The immune system (IS) is set to provide protection against pathogens, surveillance against tumor cells and promotion of healing. Such functions can be efficiently performed thanks to the presence of a large network of cells with variable degrees of specialization, which are traditionally divided into two functional branches: the innate and adaptive immunity. Innate immunity is a non-specific response and the first line of defense against pathogens. It is based on physical barriers, activity of immune cells including phagocytes and natural killer cells, serum proteins including acute-phase proteins and complement factors. Adaptive immunity is an antigen-specific response, depending on the action of antigen-presenting cells, T-lymphocytes, B-lymphocytes and antibodies. During the inflammatory response, other molecules are involved: membrane proteins (CD3, CD22), cell adhesion and migration molecules (integrins, selectins, matrix metalloproteinases), cytokines and chemokines.
The interplay among defensive cells, but also with the local microenvironment on one side and the detrimental stimuli on the other, are finely regulated in all body tissues. Nonetheless, the communication between the immune and nervous system, especially its central component, provides further complexity even in healthy conditions. This is partly due to some peculiar features of the central nervous system (CNS), such as the presence of the blood-brain barrier and the lack of both lymphatic vessels and professional antigen presenting cells. The immune-nervous cross talk becomes more intricate when neurodegeneration is involved: depending on disease type and stage and other variables such as the local environment and the host genetic background, the contribution of IS activation in neurodegenerative diseases can vary from detrimental to beneficial (Cappellano et al., 2013). In the following paragraphs we will focus on the protective functions of both innate and adaptive immune response.
Microglia are the resident macrophages of the CNS exerting protective functions through phagocytosis of pathogens and cellular debris. In neurodegenerative diseases, microglia play a role in removing apoptotic cells and protein aggregates. In the early stages of Alzheimer's disease (AD), the demolition of protein aggregates is fulfilled through the release of proteolytic enzymes, such as matrix metalloproteinases. Phagocytosis of debris then occurs thanks to specific receptors, such as the scavenger receptors. If, initially, these mechanisms are sufficient to protect neuronal homeostasis, the persistent deposition of Aβ may favor a detrimental inflammation (Cappellano et al., 2013).
In addition to phagocytosis, microglia can exert neuroprotective functions through the production of anti-inflammatory cytokines, chemokines, and neurotrophic factors, stimulating tissue repair and neuron survival. For example, concentration of growth arrest specific 6 (Gas6) (Figure 1), an anti-inflammatory molecule involved in the macrophage-microglia network, was found to be increased in early AD patients compared to age matched controls, and such an increase was also shown to confer protection against cognitive decline in patients who were prospectively followed-up over one year from AD diagnosis (Sainaghi et al., 2016).
Figure 1.
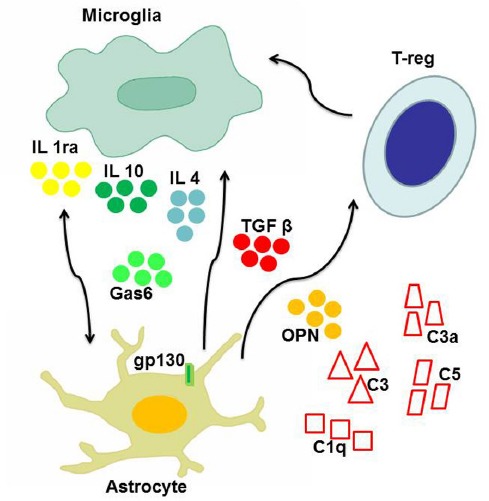
The main players of neuroprotective inflammation.
The figure shows the main cell types involved in protective pathways counteracting neurodegeneration: microglia, astrocytes, T-reg cells, complement system. Intercellular cross talk, as well as down-modulation of detrimental inflammatory reactions is essentially mediated by cytokines, including interleukin 1 receptor antagonist (IL1ra), interleukin 10 (IL10) and interleukin 4 (IL4), transforming growth factor beta (TGFβ), and osteopontin (OPN). Also other soluble molecules with growth factor properties, such as growth arrest specific 6 (Gas6), may contribute to neuroprotection.
