Keywords: nerve regeneration, brain injury, nicotiflorin, ischemic stroke, cerebral ischemia/reperfusion injury, treatment, cell apoptosis, terminal deoxynucleotidyl transferase dUTP nick end labeling, JAK2/STAT3 pathway, Bcl-2, Bax, caspase-3, neural regeneration
Abstract
Nicotiflorin is a flavonoid extracted from Carthamus tinctorius. Previous studies have shown its cerebral protective effect, but the mechanism is undefined. In this study, we aimed to determine whether nicotiflorin protects against cerebral ischemia/reperfusion injury-induced apoptosis through the JAK2/STAT3 pathway. The cerebral ischemia/reperfusion injury model was established by middle cerebral artery occlusion/reperfusion. Nicotiflorin (10 mg/kg) was administered by tail vein injection. Cell apoptosis in the ischemic cerebral cortex was examined by hematoxylin-eosin staining and terminal deoxynucleotidyl transferase dUTP nick end labeling assay. Bcl-2 and Bax expression levels in ischemic cerebral cortex were examined by immunohistochemial staining. Additionally, p-JAK2, p-STAT3, Bcl-2, Bax, and caspase-3 levels in ischemic cerebral cortex were examined by western blot assay. Nicotiflorin altered the shape and structure of injured neurons, decreased the number of apoptotic cells, down-regulates expression of p-JAK2, p-STAT3, caspase-3, and Bax, decreased Bax immunoredactivity, and increased Bcl-2 protein expression and immunoreactivity. These results suggest that nicotiflorin protects against cerebral ischemia/reperfusion injury-induced apoptosis via the JAK2/STAT3 pathway.
Introduction
Stroke is a serious leading cause of death that causes financial burden, especially in low-income and middle-income countries (Feigin et al., 2014; Levine et al., 2015; Banerjee and Das, 2016). Ischemic stroke accounts for 75% of all stroke patients (Zevallos et al., 2015). Within a certain time window, thrombolysis is thought to be the most effective treatment method, but many people cannot arrive at hospital within 4.5–6.0 hours, therefore systemic recombinant tissue plasminogen activator is limited (Zaidat et al., 2012; Akbik et al., 2016). Due to developments in pathophysiological stroke research, different mechanisms provide varied treatment opportunities (Lo et al., 2003; Hachinski et al., 2010; Liu et al., 2014).
In recent decades, studies have shown that significant blood flow reductions within the ischemic core accompany irreversible nerve cell necrosis, while programmed cell death (namely apoptosis) appears within the ischemic penumbra, and is reversible until a few hours after cerebral ischemia (Xu and Zhang, 2011; Ghosh et al., 2012; Kalogeris et al., 2012). Hence, saving apoptotic cells is an important strategy in stroke treatment.
Cerebral ischemia/reperfusion (I/R) injury triggers multiple cell apoptotic pathways (Li et al., 1997; Polster and Fiskum, 2004; Wang et al., 2013; Feng et al., 2016). Indeed, there is overwhelming evidence that ischemia-induced oxidative stress and inflammation are principally responsible for subsequent cell death by necrotic or apoptotic mechanisms (Nita et al., 2001; Jin et al., 2013). Reactive oxygen species regulate cell survival/death by activating various cell signaling pathways, such as p38, c-Jun N-terminal kinases, nuclear factor-kappa B, and Janus kinase/signal transducers and activators of transcription JAK/STAT (Nakka et al., 2008; Wang et al., 2014; Hou et al., 2016). Studies show that JAK2/STAT3 activation contributes to cell apoptosis following transient focal cerebral ischemia (Satriotomo et al., 2006; Xie et al., 2007).
