Neurological diseases and the neuroinflammatory response: Neurological diseases are usually accompanied by dramatic changes in the tissue homeostasis, inducing a neuroinflammatory environment that leads to the progressive activation of central nervous system (CNS) resident cells, and in certain cases, to the infiltration of leukocytes into the CNS. If this neuroinflammatory response persists, it is toxic for CNS resident cells, especially for neurons, and consequently is detrimental for the progression and outcome of neurological diseases. The blood-brain barrier (BBB) is a physical and functional barrier that facilitates the maintenance of homeostasis restricting the traffic of substances and cells from the blood to the CNS parenchyma. CNS resident cells (mainly glial cells) actively monitor and detect any alteration in tissue homeostasis. Any unbalance (such as infections, trauma, stroke, and neurodegeneration) leads to the activation of CNS glial cells (mainly astrocytes and microglia), to counterbalance the alteration and bring back homeostasis (Tian et al., 2012). Upon activation, microglial cells and astrocytes undergo severe morphological and structural changes, ranging from a resting to a reactive state. Reactive glial cells show enhanced release of pro- and anti-inflammatory mediators, phagocytic capacity and increased migration to the insult site. If this glial response cannot restore proper physiological condition, the inflammatory status is maintained by the secretion of pro-inflammatory mediators, resulting in chronic neuroinflammation and the loss of white and gray matter distinctive of CNS pathologies (Popovich et al., 2002). Moreover, pro-inflammatory cytokines and chemokines alter the BBB permeability, allowing the activation and recruitment of leukocytes to the CNS parenchyma (Weiss et al., 2009; Tian et al., 2012) that help to maintain the chronic neuroinflammation as a feed-back mechanism with detrimental effects on the progression and outcome of neurological diseases.
Tauroursodeoxycholic acid (TUDCA) is a neuroprotective bile conjugate: TUDCA is a conjugated derivative of the ursodeoxycholic bile acid, with neuroprotective effects in several animal models of neurodegenerative diseases, such as Alzheimer's disease (Nunes et al., 2012), Huntington's disease (Keene et al., 2002), Parkinson's disease (Castro-Caldas et al., 2012) and stroke (Rodrigues et al., 2002). Bile acids are very interesting therapeutic tools because they cross the BBB, and they are used for the chronic treatment of primary biliary cirrhosis, with no side effect attached (Lindor et al., 1994). Indeed, TUDCA treatment has a beneficial effect on amyotrophic lateral sclerosis patients (Elia et al., 2016).
TUDCA inhibits nuclear factor-kappa B (NF-κB) activation in glial cells under pro-inflammatory conditions: In addition to the direct neuroprotective effect, TUDCA exerts a direct anti-inflammatory effect on both astrocytes and microglial cells in vitro and in a mouse model of acute neuroinflammation (Yanguas-Casás et al., 2014). Under pro-inflammatory conditions, TUDCA reduces NF-κB activation both in astrocytes and microglia, leading to a decrease in the nitrite release by these cells, through a transcriptional and translational diminution of the inducible enzyme nitric oxide synthase (iNOS). Mice treated with both the bacterial lipopolysaccharide (LPS, a single intracerebroventricular injection of 2 mg/kg) and TUDCA (a daily intraperitoneal injection of 500 mg/kg for three days) reduced microglia activation to control levels 3 days after LPS injection (Yanguas-Casás et al., 2014). Apart from restricting the activation of these cells, the decrease in nitrite secretion limits the spreading and the chronification of the pro-inflammatory response to surrounding cells. Besides, TUDCA treatment reduces the migratory capacity of microglial cells, preventing an exacerbated pro-inflammatory response in the insult site (Yanguas-Casás et al., 2014). The treatment with TUDCA reduces endothelium activation and decreases the expression of vascular adhesion proteins (e.g., VCAM-1). Moreover, TUDCA also reduces the monocyte chemoattractant protein 1 (MCP-1) expression in microglia and astrocytes under pro-inflammatory conditions (Yanguas-Casás et al., 2014). These proteins are required for both microglial migration and leukocyte transmigration into the CNS parenchyma (Weiss et al., 2009). These results suggest that TUDCA might reduce leukocyte extravasation to the inflammation site in the CNS.
