Abstract
Therapies such as direct tension-free microsurgical repair or transplantation of a nerve autograft, are nowadays used to treat traumatic peripheral nerve injuries (PNI), focused on the enhancement of the intrinsic regenerative potential of injured axons. However, these therapies fail to recreate the suitable cellular and molecular microenvironment of peripheral nerve repair and in some cases, the functional recovery of nerve injuries is incomplete. Thus, new biomedical engineering strategies based on tissue engineering approaches through molecular intervention and scaffolding offer promising outcomes on the field. In this sense, evidence is accumulating in both, preclinical and clinical settings, indicating that platelet-rich plasma products, and fibrin scaffold obtained from this technology, hold an important therapeutic potential as a neuroprotective, neurogenic and neuroinflammatory therapeutic modulator system, as well as enhancing the sensory and motor functional nerve muscle unit recovery.
Keywords: peripheral nerve injuries (PNI); Schwann cells; axons, platelet-rich plasma; biomolecules; fibrin; scaffold; intraneural; perineural; microenvironment
Introduction
Every year, 350,000 patients are affected by traumatic peripheral nerve injuries, which accounts for $150 billion in annual health care costs (Griffin et al., 2013). Direct tension-free microsurgical repair and/or the transplantation of a nerve autograft to bridge the gap are the gold standard treatments aimed at enhancing the intrinsic regenerative potential of injured axons (Fowler et al., 2015). However, such treatments do not recreate the suitable cellular and molecular microenvironment and in some cases, the functional recovery of nerve injuries is incomplete (Faroni et al., 2015). Platelet-rich plasma (PRP) products hold an important therapeutic potential as a neuroprotective, neurogenic, and neuroinflammatory therapeutic modulator system (Anitua et al., 2013; Kuffler, 2014; Anitua et al., 2015a; Zheng et al., 2016) and as an enhancer of sensory and motor functional nerve-muscle unit recovery (Anjayani et al., 2014; Kuffler, 2015; Sanchez et al., 2015), emerging as a biological adjuvant in peripheral nerve injuries (PNIs) and neuropathies. These autologous products are applied, as a filler of nerve conduits or vein-muscle grafts across nerve gaps post trauma by infiltrating the nerve stumps perineurally and intraneurally which is guided with ultrasound probes, or as scaffolds to bridge or wrap the injured nerve stumps (Farrag et al., 2007; Giannessi et al., 2014; Kim et al., 2014; Malahias et al., 2015). Moreover, there are non-traumatic peripheral injuries such as compression, adhesion and fibrosis (as in the case of carpal tunnel syndrome and fibrotic post-surgical side effects) (Dodla et al., 2008), where this novel approach applied may additionally diminish undesirable consequences such as fibrotic scars and denervated organ atrophy since this adjuvant therapy can speed up the functional recovery of the nerve-muscle unit (Sariguney et al., 2008; Takeuchi et al., 2012; Wu et al., 2012; Ye et al., 2012; Sanchez et al., 2014). Therefore, PRPs may be applied to assist and synergize with the gold standard therapies in nerve regeneration and neuropathies, and may be harnessed by surgeons in the operating room and in the clinical setting as an “off the shelf” alternative.
Degeneration and Regeneration after PNI: Molecular and Cellular Events
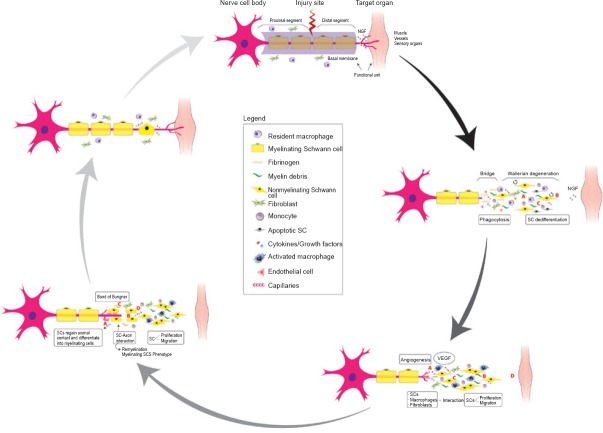
Following a PNI, an orchestrated multicellular and pleiotropic molecular response will ensue. This response consists in the interplay among Schwann cells (SCs), resident macrophages, endothelial cells (ECs), and fibroblasts, mainly modulated by injured axons, myelin breakdown products, soluble factors, and hypoxia as main signals. It will end up regrowing and guiding axons, and reconnecting them with the target organs at a rate of about 1 mm per day in humans (Parrinello et al., 2010; Zochodne, 2012; Cattin et al., 2015) (Figure 1).
Figure 1.
Spontaneous peripheral nerve regeneration is a multicellular and pleiotropic process.