Traditional Chinese medicines are effective and have less clinical side-effects for cerebral ischemia patients, but their mechanisms and targets need further investigation (Murphy, 2003; Sun et al., 2015). Nicotiflorin, kaempferol-3-O-rutinoside, a flavonoid glycoside extracted from Carthamus tinctorius, has shown protective effects against brain injury in a multi-infarct dementia model (Xie et al., 2007). Similarly, other studies have shown that nicotiflorin improves ischemic brain damage after transient focal cerebral ischemia (Li et al., 2006; Huang et al., 2007). Nicotiflorin is neuroprotective against hypoxia-, glutamate- or oxidative stress-induced retinal ganglion cell death (Nakayama et al., 2011). In addition, we have previously shown protective effects of nicotiflorin in brain injury and neuroinflammation by inhibiting STAT3 activation (Yu et al., 2013). As already noted, JAK2/STAT3 activation contributes to cell apoptosis following transient focal cerebral ischemia. However, it remains poorly understood whether nicotiflorin protects against cerebral I/R-induced cell apoptosis through the JAK2/STAT3 pathway. Accordingly, this is the focus of our present study, and indeed has not previously been investiagted. Thus, we examined the anti-apoptopic effect and underlying nicotiflorin signaling pathway in rats following transient ischemia induced by I/R.
Materials and Methods
Animals
Twenty-four male specific-pathogen-free Sprague-Dawley rats weighing 260–310 g and aged 13–15 weeks were provided by the Experimental Animal Center, Southwest Medical University, China (license No. SCXK(Chuan)2013-17). The rats were equally and randomly allocated to three groups: sham, I/R, and nicotiflorin. Rats were maintained under standardized temperature and humidity with a 12-hour light/dark cycle, and free access to food and water. All procedures were performed in accordance with China Animal Welfare Legislation. Protocols were approved by the Ethics Committee of Southwest Medical University, China.
Surgical operation
Transient middle cerebral artery occlusion (MCAO) was performed on the right side using a nylon filament, as previously described (Longa et al., 1989). Briefly, rats were anesthetized with 10% chloral hydrate (350 mg/kg, intraperitoneally). The internal carotid artery and external carotid artery were carefully detached and a prepared segment of 4-0 monofilament fiber was inserted from the external carotid artery into the internal carotid artery to block the origin of the middle cerebral artery. The sham operation involved a similar surgical procedure except for MCAO. After 2 hours of ischemia, the fiber was gently removed to enable reperfusion of the middle cerebral artery. Body temperature was maintained at 37°C throughout the operation. Rats with the following symptoms were assumed to be successful models: failure to fully extend left forepaw, circling to the left, or falling to the left. After 24 hours of reperfusion, rats were executed.
Drug administration
Nicotiflorin (Shanghai Winherb Medical Technology Co., Ltd., Shanghai, China) was dissolved in 25% polyethylene glycol 400. Nicotiflorin (10.0 mg/kg) was administered by tail vein injection to the nicotiflorin group at the beginning of reperfusion. Vehicle was administered to the I/R and sham groups in the same manner. Rats were executed after 24 hours of nicotiflorin treatment.
Brain tissue extraction
Brain tissue was removed after reperfusion for 24 hours, then fixed, embedded in paraffin blocks, and cut into 5 μm-thick coronal sections (0.26–0.51 mm anterior to bregma) for conventional hematoxylin-eosin staining, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, and immunohistochemical staining.
Hematoxylin-eosin staining
Briefly, slices were deparaffinized, hydrated in water, then stained with hematoxylin for 15 minutes, and washed in running tap water for 20 minutes. Next, slices were counterstained with eosin for 2 minutes, dehydrated in 95% absolute alcohol until excess eosin was removed, permeabilized in xylene, and mounted. Pathological changes were observed under a light microscope.
