Microglial cells (or microglia) are the mononuclear phagocytes residing in the central nervous system (CNS). In homeostasis, they showed ramified morphology with relative small cell bodies and long processes (Figure 1A). They detect injury signals in the CNS and get activated. In the brain they undergo different stages of activation, which can be classified according to the morphological and immune-reactive diversities. In the traumatic brain injury, the phagocytic and amoeboid shape microglial cells (Figure 1B) are of particular interest, since they are considered as the fully activated form of microglia, and are important features for brain tissue destruction and inflammation.
Figure 1.
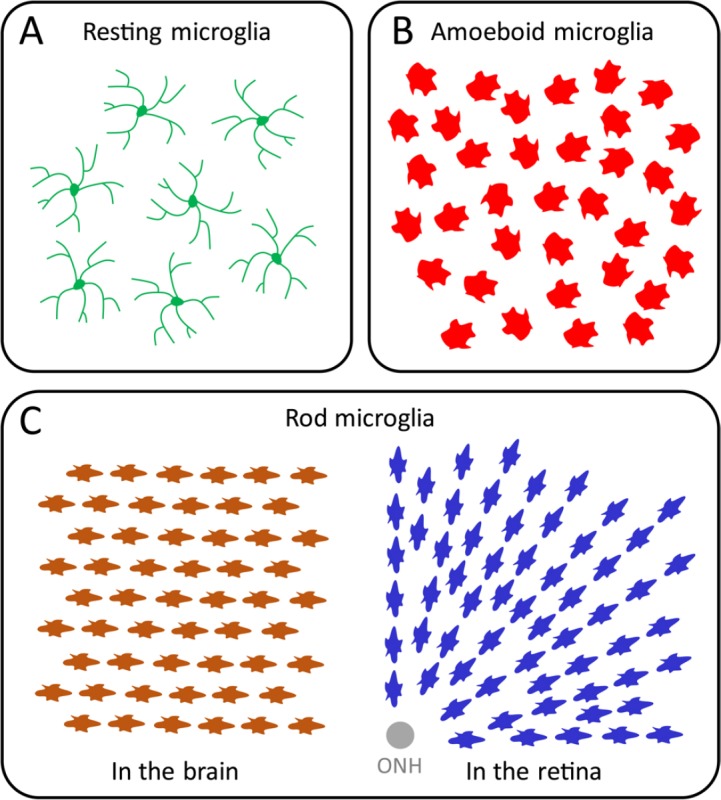
The diverse morphologies of microglia in the central nervous system (CNS).
(A) The resting microglial cells. In the normal homeostasis, the microglia of the resting state show ramified morphology with relatively small soma and long thin processes. (B) The amoeboid microglial cells. The activated microglia of amoeboid shape without obvious cellular orientation or alignment. (C) The rod microglial cells. The activated microglia of rod shape in the brain and retina exhibit elongated sausage-like soma, and get aligned at certain orientations. ONH: Optic nerve head.
Rod microglia were firstly reported by Franz Nissl in 1899, from general paresis patients (Nissl, 1899). They represent a unique cell type of activated microglia with distinct morphology from amoeboid microglia. They exhibit a rod-like cell body without polarized processes. Usually, the rod microglia get aligned one another along their elongated axis (Figure 1C) (Taylor et al., 2014). The unique morphology implies they exert specific functions to neuroinflammation; however, this population is largely neglected during the past decades. There are occasional reports of rod microglia in different types of neuropathies, such as viral-encephalitis, lead encephalopathy, and slowly progressed hippocampal ischemia (Graeber and Mehraein, 1994). It has been suggested that the morphological integrity of tissue microstructure was one pre-requisite for the rod phenotype; while in rapidly progressed neurological conditions, such as traumatic brain injury and stroke, only phagocytic and amoeboid shape microglial cells were found. In fact, recent studies demonstrated the existence of rod microglia in postmortem brain samples from patients with Alzheimer's disease and other neurodegenerative disorders (Odawara et al., 1995). Notably, rod microglia expressed high level of MHC class II beta-chain (Graeber and Mehraein, 1994), which might be important in maintaining their morphology within the extracellular matrix.
In past decade, several animal studies revisited the potential functions of rod microglia in the nervous system. For instance, in experimental brain injury induced by fluid percussion, rod microglia was observed at the injured cortex 2 days after injury, expressing markers of activated microglia, including CD68 and OX-6 (Ziebell et al., 2012). It is found that rod microglia became bipolarized and attached to neuronal processes or nerve fibers to form trains (Figure 1C) (Taylor et al., 2014). It is also observed that rod microglia seem to get aligned with each other; however, the potential interaction (e.g., gap junctions) between different rod microglia remains elusive. Notably, the unique morphology and alignment make the definition of rod microglia ambiguous and subjective. In that scenarios, researchers characterize microglial phenotype rely on their personal experiences, which are subjective and time consuming. In a recent study, the researchers utilized customized computer software to quantitatively analyze the morphology (e.g., orientation angles) (Zhang et al., 2016). This thus provides descriptive definition makes rod microglial phenotype unambiguous and objective. Moreover, the automatic process allows high throughput analysis with precise details.