Schwann cells are the master and servant in peripheral nerve regeneration while macrophages act as “Jack of all trades”. The partnership between the transdifferentiated SCs and macrophages induce the latter to synthesize VEGF. In addition to stimulating the proliferation of endothelial cells, promoting new vessels that guide the axon growth, thereby serving as tracks for migrating and proliferating SCs to from a Band of Bungner, VEGF enhances the survival, migration and proliferation of SCs, all of which contribute to the outgrowth of axons, restoration of basal lamina and facilitation of the formation of Band of Bungner at both nerve stumps. NGF: Nerve growth factor; SCs: Schwann cells; VEGF: vascular endothelial growth factor.
Disruption of the regeneration unit by the noxious agent results in loss of axonal contact with SCs whose phenotype is drastically modified, thereby contributing to SC activation or transdifferentiation. Macrophages will collaborate with the activated-dedifferentiated SCs in clearing the myelin and other tissue debris. Moreover, these SCs come into direct contact with resident fibroblasts that accumulate in large numbers at the site of injury influencing SC migration and dedifferentiation (Parrinello et al., 2010; Arthur-Farraj et al., 2012; Jessen et al., 2015). SCs show a striking plastic response to the biological battlefield they are exposed to inside a damaged nerve and are the early detectors of damage (Figure 1). In a context- and time-dependent manner, transdifferentiated SCs perform a variety of cell repair tasks from phagocytosing myelin debris to secreting neurotrophic and neurotropic factors (laminin), proliferation and migration, which results in the formation of SC cords and Bungner Bands in the proximal and distal nerve segment, respectively (Gaudet et al., 2011; Zochodne, 2012; Jessen et al., 2015). Although SCs have the reputation of being the engine of peripheral nerve repair, in the nerve repair complex process, they are fuelled by axon growth cones and supportive stromal cells such as macrophages and fibroblasts, the very elements of Wallerian degeneretion as a neuroinflammatory process (Figure 1) (Parrinello et al., 2010; Gaudet et al., 2011; Cattin et al., 2015; Chen et al., 2015; Jessen et al., 2015). Emerging evidence suggests that macrophage plasticity contributes to peripheral nerve regeneration via distinct mechanisms: by phagocyitosing myelin debris, synthesizing trophic factors such as vascular endothelial growth factor (VEGF) and promoting angiogenesis, producing collagen type VI, modulating the proliferation and migration of SCs, and influencing the resolution of inflammation through the polarization from M1 to M2 phenotype (Mokarram et al., 2012; Cattin et al., 2015; Chen et al., 2015). Cattin et al. (2015) confirmed an idea suggested by Chen et al. (2005) that blood vessels might provide substrate or signalling for axon growth guidance and SC migration, by showing that macrophages selectively sense hypoxia in the area of nerve bridge and drive angiogenesis via the VEGF-secretion pathway at the nerve bridge (Figure 1). Despite the robust repair capacity to regrow peripheral nervous axons shown in the adult mammal (Gaudet et al., 2011; Cattin et al., 2015) and meticulous microsurgical nerve repair techniques there are some limiting factors, including the poor vascularization, the patients age, the chronic denervation of SCs, the endoneurial and perineurial fibrosis, the misguided axonal growth, the vast distance that axon growth cones must cover to reinnervate target organs/tissues, as well as their atrophy, and the rate of regeneration (Hall, 2005; Zochodne, 2012; Scheib and Hoke, 2013; Painter et al., 2014).
Plasma Rich in Growth Factors: an Injectable Scaffold to Assist in Nerve Repair
Plasma rich in growth factors (PRGFs) consist of a pool of growth factors (GFs), microparticles, and other bioactive mediators many of them trapped, through fibrin heparan sulfate-binding domains, in a three-dimensional transient fibrin matrix (Figure 2) (Anitua et al., 2015b). Once PRP is infiltrated intraneurally as a liquid-to-gel injectable scaffold, or wrapped around the injured nerve gap as a matrix-like viscous and malleable structure, or both, (Sanchez et al., 2015) (Figure 3) tissue fibrinolysis breaks the fibrin down, thereby releasing cell signalling molecules such as neurotrophic (nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), insulin-like growth factor 1 (IGF-1), platelet-derived growth factor (PDGF), VEGF, hepatocyte growth factor (HGF)) and neurotropic factors (fibrin, fibronectin, and vitronectin) (Anitua et al., 2015c).
Figure 2.