A potential neuroprotective activity has also been acknowledged to astrocytes, since it was shown that they can both promote cell debris degradation and inhibit adaptive response suppressing lymphocytes activation and proliferation. Astrocytes are the most numerous glial population. They play immune functions recognizing pathogens and producing cytokines and chemokines, and neuronal supporting function by producing growth factors, adjusting extracellular fluids, and conditioning synaptic transmission. Recent studies in murine models have shown that astrocytes may play a key role in various protective mechanisms against immune-mediated damage (Colombo and Farina, 2016). One of the protective mechanisms is carried out by the glycoprotein gp130, which transduces survival signals for glial cells and acts in inflammation switching off. Another mechanism is mediated by transforming growth factor-β (TGF-β), a pleiotropic cykokine showing anti-inflammatory properties. TGF-β can inhibit the production of inflammatory mediators by astrocytes, the recruitment of T cells and, ultimately, the immune-mediated damage, through the inhibition of the pro-inflammatory NF-κB pathway (Figure 1). Finally, astrocytes can turn off also the adaptive response, being able to induce apoptosis in T-cells, favoring their elimination through Fas-Fas ligand pathway.
Cytokines are an heterogeneous group of small secreted signaling molecules important for inter- and intracellular communication during the immune response. They also contribute to maintenance of neural balance, neurogenesis, cell proliferation, migration and apoptosis. Cytokines can play, alternately, pro-inflammatory or anti-inflammatory roles. Interleukin-1 (IL-1) receptor antagonist and IL-10 display a clear anti-inflammatory function, conferring neuroprotection in both AD and Parkinson's disease (PD): they attenuate microglia activation and inhibit release of other pro-inflammatory cytokines (Cappellano et al., 2013). Other cytokines can perform a protective activity in neurodegenerative disease. This is the case of IL-4 and TGF-β: the former inhibits in vitro the production of pro-inflammatory cytokines, and in vivo appears to stimulate a quiescent state of glia while facilitating phagocytosis (Figure 1). The latter can protect neurons through the decrease of IL-17 production and facilitate the removal of amyloid plaques in AD (Zheng et al., 2016). Even cytokines with a predominantly pro-inflammatory action, such as tumor necrosis factor- α (TNF-α), IL-1 and IL-6, were reported to display anti-inflammatory activity in peculiar contexts (Cappellano et al., 2013). TNF-α was proven effective in protecting hippocampal neurons from excitotoxicity-mediated damage in animal models of neurodegeneration; In AD animal models, IL-1 can reduce the accumulation of amyloid plaques favoring its degradation or limiting its production; IL-6 may stimulate an activated phenotype of microglia, inclined to the elimination of amyloid plaques, rather than to stimulation of the inflammatory state. A further example is displayed by osteopontin (OPN), a pro-inflammatory cytokine which was shown to be involved in several neurodegenerative diseases (Carecchio and Comi, 2011). Robust evidence points to a detrimental role of OPN in multiple sclerosis, since it was shown that polymorphic variants of its gene are associated with faster disease progression, but also that increased levels of circulating OPN are associated with disease relapses (Comi et al., 2012). A similar scenario was detected in AD: AD patients showed higher OPN concentration in the cerebrospinal fluid compared to matched controls, and such increase was more evident in patients showing faster cognitive decline (Carecchio and Comi, 2011). On the contrary, evidence on the role of OPN in PD is somehow conflicting, since both detrimental and protective functions were detected (Carecchio and Comi, 2011) (Figure 1). Recent reports suggest that the pleiotropic activity of this protein might depend, at least in part, by post-translational modifications (Boggio et al., 2016).