TUNEL assay
TUNEL staining was used to detect fragmented nuclear DNA during apoptosis (ArunaDevi et al., 2010; Bahmani et al., 2011), according to standard protocols for the TUNEL assay kit (Boster, Wuhan, China). Briefly, slices were deparaffinized, rehydrated, and then incubated in proteinase K for 15 minutes to digest DNA. After washing in a Tris buffer, sections were incubated in labeling buffer mixed with TdT and digoxigenin (DIG)-d-UTP at 37°C for 2 hours. Blocking reagent was added at room temperature for 30 minutes. Next, sections were incubated in anti-DIG-biotin for 30 minutes, with Strept Avidin Biotin Complex (SABC)-FITC used for final detection. Stained cells were counted from three different views per section using a fluorescence microscope (AMG EVos FL Microscopy, Seattle, WA, USA). The average count number was used for quantification and comparison between groups.
Immunohistochemical staining
Immunohistochemistry of coronal sections was performed as described previously (Yu et al., 2013). Rabbit anti-B-cell lymphoma 2 (Bcl-2) associated X (Bax) polyclonal antibody (Boster; 1:200), and rabbit anti-Bcl-2 polyclonal antibody (Boster; 1:100) were used. Briefly, sections were dewaxed, rinsed with 3% H2O2 for 20 minutes, and incubated in 5% bovine serum albumin for 30 minutes to block nonspecific binding. Sections were then separately incubated in Bax and Bcl-2 antibodies overnight at 4°C. Next, ready-to-use goat anti-rabbit IgG secondary antibody (Boster) was incubated at 37°C for 1 hour. Sections were then stained with 3,3′-diaminobenzidine and counterstained with hematoxylin. For each antibody staining, immunoreactive cells per 1 mm2 of the cortex were counted using an optical microscope (Leica DM750, Solms, Germany) (Chu et al., 2006). The average count number was used for quantification and comparison between groups.
Western blot assay
After reperfusion for 24 hours, ischemic cortex was collected for total protein extraction (n = 3 each group), according to the manufacturer's instructions for the protein extraction kit (Boster). Equivalent amounts of protein were loaded onto 10% sodium dodecyl sulfate-polyacrylamide electrophoresis gels for standard electrophoresis. Afterwards, gels were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% skimmed milk in Tris-buffered saline for 1 hour at room temperature, and then incubated overnight at 4°C with the following antibodies: monoclonal phospho-JAK2 (Cell Signaling Technology, Danvers, MA, USA; 1:1,000), monoclonal phospho-STAT3 (Cell Signaling Technology; 1:1,000), polyclonal Bax (Boster; 1:500), polyclonal caspase-3 (Boster; 1:500), polyclonal Bcl-2 (Boster; 1:500), and an internal control, monoclonal beta-actin antibody (Cell Signaling Technology; 1:1,000). Next, membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG (1:1,000; Nantong, China) for 1 hour at room temperature. Protein bands were quantified by scanning of visualized bands using enhanced chemiluminescence (Millipore, Bedford, MA, USA) and gel imaging equipment (Bio-Rad, Hercules, CA, USA). Band optical density was measured using Quantity One 4.6.2 software (Bio-Rad).
Statistical analysis
All data are presented as the mean ± SEM, and were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Differences among groups were analyzed by one-way analysis of variance followed by post hoc Tukey test. In all analyses, values of P < 0.05 were considered statistically significant.
Results
Nicotiflorin inhibited neuronal pathological changes
Hematoxylin-eosin staining showed that normal neurons in the sham group exhibited regulatory round and bright blue nuclei (Figure 1). While in the I/R group, neurons showed apparent disorder, and part of them presented apoptosis features: nuclear chromatin pyknosis, side scatter, or fracture. Nicotiflorin reduced pathological neuronal injury and the number of dead neurons (necrosis and apoptosis) induced by cerebral I/R.
Figure 1.
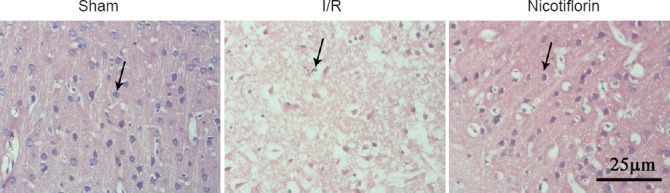
Representative hematoxylin-eosin (HE) stained micrographs of the cortical ischemic penumbra.