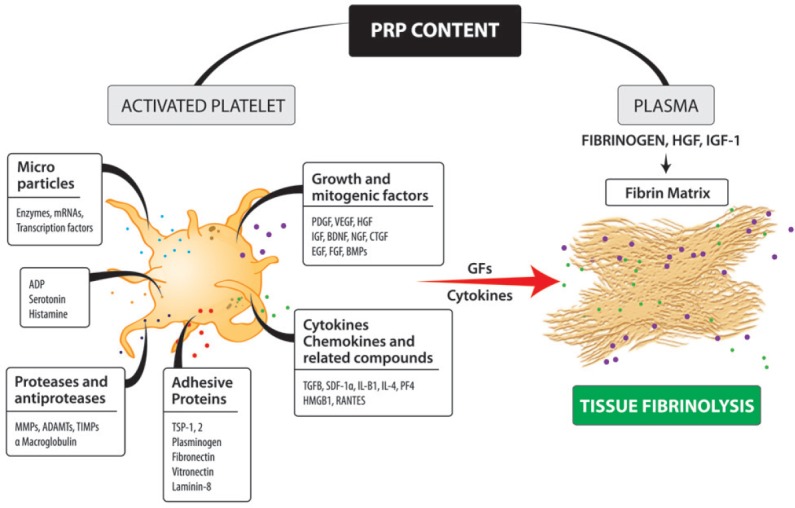
Illustration of some biological mediators of platelet-rich plasma (PRP) that govern tissue repair by still poorly undestood mechanisms.
There are biomolecules and several growth factors which come either from platelet activation and plasma or both. Several of these bioactive mediators and other growth factors or proteins remain trapped through fibrin heparan sulfate-binding domains, in a three-dimensional transient fibrin matrix to be released later by tissue fibrinolysis. ADAMTs: A disintegrin and metalloprotease with thrombospondin motifs; ADP: adenosine diphosphate; BDNF: brain-derived neurotrophic factor; BMPs: bone morphogenetic proteins; CTGF: connective tissue growth factor; EGF: epidermal growth factor; FGF: fibroblast growth factor; GFs: growth factors; HGF: hepatocyte growth factor; HMGB1: high mobility group box 1; IGF: insulin-like growth factor; IL-β1: interleukin-β1; MMPs: matrix metalloproteinases; NGF: nerve growth factor; PDGF: platelet-derived growth factor; PF4: platelet factor 4; RANTES: regulated upon activation, normal T cell expressed and presumably secreted; SDF-1α: stromal cell-derived factor-1α; TGFB: transforming growth factor beta; TIMPs: tissue inhibitors of metalloproteinases; TSP-1: thrombospondin-1; VEGF: vascular endothelial growth factor.
Figure 3.
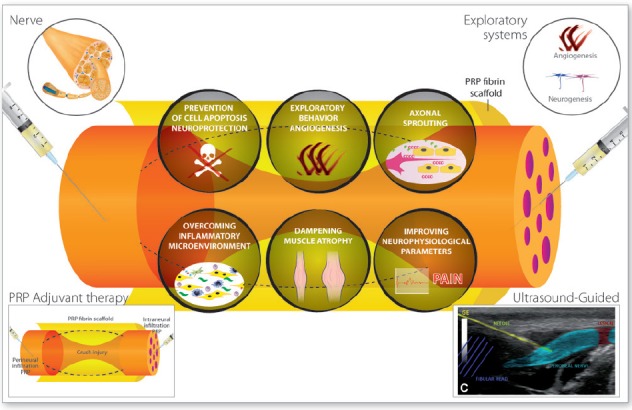
Six lines of evidence suggest the therapeutic potential use of PRPs on neural tissue repair and regeneration.
These include the prevention of cell apoptosis and neuroprotection, the stimulation of angiogenesis, the modulation of inflammatory microenvironment, the enhancement of axonal outgrowth and nerve guidance, the dampening of both denervated muscle atrophy and scarring that follow peripheral nerve trauma and damage, and the improvement of neurologic parameters in humans. PRP: Platelet-rich plasma.
Growing in vitro and in vivo evidence suggests that the biomolecules conveyed by PRPs are instrumental agents that modulate early inflammation, stem cell-like myelinating SC activation, macrophage polarization, as well as the active resolution of inflammation, angiogenesis, and fibrogenesis, thereby acting as key drivers of full nerve functional recovery (Sondell et al., 1999; Jiang et al., 2013; Zheng et al., 2014; Cattin et al., 2015; Jessen et al., 2015). There are so far six lines of evidence pointing the therapeutic potential of PRPs as follows (Figure 3).