Additional anti-inflammatory effect of TUDCA inducing TGF-β pathway: Recently, we found that TUDCA has an additional anti-inflammatory effect in neuroinflammation through the regulation of the transforming growth factor β (TGF-β) pathway (Yanguas-Casás et al., 2016; Figure 1). TGF-β is a pleiotropic cytokine involved in a wide variety of physiological and pathological conditions that actively modulate the inflammatory process by a direct effect on immune and CNS resident cells under physiological and neuropathological conditions (Blobe et al., 2000). TGF-β inhibits the activation of pro-inflammatory pathways, reducing the production and release of pro-inflammatory mediators. Besides, TGF-β drives microglial cells towards an anti-inflammatory phenotype, contributing to homeostasis restoration. In a mouse model of acute neuroinflammation by intracerebraventricular injection of LPS, TUDCA increases the transcription of TGF-β2 and TGF-β3 in the hippocampus of LPS treated mice, compared to those treated with LPS alone. We found that TGF-β3 protein expression was increased in the brains of those mice. Interestingly, TGF-β3 expression was limited to certain cell types, such as microglia, endothelial cells and some neurons, but was absent in astrocytes (Yanguas-Casás et al., 2016). The inhibition of TGF-β receptor (ALK5) in mice treated with TUDCA reduced TGF-β3 expression and restored microglia activation to the same levels as mice treated with LPS alone. This result suggests that TUDCA-induced TGF-β3 expression might be regulated by TGF-β pathway as a positive feed-back. Moreover, TGF-β pathway activation is required for TUDCA-induced inhibition of microglia activation in mice treated with LPS (Yanguas-Casás et al., 2016). We cannot exclude that the increasing expression of TGF-β3 in endothelial cells might participate in the effects of TUDCA on VCAM-1 expression and leukocyte infiltration into the CNS.
Figure 1.
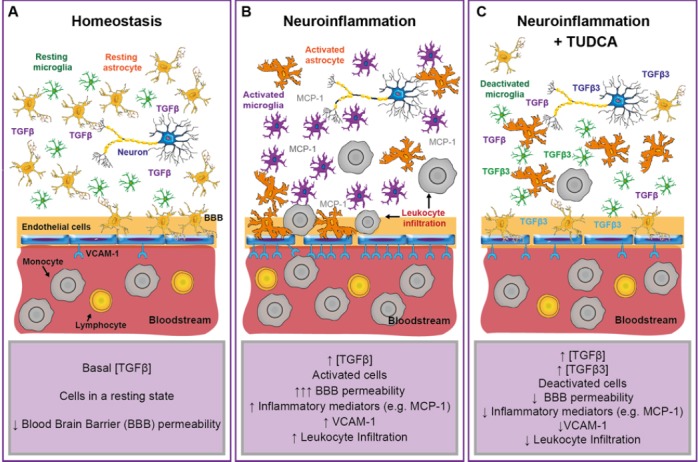
Proposed model for the effect of tauroursodeoxycholic acid (TUDCA) in the neuroinflammatory process.