Cytokine production occurs under the control of several cellular pathways, including MAPK, NF-κB, and peroxisome proliferator activated receptor γ (PPAR-γ) pathways, the latter related to a strong anti-inflammatory activity, consisting in suppression of inflammatory genes expression and blockade of inflammatory cytokines production (Cappellano et al., 2013). Conversely, NF-κB pathway was shown to be primarily involved in pro-inflammatory responses, and its inhibition has been proved useful in reducing detrimental neuroinflammation (Colombo and Farina, 2016).
The role of the complement system is mainly regarded as pro-inflammatory, since it favors the recognition of pathogens by phagocytic cells and stimulates chemotaxis. Nonetheless, it was also shown that C3a can provide protection against glutamate mediated excitotoxicity in murine models of neurodegeneration (Cappellano et al., 2013). Furthermore, inhibition of C5, C3 and C1q may favor amyloid β deposition (Cappellano et al., 2013). In this context, the anti-inflammatory activity of complement is expressed through the inhibition of pro-inflammatory cytokines, removal of apoptotic cells and stimulation of neuronal survival (Cappellano et al., 2013).
In the CNS, the adaptive immune response is essentially orchestrated by CD4+ lymphocytes. CD4+ cells can exert pro-inflammatory functions, as in the case of Type 1 and Type 17 Helper cells (Th1 and Th17), or alternatively anti-inflammatory or regulatory functions, when Type 2 Helper cells (Th2) or T-reg cells are involved (Figure 1). Infiltration of CD4+ T-reg cells confers neuroprotection in AD through the modulation of microglia, especially inhibiting the production of pro-inflammatory factors. The switch to an anti-inflammatory phenotype is essentially regulated by cytokines (Figure 1). Findings from AD animal models indicate that IL-2 is effective in inducing expansion of T-reg cells. These cells are able, in turn, to stimulate phagocytosis of amyloid plaques by microglial cells, with a maximum effect occurring in the early stages of disease, when cognitive functions can still be restored. The concept of a beneficial role of the immune system in coping to external/internal insults and favoring neuroplasticty was previously defined as “protective autoimmunity”. According to this notion, aging of the immune system would impair its ability to protect the CNS, thus favoring neurodegeneration (Schwartz and Raposo, 2014).
A protective activity is not an exclusive prerogative of T-lymphocytes. Indeed, in immunodeficient AD murine models, the decrease not only of T-lymphocytes, but also of B-lymphocytes and natural killer cells can favor a detrimental loop, driven by the overproduction of pro-inflammatory cytokines and concomitant reduction of phagocytic capacity of microglia (Zheng et al., 2016).
Another important aspect of the adaptive immune response is the presence of receptors for neurotransimetters on T cells. This is of particular relevance in PD, since dopaminergic treatment was shown to modulate patients’ T cell proteome (Alberio et al., 2012), and dopamine receptor expression on patients’ T cells was associated with motor dysfunction measured by the Unified Parkinson's Disease Rating Scale (Kustrimovic et al., 2016).
Indeed, a fine and targeted modulation of immunity might represent an effective therapeutic strategy for neurodegenerative diseases, especially considering that previous attempt to broadly counteract neuroinflammation with non-steroidal anti-inflammatory drugs (NSAIDs) have yielded disappointing results (Cappellano et al., 2013). Consistently, studies selectively targeting innate and/or adaptive immunity are ongoing. As regards innate immunity, one strategy may involve up-regulation of microglial phagocytic capacity, through the administration of colony-stimulating factors. Granulocyte macrophage colony stimulating factor (GM-CSF) is increased in patients with rheumatoid arthritis, a disease conferring some protection from AD development. The administration of GM-CSF in AD mice was effective in reducing beta amyloid deposition and protecting hippocampal synapses (Boyd et al., 2010). Encouraging results were obtained also in humans: in cancer patients undergoing hematopoietic cell transplantation, supportive care with GM-CSF induced an improvement of cognitive performance (Jim et al., 2012). Such preliminary observations led the recent launch of a phase 2 trial on GM-CSF use in AD. On the side of adaptive immunity, clinical trials with active or passive immunization strategies have been conducted, especially targeting AD. The first and second era of AD immunotherapy did not meet the expectations. The first vaccination protocol failed due to immunologic side effects, while in the second course of trials it is likely that the target population was in a too advanced disease phase to show significant benefit. The next step consisted in enrolling very early AD patients, in order to act when neurodegeneration was in its initial phases. Some preliminary results are promising and the strategy of anticipating drug administration has a strong rationale. Furthermore, clinical trials with immunotherapy targeting alpha-synuclein in PD are ongoing.
