada-Perez M, Harrison N, Hidalgo A. Molecular mechanism of central nervous system repair by the Drosophila NG2 homologue kon-tiki. J Cell Biol. 2016;214:587–601. doi: 10.1083/jcb.201603054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer F, Kalchhauser I, Dickson BJ. The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila. Dev Cell. 2007;12:751–766. doi: 10.1016/j.devcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Howes EA, Treherne JE. Mechanisms of glial regeneration in an insect central nervous system. J Exp Biol. 1987;132:59–78. doi: 10.1242/jeb.132.1.59. [DOI] [PubMed] [Google Scholar]
- Stegmuller J, Werner H, Nave KA, Trotter J. The proteoglycan NG2 is complexed with alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells. Implications for glial-neuronal signaling. J Biol Chem. 2003;278:3590–3598. doi: 10.1074/jbc.M210010200. [DOI] [PubMed] [Google Scholar]
- Trotter J, Karram K, Nishiyama A. NG2 cells: properties, progeny and origin. Brain Res Rev. 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Zuo H, Nishiyama A. Polydendrocytes in development and myelin repair. Neurosci Bull. 2013;29:165–176. doi: 10.1007/s12264-013-1320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