Neuroprotection and prevention of cell apoptosis
Several growth factors present in PRP including NGF, BDNF, PDGF, VEGF, IGF-1, transforming growth factor beta (TGFB) alone or in combination have been shown to exert an antiapoptotic and neuroprotective effect on mesenchymal stem cells (MSCs), neurons, SCs, and human neural stem cells (Sondell et al., 1999; Lee et al., 2003; Borselli et al., 2010; Emel et al., 2011; Luo et al., 2012; Rao and Pearse, 2016). PRP fibrin scaffolds enriched with NGF, BDGF, and retinoic acid and loaded with bone marrow stromal cells (BMSCs), enhance their survival and differentiation into the neural phenotype (Zurita et al., 2010). In addition, when this PRP scaffold was transplanted into the brain, the viability and biologic activity of allogenic BMSC increased (Vaquero et al., 2013). Moreover, neuroprotective and antifibrotic beneficial effects (Cho et al., 2010; Wu et al., 2012) were reported with the injection of PRP into the corpus cavernosum in a bilateral cavernous nerve injury rat model and applying PRP in a facial nerve suture in a guinea pig model. A recent in vitro study on neuronal cultures from mouse model of Alzheimer's disease (Anitua et al., 2015a) showed that the neurotoxicity induced by aggregated β-amyloid added in primary neuronal cultures was significantly reduced, and the living cell number after the co-treatment with PRP increased. In addition, chronic intranasal administration of PRP in Alzheimer's disease mouse model elicits neuroprotection which is likely mediated by the activation of the antiapoptotic PIEK/Akt signalling pathway (Anitua et al., 2014).
Stimulation of angiogenesis
Borselli et al. (2010) showed in an ischemic limb rodent model with loss of neuromuscular junction (NMJ) innervation that an injectable scaffold loaded with VEGF and IGF-1 accelerated regeneration of damaged NMJs together with an enhancement of angiogenesis. In a rat model it has been reported that sciatic nerve gaps of 10 mm repaired with vein graft filled with PRP exhibited a more prominent early neoangiogenesis than sciatic nerve gaps treated with nerve autograft alone (Kim et al., 2004). In this regard, it should be taken into account that fibrin is a pivotal element within PRP that provides ECM tissue with a robust and permissive 3D matrix for angiogenesis (Hall et al., 2007).
Enhancing axonal outgrowth capacity
The crucial role played by GF within the PRP has been highlighted in a rat brain-spinal cord cocultured system, where the addition of PRP supernatant promoted an increase in the size and number of axons, a positive effect that was significantly suppressed when antibodies against IGF-1 and VEGF were added (Takeuchi et al., 2012). As a cellular carrier, two studies in acute nerve injury model in guinea pig and rabbits applied PRP and seeded the acellular carrier with either MSCs or SCs, reporting beneficial effects on axonal counts, myelination and electrophysiological parameters (Cho et al., 2010; Ye et al., 2012). One example of the use of PRP as a filler of acellular nerve allografts (ANA PRP) represents the work of Zheng et al. (2016) that, having previously shown a dose-dependent effect of PRP on the proliferation, migration and, neurotrophic function in rat SCs cutured with PRP, they showed significant improvements in diameter, thickness, and numbers of myelinating axons as well as an enhancement of electrophysiological parameters in sciatic nerve injury repaired with autografts and ANA PRP in a rat model (Zheng et al., 2014). Using a simple inside-out vein autograft or an inside-out vein autograft filled with PRP to bridge the sciatic nerve gap in a rat model, Kim et al. (2014) observed that the number of myelinated axons, the axon diameter and myelin sheath were significantly superior when PRP was used as a filler. These results are in accord with the work of Kaplan et al. (2011), who used platelet gels as filler of collagen nerve conduit with improvement in functional and structural outcomes in an injury model of rat sciatic nerve. Using platelet-rich fibrin (PRF) as a filler of silicon nerve guidance (Lichtenfels et al., 2013) or nerve grafts (Sabongi et al., 2014) in a rat model, animals treated with PRP improved functional recovery and showed a superior sciatic functional index compared with non-treated animals. However the researchers did not find morphometric or structural improvements (Lichtenfels et al., 2013; Sabongi et al., 2014). The application of PRP as a fibrin membrane to wrap the neurorraphy in an acute injury model of sciatic nerve neurotmesis showed diverse positive effects. Gianessi et al. (2014) observed a stronger EMG signal, a significantly larger axonal density, and a lower scar tissue in animals treated with PRP fibrin membranes, and remains of PRP membranes were still present after 6 weeks post-surgery. In this sense, two studies reported the positive effects of using PRP as adjuvant in nerve suture. Farrag et al. (2007) reported that PRP may enhance the myelin thickness and increase the axon counts when injured nerve is sutured and assisted with PRP, whereas Sariguney et al. (2008) found no positive effects on axonal size in sutured nerves assisted with PRP. However, they showed a better functional outcome associated with improvement in the myelin thickness and the onset latency. Sanchez et al. (2008) applying PRP as both filler of the injured nerve and as a scaffold to coat the nerve crush on sheep, reported an earlier electrophysiological response, a higher axonal density, and lower muscle atrophy in treated animals compared with the saline or spontaneous regeneration groups.