(A) Under homeostatic conditions, the blood-brain barrier (BBB) restricts the entry of circulating blood cells into the central nervous system (CNS) parenchyma. The low permeability to circulating blood substances and leukocytes and the basal concentration of anti-inflammatory mediators (such as transforming growth factor β (TGFβ)) in the CNS allow a resting-homeostatic state in CNS resident cells. (B) CNS neuroinflammation drives the activation of resident glial cells (such as microglia and astrocytes) and the secretion of inflammatory mediators (e.g., monocyte chemoattractant protein 1 (MCP-1)) that promote the infiltration of peripheral leukocytes into the injured parenchyma. Endothelial cells increase the expression of surface adhesion molecules (e.g., vascular cell adhesion molecule 1 (VCAM-1)). Both, reactive astrocytes and activated endothelium, increase the permeability of the BBB. Circulating leukocytes adhere to the activated endothelium and infiltrate into the CNS, perpetuating the inflammatory response by a direct interaction with neurons and glial cells. During early inflammation, the activation of matrix metalloproteases releases TGFβ from the extracellular matrix, increasing the activation of the TGFβ pathway and counterbalancing the pro-inflammatory response. (C) Under inflammatory conditions, TUDCA treatment increases the activation of the TGFβ pathway and the expression of TGFβ3 in neurons, microglia and endothelial cells. The activation of TGFβ pathway drives the deactivation of glial cells and the endothelium, leading to an anti-inflammatory microenvironment with reduced expression of vascular adhesion molecules (e.g., VCAM-1) and inflammatory mediators (e.g., MCP-1), and therefore to a reduced BBB permeability and leukocyte infiltration.
Conclusions: Although the neuroprotective effects of TUDCA have been widely described, little is known about the effect of TUDCA on glial cells. In addition to its direct neuroprotective effect on different animal models of neurological diseases, TUDCA might have an indirect neuroprotective effect through the inhibition of glia and endothelium activation under pro-inflammatory conditions. TUDCA treatment inhibits NF-κB pathway and increases further TGF-β pathway favoring the resolution of the inflammatory process. The anti-inflammatory environment promoted by glial cells might lead to an increase of neuronal survival and a faster restoration of neural function in the CNS. Our results have therapeutical implications for those neuropathologies that course with neuroinflammation.
This work was supported by grants from the Spanish Ministry of Science and Innovation (SAF2009-11257), the Spanish Ministry of Economy and Competitivity (SAF2012-40126) and grants PI2008/19 and PI2009/51 from the FISCAM-Castilla- La Mancha Community.
References
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas M, Carvalho AN, Rodrigues E, Henderson CJ, Wolf CR, Rodrigues CM, Gama MJ. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson's disease. Mol Neurobiol. 2012;46:475–486. doi: 10.1007/s12035-012-8295-4. [DOI] [PubMed] [Google Scholar]
- Elia AE, Lalli S, Monsurro MR, Sagnelli A, Taiello AC, Reggiori B, La Bella V, Tedeschi G, Albanese A. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur J Neurol. 2016;23:45–52. doi: 10.1111/ene.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington's disease. Proc Natl Acad Sci U S A. 2002;99:10671–10676. doi: 10.1073/pnas.162362299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Harrison JM, Wiesner RH, Anderson ML, Lange SM, Lesage G, Rossi SS, Hofmann AF. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–1290. doi: 10.1016/0016-5085(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Nunes AF, Amaral JD, Lo AC, Fonseca MB, Viana RJ, Callaerts-Vegh Z, D’Hooge R, Rodrigues CM. TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-beta deposition in APP/PS1 mice. Mol Neurobiol. 2012;45:440–454. doi: 10.1007/s12035-012-8256-y. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Spellman SR, Sola S, Grande AW, Linehan-Stieers C, Low WC, Steer CJ. Neuroprotection by a bile acid in an acute stroke model in the rat. J Cereb Blood Flow Metab. 2002;22:463–471. doi: 10.1097/00004647-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Tian L, Ma L, Kaarela T, Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J Neuroinflammation. 2012;9:155. doi: 10.1186/1742-2094-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Yanguas-Casás N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramírez L. Tauroursodeoxycholic acid reduces glial cell activation in an animal model of acute neuroinflammation. J Neuroinflammation. 2014;11:50. doi: 10.1186/1742-2094-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanguas-Casás N, Barreda-Manso MA, Perez-Rial S, Nieto-Sampedro M, Romero-Ramírez L. TGFβ contributes to the anti-inflammatory effects of tauroursodeoxycholic acid on an animal model of acute neuroinflammation. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0142-6. doi:10.1007/s12035-016-0142-6. [DOI] [PubMed] [Google Scholar]