In the sham group, normal neurons show round and blue nuclei (arrow). In the I/R group, apoptotic or dead neurons exhibit pyknotic nuclei or side scatter (arrow). Nicotiflorin treatment significantly decreased pathological injury. Scale bar: 25 μm. I/R: Ischemia/reperfusion
Nicotiflorin decreased cerebral I/R-induced cell apoptosis
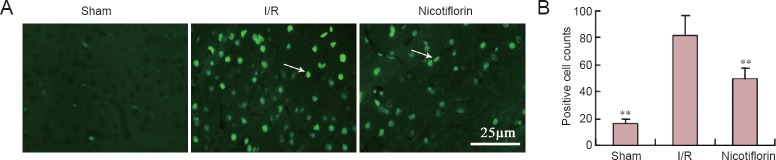
In the sham group, there were only a few TUNEL-positive cells (bright green fluorescence) in the cerebral cortex (Figure 2A). Further, in the I/R group, the TUNEL-positive cell number markedly increased after 2 hours of MCAO and 24 hours of reperfusion. In contrast, compared with the I/R group, TUNEL-positive cells significantly decreased after nicotiflorin treatment (P < 0.01; Figure 2B).
Figure 2.
Effect of nicotiflorin on cell apoptosis in the I/R model.
(A) TUNEL staining in cortical cells under fluorescence microscopy (40×). Apoptotic cells are marked by arrows. Scale bar: 25 μm. (B) TUNEL-positive cell number was counted under fluorescent microscopy. **P < 0.01, vs. I/R group (mean ± SEM, n = 3, one-way analysis of variance followed by post hoc Tukey test). TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling; I/R: ischemia/reperfusion.
Nicotiflorin inhibited JAK2 and STAT3 phosphorylation
Phosphorylation of JAK2 and STAT3 is thought to play an important role in apoptosis regulation. Therefore, both proteins were assessed by western blot assay. Activited pJAK2 and pSTAT3 both increased after ischemic injury in the I/R group compared with the sham group (P < 0.01). However, compared with the I/R group, nicotiflorin inhibited pJAK2 and pSTAT3 activation (Figure 3A, B).
Figure 3.
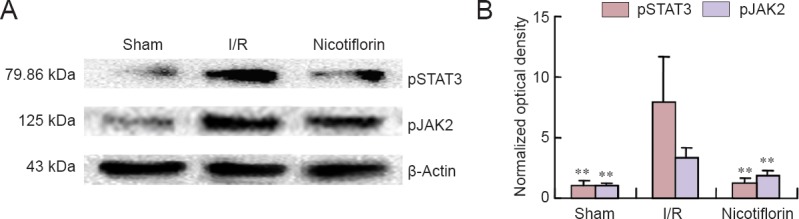
Nicotiflorin effect on expression of JAK2 and STAT3 phosphorylation in the ischemic cerebral cortex of I/R rats.
(A) Western blot images of pJAK2 and pSTAT3. (B) The resulting histogram shows increased pJAK2 and pSTAT3 expression in brain tissue from I/R rats. Nicotiflorin significantly reduced JAK2 and STAT3 phosphorylation. **P < 0.01, vs. I/R group (mean ± SEM, n = 3, one-way analysis of variance followed by post hoc Tukey test). I/R: Ischemia/reperfusion.