Overcoming the inflammatory microenvironment
Though indirect, two important pieces of evidence in neural tissue support the antiinflammatory effect of PRP. Anitua et al. (2014) reported that astrocytes cultured with β-amyloid expressed proinflammatory cytokines, but this effect was completely blocked when the culture was suplemented with PRP, an effect mediated by the supression of thenuclear transcription factor-κB (NF-κB) on astrocytes. In a mouse model of Parkinson's disease, Anitua et al. (2015a) showed that the neuroinflammatory process, mediated by microglia, was reduced, together with an improvement in motor performance, responses that were associated with a robust reduction in NF-κB activation, nitric oxide, cyclooxygenase, and tumor necrosis factor expression in the brain. In a rabbit model of dextrose-induced median nerve injury, the injection of PRP into the carpal tunnel of rabbits injured 4 weeks before, exerted a significant reduction in nerve swelling compared with the control group (Park and Kwon, 2014).
Dampening the denervated target muscle atrophy
Several animal studies have demostrated that the application of PRP as a filler, a fibrin membrane, or both, induce an earlier axonal regeneration and functional recovery (Farrag et al., 2007; Sariguney et al., 2008; Emel et al., 2011; Wu et al., 2012; Gianessi et al., 2014; Kim et al., 2014; Sanchez et al., 2015). This is the case reported by Sanchez et al. (2015) on sheep, where nerves repaired with PRP were associated with an earlier electrophysiological recovery and lower muscle atrophy, suggesting that PRP application may dampen the target muscle atrophy. In addition, another recovery burden in nerve repair is scarring, which has been reported to be minimized by the repair of sciatic injured nerve assisted with PRP (Gianessi et al., 2014). Anitua et al. (2015d) showed that intramuscular injection of PRP 24 hours after the induction of limb ischemia in mice, mitigates fibrosis and muscle atrophy. These results are in agreement with the reduction of atrophy in denervated muscle reported when muscle was infiltrated with cells (Schaakxs et al., 2013), effects suggested to be mediated by the IGF-1 (Shavlakadze et al., 2005). Moreover, TGFB, an important GF within PRP, attenuates the adverse effects of chronically denervated Schwann cells, and reactivated SCs support axon regeneration in vivo (Sulaiman and Gordon, 2012).
The improvement of neurologic parameters in humans
In the wake of promising results in animal experimentation, PRP has been applied either as filler of nerve conduits across post traumatic nerve gaps (Kuffler, 2011, 2014), as a liquid dynamic scaffold infiltrated perineurally (Hibner et al., 2012; Anjayani et al., 2014; Malahias et al., 2015), intraneurally, or both (as in the case of a peroneal nerve palsy (Sanchez et al., 2014) and other damaged nerves. Furthermore, it also has been applied as scaffold or fibrin membranes (Kuffler, 2011, 2014; Scala et al., 2014) with beneficial outcomes and better functional recovery. Kuffler applied autologous platelet rich fibrin as a filler of a collagen tube, proceeding to bridge the 12 cm nerve gap 3.25 years after an ulnar nerve trauma, and to recovery of both muscle and sensory function (Kuffler, 2011). In a recent series of cases of surgical nerve repair, Kuffler (2014) reported functional recovery in patients under 58 years whose nerve gaps of 2–16 cm were treated with collagen tube filled with PRP, from 0.5–3 years post trauma.
In a double-blind, randomized, clinical trial, the application of perineural PRP injections in tibial and ulnar nerves has shown sensory improvement in leprosy peripheral neuropathy (Anjayani et al., 2014). In a retrospective analysis of 10 patients with persistent pudendal neuralgia, who underwent a second trans-gluteal decompression of the pudendal nerve, they injected activated PRP around the coated nerve, reporting a significant reduction in pain (Hibner et al., 2012). In a case series of 14 patients with carpal tunnel syndrome, a single ultrasound-guided injection of PRP around the median nerve led to the disappearance of pain in eight patients, and pain alleviation in three patients at three months of follow-up (Malahias et al., 2015). Another case report, in this case applying sequential proximal and distal ultrasound-guided PRP injections intraneurally and perineurally (Figure 3) in a common peroneal nerve palsy, Sanchez et al. (2014) reported a significant functional recovery assessed by electromyographic signs of reinnervation for both peroneus longus and tibialis anterior muscles as well as almost full recovery of sensiviity. It has been reported that the intravenous injection of 25 cc of concentrated PRP in a 6-year-old-boy with perinatal cerebral palsy is safe, and can significantly improve the cognitive and language functions (Alcaraz et al., 2015).
Conclusion
The ultimate goal of any peripheral nerve repair strategy is the restoration of nerve-target organ function, while minimizing therapeutic side effects. PRPs are versatile and safe biological products to be harnessed by surgeons and clinicians as an adjuvant therapeutic tool to enhance the robust intrinsic nerve repair processes and overcome post-traumatic and neuropathic inhibitory microenvironment by combinatorial strategy of delivering neurotrophic and neurotropic factors. They may assist nerve conduit guidances and grafts as a filler, as a liquid in intraneural and perineural ultrasound-guided injections in nerve entrapments and fibrosis, and as a scaffold to bridge or wrap the injured nerve gap.