In a different set of studies, rod microglia have been reported in the retina undergoing retinal ganglion cell (RGC) loss in rat glaucoma models, a type of chronic ocular degeneration associated with optic neuropathy (Rojas et al., 2014). The retinal rod microglia have been found to be restricted in the nerve fiber layer (NFL)/ganglion cell layer (GCL), with a close relationship to RGC axon bundles. Therefore, they were radially aligned pointing to the optic nerve head (ONH) (Figure 1C). It has also been found that retinal rod microglial cells are likely to express M1- or M2-like microglial markers, which are actually expressed by amoeboid morphology microglial cells. Interestingly, in another study with acute ocular injury model, retinal rod microglial cells were observed 3 days after optic nerve transection, peaked at 3 weeks and disappeared after 6 weeks following the injury (Yuan et al., 2015). These rod microglial cells were phagocytic, and acted as the majority of activated microglial cells in the injured retina. The study further showed that the rod microglia cells are actually proliferating, especially in the period of 3 days to 2 weeks after injury. In contrast, infiltrating microglia differentiated from the myeloid precursor cells in the blood only have a minimal contribution (Yuan et al., 2015). In another study of glaucoma model by the acute elevation of intra-ocular pressure, activated microglia in the NFL/GCL also exhibited the rod phenotype, though the authors did not use this terminology (Wang et al., 2014). In this study, inhibition of rod microglia by minocycline significantly ameliorated the RGC loss. Collectively, these results suggested that rod microglia might be a common neuropathological feature in RGC insults of the retina, and worth more attention.
It has been believed that the rod morphology microglia might represent a mild level of neural injury and relative integrity of the surrounding tissues. In fact, in vitro cultured rod microglia exhibited low levels of M1-/M2-like microglial markers, and showed cell proliferation. With LPS challenge, they were further activated, became amoeboid shape and express pro-inflammatory M1-like markers (Tam and Ma, 2014). Therefore, the appearance of rod microglia in neural tissues could indicate a mild progression of the injury signaling; the use of rod-to-amoeboid microglia ratio therefore might be of interest to pathological analyses in clinical trials, especially in neurodegenerative diseases.
In summary, rod microglia represent a unique kind of activated microglial cells during neurodegenerative disorders. They get aligned along with axonal fibers in the brain and retina. They originate from resident microglial proliferation in responding to neural insults and exert phagocytic functions. They usually appear in mild neurodegeneration.
The authors thank Prof. Ti-Fei Yuan at Nanjing Normal University for critical reading and advices for improving the manuscript. This work was supported by National Natural Science Foundation of China (31600839 to BP), grants from Guangdong Innovative and Entrepreneurial Research Team Program (2013S046 to BP) and Shenzhen Peacock Plan (to BP).
References
- Graeber MB, Mehraein P. Microglial rod cells. Neuropathol Appl Neurobiol. 1994;20:178–180. [PubMed] [Google Scholar]
- Nissl F. Ueber einige Beziehungen zwischen Nervenzellerkrankungen und gliosen Erscheinungen bei verschiedenen Psychosen. Arch Psychiatr. 1899;32:656–676. [Google Scholar]
- Odawara T, Iseki E, Kosaka K, Akiyama H, Ikeda K, Yamamoto T. Investigation of tau-2 positive microglia-like cells in the subcortical nuclei of human neurodegenerative disorders. Neurosci Lett. 1995;192:145–148. doi: 10.1016/0304-3940(95)11595-n. [DOI] [PubMed] [Google Scholar]
- Rojas B, Gallego BI, Ramirez AI, Salazar JJ, de Hoz R, Valiente-Soriano FJ, Aviles-Trigueros M, Villegas-Perez MP, Vidal-Sanz M, Trivino A, Ramirez JM. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J Neuroinflammation. 2014;11:133. doi: 10.1186/1742-2094-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WY, Ma CH. Bipolar/rod-shaped microglia are proliferating microglia with distinct M1/M2 phenotypes. Sci Rep. 2014;4:7279. doi: 10.1038/srep07279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Morganti-Kossmann C, Lifshitz J, Ziebell JM. Rod microglia: a morphological definition. PLoS One. 2014;9:e97096. doi: 10.1371/journal.pone.0097096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Peng B, Lin B. Fractalkine receptor regulates microglial neurotoxicity in an experimental mouse glaucoma model. Glia. 2014;62:1943–1954. doi: 10.1002/glia.22715. [DOI] [PubMed] [Google Scholar]
- Yuan TF, Liang YX, Peng B, Lin B, So KF. Local proliferation is the main source of rod microglia after optic nerve transection. Sci Rep. 2015;5:10788. doi: 10.1038/srep10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Peng B, Wang S, Liang YX, Yang J, So KF, Yuan TF. Image processing methods to elucidate spatial characteristics of retinal microglia after optic nerve transection. Sci Rep. 2016;6:21816. doi: 10.1038/srep21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell JM, Taylor SE, Cao T, Harrison JL, Lifshitz J. Rod microglia: elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J Neuroinflammation. 2012;9:247. doi: 10.1186/1742-2094-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]