Therefore, it is reasonable to believe that early, fine, and specific modulation of crucial players of the immune response could be a winning strategy for counteracting neurodegenerative diseases.
References
- Alberio T, Pippione AC, Comi C, Olgiati S, Cecconi D, Zibetti M, Lopiano L, Fasano M. Dopaminergic therapies modulate the T-CELL proteome of patients with Parkinson's disease. IUBMB Life. 2012;64:846–852. doi: 10.1002/iub.1073. [DOI] [PubMed] [Google Scholar]
- Boggio E, Dianzani C, Gigliotti CL, Soluri MF, Clemente N, Cappellano G, Toth E, Raineri D, Ferrara B, Comi C, Dianzani U, Chiocchetti A. Thrombin cleavage of osteopontin modulates its activities in human cells in vitro and mouse experimental autoimmune encephalomyelitis in vivo. J Immunol Res 2016. 2016 doi: 10.1155/2016/9345495. 9345495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, Potter H. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimers Dis. 2010;21:507–518. doi: 10.3233/JAD-2010-091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellano G, Carecchio M, Fleetwood T, Magistrelli L, Cantello R, Dianzani U, Comi C. Immunity and inflammation in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2:89–107. [PMC free article] [PubMed] [Google Scholar]
- Carecchio M, Comi C. The role of osteopontin in neurodegenerative diseases. J Alzheimers Dis. 2011;25:179–185. doi: 10.3233/JAD-2011-102151. [DOI] [PubMed] [Google Scholar]
- Colombo E, Farina C. Astrocytes: Key regulators of neuroinflammation. Trends Immunol. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Comi C, Cappellano G, Chiocchetti A, Orilieri E, Buttini S, Ghezzi L, Galimberti D, Guerini F, Barizzone N, Perla F, Leone M, D’Alfonso S, Caputo D, Scarpini E, Cantello R, Dianzani U. The impact of osteopontin gene variations on multiple sclerosis development and progression. Clin Dev Immunol 2012. 2012 doi: 10.1155/2012/212893. 212893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim HS, Boyd TD, Booth-Jones M, Pidala J, Potter H. Granulocyte macrophage colony stimulating factor treatment is associated with improved cognition in cancer patients. Brain Disord Ther. 2012;1:1000101. doi: 10.4172/bdt.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustrimovic N, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, Comi C, Mauri M, Minafra B, Riboldazzi G, Sanchez-Guajardo V, Marino F, Cosentino M. Dopaminergic receptors on CD4+ T naive and memory lymphocytes correlate with motor impairment in patients with Parkinson's disease. Sci Rep. 2016;6:33738. doi: 10.1038/srep33738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainaghi PP, Bellan M, Lombino F, Alciato F, Carecchio M, Galimberti D, Fenoglio C, Scarpini E, Cantello R, Pirisi M, Comi C. Growth arrest specific 6 concentration is increased in the cerebrospinal fluid of patients with Alzheimer's disease. J Alzheimers Dis. 2017;55:59–65. doi: 10.3233/JAD-160599. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Raposo C. Protective autoimmunity: A unifying model for the immune network involved in CNS repair. Neuroscientist. 2014;20:343–358. doi: 10.1177/1073858413516799. [DOI] [PubMed] [Google Scholar]
- Zheng C, Zhou XW, Wang JZ. The dual roles of cytokines in Alzheimer's disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener. 2016;5:7. doi: 10.1186/s40035-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