Footnotes
Conflicts of interest: SP is a researcher at BTI (Biotechnology Institute) a dental implant company that investigates in the fields of oral implantology and PRGF-Endoret technology.
References
- Alcaraz J, Oliver A, Sanchez JM. Platelet-rich plasma in a patient with cerebral palsy. Am J Case Rep. 2015;16:469–472. doi: 10.12659/AJCR.893805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitua E, Pascual C, Perez-Gonzalez R, Antequera D, Padilla S, Orive G, Carro E. Intranasal delivery of plasma and platelet growth factors using PRGF-Endoret system enhances neurogenesis in a mouse model of Alzheimer's disease. PLoS One. 2013;8:e73118. doi: 10.1371/journal.pone.0073118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitua E, Pascual C, Antequera D, Bolos M, Padilla S, Orive G, Carro E. Plasma rich in growth factors (PRGF-Endoret) reduces neuropathologic hallmarks and improves cognitive functions in an Alzheimer's disease mouse model. Neurobiol Aging. 2014;35:1582–1595. doi: 10.1016/j.neurobiolaging.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Anitua E, Pascual C, Pérez-Gonzalez R, Orive G, Carro E. Intranasal PRGF-Endoret enhances neuronal survival and attenuates NF-kappaB-dependent inflammation process in a mouse model of Parkinson's disease. J Control Release. 2015a;203:170–180. doi: 10.1016/j.jconrel.2015.02.030. [DOI] [PubMed] [Google Scholar]
- Anitua E, Zalduendo MM, Prado R, Alkhraisat MH, Orive G. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015b;103:1011–1020. doi: 10.1002/jbm.a.35244. [DOI] [PubMed] [Google Scholar]
- Anitua E, Prado R, Azkargorta M, Rodriguez-Suárez E, Iloro I, Casado-Vela J, Elortza F, Orive G. High-throughput proteomic characterization of plasma rich in growth factors (PRGF-Endoret)-derived fibrin clot interactome. J Tissue Eng Regen Med. 2015c;9:E1–E12. doi: 10.1002/term.1721. [DOI] [PubMed] [Google Scholar]
- Anitua E, Pelacho B, Prado R, Aguirre JJ, Sánchez M, Padilla S, Aranguren XL, Abizanda G, Collantes M, Hernandez M, Perez-Ruiz A, Peñuelas I, Orive G, Prosper F. Infiltration of plasma rich in growth factors enhances in vivo angiogenesis and improves reperfusion and tissue remodeling after severe hind limb ischemia. J Control Release. 2015d;202:31–39. doi: 10.1016/j.jconrel.2015.01.029. [DOI] [PubMed] [Google Scholar]
- Anjayani S, Wirohadidjojo YW, Adam AM, Suwandi D, Seweng A, Amiruddin MD. Sensory improvement of leprosy peripheral neuropathy in patients treated with perineural injection of platelet-rich plasma. Int J Dermatol. 2014;53:109–113. doi: 10.1111/ijd.12162. [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130:605–618. doi: 10.1007/s00401-015-1482-4. [DOI] [PubMed] [Google Scholar]
- Chen YY, McDonald D, Cheng C, Magnowski B, Durand J, Zochodne DW. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 2005;64:613–622. doi: 10.1097/01.jnen.0000171650.94341.46. [DOI] [PubMed] [Google Scholar]
- Cho HH, Jang S, Lee SC, Jeong HS, Park JS, Han JY, Lee KH, Cho YB. Effect of neural-induced mesenchymal stem cells and platelet-rich plasma on facial nerve regeneration in an acute nerve injury model. Laryngoscope. 2010;120:907–913. doi: 10.1002/lary.20860. [DOI] [PubMed] [Google Scholar]
- Dodla M, Bellamkond R, Atala A. Peripheral nerve regeneration. Foundations of Regenerative Medicines: Clinical & Therapeutic Applications. 2008:1270–1382. [Google Scholar]
- Emel E, Ergun SS, Kotan D, Gürsoy EB, Parman Y, Zengin A, Nurten A. Effects of insulin-like growth factor-I and platelet-rich plasma on sciatic nerve crush injury in a rat model. J Neurosurg. 2011;114:522–528. doi: 10.3171/2010.9.JNS091928. [DOI] [PubMed] [Google Scholar]
- Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev. 2015;82-83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Farrag TY, Lehar M, Verhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117:157–165. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- Fowler JR, Lavasani M, Huard J, Goitz RJ. Biologic strategies to improve nerve regeneration after peripheral nerve repair. J Reconstr Microsurg. 2015;31:243–248. doi: 10.1055/s-0034-1394091. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannessi E, Coli A, Stornelli MR, Miragliotta V, Pirone A, Lenzi C, Burchielli S, Vozzi G, De Maria C, Giorgetti M. An autologously generated platelet-rich plasma suturable membrane may enhance peripheral nerve regeneration after neurorraphy in an acute injury model of sciatic nerve neurotmesis. J Reconstr Microsurg. 2014;30:617–626. doi: 10.1055/s-0034-1372483. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Hogan MV, Chhabra AB, Deal DN. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- Hall H. Modified fibrin hydrogel matrices: both, 3D-scaffolds and local and controlled release systems to stimulate angiogenesis. Curr Pharm Des. 2007;13:3597–3607. doi: 10.2174/138161207782794158. [DOI] [PubMed] [Google Scholar]
- Hall S. The response to injury in the peripheral nervous system. J Bone Joint Surg Br. 2005;87:1309–1319. doi: 10.1302/0301-620X.87B10.16700. [DOI] [PubMed] [Google Scholar]
- Hibner M, Castellanos ME, Drachman D, Balducci J. Repeat operation for treatment of persistent pudendal nerve entrapment after pudendal neurolysis. J Minim Invasive Gynecol. 2012;19:325–330. doi: 10.1016/j.jmig.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Qu W, Li Y, Zhong W, Zhang W. Platelet-derived growth factors-BB and fibroblast growth factors-base induced proliferation of Schwann cells in a 3D environment. Neurochem Res. 2013;38:346–355. doi: 10.1007/s11064-012-0925-8. [DOI] [PubMed] [Google Scholar]
- Kaplan S, Pişkin A, Ayyildiz M, Aktaş A, Köksal B, Ulkay MB, Türkmen AP, Bakan F, Geuna S. The effect of melatonin and platelet gel on sciatic nerve repair: an electrophysiological and stereological study. Microsurgery. 2011;31:306–313. doi: 10.1002/micr.20876. [DOI] [PubMed] [Google Scholar]
- Kim JY, Jeon WJ, Kim DH, Rhyu IJ, Kim YH, Youn I, Park JW. An inside-out vein graft filled with platelet-rich plasma for repair of a short sciatic nerve defect in rats. Neural Regen Res. 2014;9:1351–1357. doi: 10.4103/1673-5374.137587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler DP. An assessment of current techniques for inducing axon regeneration and neurological recovery following peripheral nerve trauma. Prog Neurobiol. 2014;116:1–12. doi: 10.1016/j.pneurobio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Kuffler DP. Platelet-rich plasma promotes axon regeneration, wound healing, and pain reduction: fact or fiction. Mol Neurobiol. 2015;52:990–1014. doi: 10.1007/s12035-015-9251-x. [DOI] [PubMed] [Google Scholar]
- Kuffler DP, Reyes O, Sosa IJ, Santiago-Figueroa J. Neurological recovery across a 12-cm-long ulnar nerve gap repaired 3.25 years post trauma: case report. Neurosurgery. 2011;69:E1321–1326. doi: 10.1227/NEU.0b013e31822a9fd2. [DOI] [PubMed] [Google Scholar]
- Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- Lichtenfels M, Colomé L, Sebben AD, Braga-Silva J. Effect of Platelet Rich Plasma and Platelet Rich Fibrin on sciatic nerve regeneration in a rat model. Microsurgery. 2013;33:383–390. doi: 10.1002/micr.22105. [DOI] [PubMed] [Google Scholar]
- Luo H, Zhang Y, Zhang Z, Jin Y. The protection of MSCs from apoptosis in nerve regeneration by TGFβ1 through reducing inflammation and promoting VEGF-dependent angiogenesis. Biomaterials. 2012;33:4277–4287. doi: 10.1016/j.biomaterials.2012.02.042. [DOI] [PubMed] [Google Scholar]
- Malahias MA, Johnson EO, Babis GC, Nikolaou VS. Single injection of platelet-rich plasma as a novel treatment of carpal tunnel syndrome. Neural Regen Res. 2015;10:1856–1859. doi: 10.4103/1673-5374.165322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter MW, Brosius Lutz A, Cheng YC, Latremoliere A, Duong K, Miller CM, Posada S, Cobos EJ, Zhang AX, Wagers AJ, Havton LA, Barres B, Omura T, Woolf CJ. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron. 2014;83:331–343. doi: 10.1016/j.neuron.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GY, Kwon DR. Platelet-rich plasma limits the nerve injury caused by 10% dextrose in the rabbit median nerve. Muscle Nerve. 2014;49:56–60. doi: 10.1002/mus.23863. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SN, Pearse DD. Regulating axonal responses to injury: the intersection between signaling pathways involved in axon myelination and the inhibition of axon regeneration. Front Mol Neurosci. 2016;9:33. doi: 10.3389/fnmol.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabongi RG, De Rizzo LALM, Fernandes M, Valente SG, Gomes dos Santos JB, Faloppa F, Leite VM. Nerve regeneration: is there an alternative to nervous graft? J Reconstr Microsurg. 2014;30:607–616. doi: 10.1055/s-0034-1372477. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Yoshioka T, Ortega M, Delgado D, Anitua E. Ultrasound-guided platelet-rich plasma injections for the treatment of common peroneal nerve palsy associated with multiple ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:1084–1089. doi: 10.1007/s00167-013-2479-y. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Anitua E, Delgado D, Prado R, Sánchez P, Fiz N, Guadilla J, Azofra J, Pompei O, Orive G, Ortega M, Yoshioka T, Padilla S. Ultrasound-guided plasma rich in growth factors injections and scaffolds hasten motor nerve functional recovery in an ovine model of nerve crush injury. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.2079. doi:10.1002/term.2079. [DOI] [PubMed] [Google Scholar]
- Sariguney Y, Yavuzer R, Elmas C, Yenicesu I, Bolay H, Atabay K. Effect of platelet-rich plasma on peripheral nerve regeneration. J Reconstr Microsurg. 2008;24:159–167. doi: 10.1055/s-2008-1076752. [DOI] [PubMed] [Google Scholar]
- Scala M, Mereu P, Spagnolo F, Massa M, Barla A, Mosci S, Forno G, Ingenito A, Strada P. The use of platelet-rich plasma gel in patients with mixed tumour undergoing superficial parotidectomy: a randomized study. In Vivo. 2014;28:121–124. [PubMed] [Google Scholar]
- Schaakxs D, Kalbermatten DF, Raffoul W, Wiberg M, Kingham PJ. Regenerative cell injection in denervated muscle reduces atrophy and enhances recovery following nerve repair. Muscle Nerve. 2013;47:691–701. doi: 10.1002/mus.23662. [DOI] [PubMed] [Google Scholar]
- Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, White JD, Davies M, Hoh JF, Grounds MD. Insulin-like growth factor I slows the rate of denervation induced skeletal muscle atrophy. Neuromuscul Disord. 2005;15:139–146. doi: 10.1016/j.nmd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman OA, Gordon T. Transforming growth factor-beta and forskolin attenuate the adverse effects of long-term Schwann cell denervation on peripheral nerve regeneration in vivo. Glia. 2002;37:206–218. doi: 10.1002/glia.10022. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Kamei N, Shinomiya R, Sunagawa T, Suzuki O, Kamoda H, Ohtori S, Ochi M. Human platelet-rich plasma promotes axon growth in brain-spinal cord coculture. Neuroreport. 2012;23:712–716. doi: 10.1097/WNR.0b013e3283567196. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Otero L, Bonilla C, Aguayo C, Rico MA, Rodriguez A, Zurita M. Cell therapy with bone marrow stromal cells after intracerebral hemorrhage: impact of platelet-rich plasma scaffolds. Cytotherapy. 2013;15:33–43. doi: 10.1016/j.jcyt.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Wu CC, Wu YN, Ho HO, Chen KC, Sheu MT, Chiang HS. The neuroprotective effect of platelet-rich plasma on erectile function in bilateral cavernous nerve injury rat model. J Sex Med. 2012;9:2838–2848. doi: 10.1111/j.1743-6109.2012.02881.x. [DOI] [PubMed] [Google Scholar]
- Ye F, Li H, Qiao G, Chen F, Tao H, Ji A, Hu Y. Platelet-rich plasma gel in combination with Schwann cells for repair of sciatic nerve injury. Neural Regen Res. 2012;7:2286–2292. doi: 10.3969/j.issn.1673-5374.2012.29.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Zhu Q, Liu X, Huang X, He C, Jiang L, Quan D. Improved peripheral nerve regeneration using acellular nerve allografts loaded with platelet-rich plasma. Tissue Eng Part A. 2014;20:3228–3240. doi: 10.1089/ten.tea.2013.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Zhu Q, Liu X, Huang X, He C, Jiang L, Quan D. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J Tissue Eng Regen Med. 2016;10:428–436. doi: 10.1002/term.1756. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. The challenges and beauty of peripheral nerve regrowth. J Peripher Nerv Syst. 2012;17:1–187. doi: 10.1111/j.1529-8027.2012.00378.x. [DOI] [PubMed] [Google Scholar]
- Zurita M, Otero L, Aguayo C, Bonilla C, Ferreira E, Parajón A, Vaquero J. Cell therapy for spinal cord repair: optimization of biologic scaffolds for survival and neural differentiation of human bone marrow stromal cells. Cytotherapy. 2010;12:522–537. doi: 10.3109/14653241003615164. [DOI] [PubMed] [Google Scholar]