Nicotiflorin altered caspase-3, Bcl-2, and Bax immunoreactivity
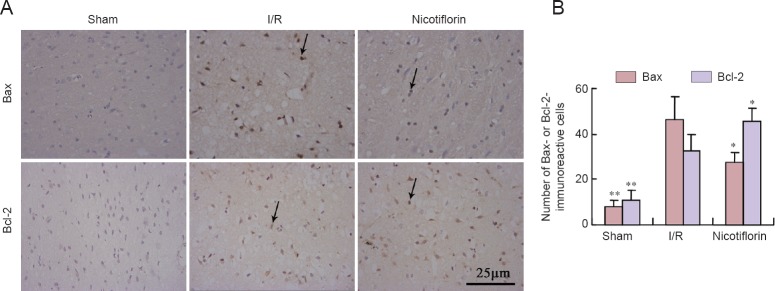
Bcl-2 family members play important regulatory roles in cerebral I/R injury-induced apoptosis (Hardwick et al., 2012; Kalogeris et al., 2012; Kvansakul and Hinds, 2013; Troy and Jean, 2015). Therefore, we examined changes in Bcl-2 and Bax immunoreactivity. Immunohistochemical staining showed increased Bax immunoreactivity and lower Bcl-2 immunoreactivity in the cerebral cortex of the I/R group (Figure 4A). In contrast, nicotiflorin significantly reduced Bax immunoreactivity, but increased Bcl-2 immunoreactivity (P < 0.05 or P < 0.01; Figure 4B).
Figure 4.
Nicotiflorin effect on Bcl-2 and Bax immunreactivity in the ischemic cerebral cortex of I/R rats (immunohistochemical staining).
(A) High-magnification images of Bcl-2 and Bax immunohistochemical staining (arrows show immunoreactive cells). Scale bar: 25 μm. (B) For each antibody staining, immunoreactive cells in a 1 mm width were counted under optical microscopy. The average count number was used for quantitation and comparison between groups. Respective numbers of Bcl-2- and Bax-positive cells show that nicotiflorin decreases Bax immunoreactivity and increases Bcl-2 immunoreactivity. *P < 0.05, **P < 0.01, vs. I/R group (mean ± SEM, n = 3, one-way analysis of variance followed by post hoc Tukey test). I/R: Ischemia/reperfusion.
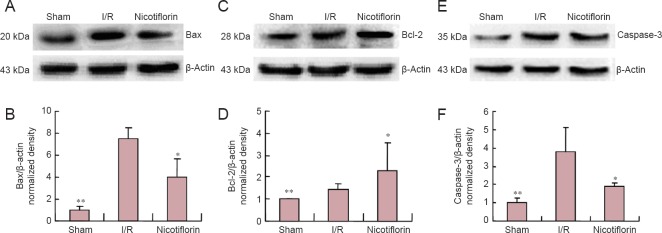
To further examine this anti-apoptotic effect of nicotiflorin, caspase-3, Bax, and Bcl-2 expression was examined by western blot assay in brain tissue after ischemia. Bax and Bcl-2 confirmed the changes observed by immunohistochemistry. Following transient MCAO and 24 hours of reperfusion in the I/R group, increased Bax and caspase-3 expression, and relatively less Bcl-2 expression was detected. However, in the nicotiflorin group, decreased Bax and caspase-3, and increased Bcl-2 expression was observed (P < 0.05 or P < 0.01, n = 3; Figure 5).
Figure 5.
Nicotiflorin effect on Bcl-2, Bax, and caspase-3 expression in the ischemic cerebral cortex of I/R rats (western blot assay).
(A, C, E) Western blot images of Bax, Bcl-2, and caspases-3 protein; (B, D, F) Western blot assay shows up-regulation of caspase-3 and Bax expreesssion and down-regulation of Bcl-2 expression in the I/R group (*P < 0.05, **P < 0.01, vs. I/R group). Both Bax and caspase-3 expression were decreased and Bcl-2 expreesssion increased in the nicotiflorin group. Data are expressed as the mean ± SEM, n = 3, one-way analysis of variance followed by post hoc Tukey test. I/R: Ischemia/reperfusion.
Discussion
Neuronal damage includes both apoptosis and necrosis (Charriaut-Marlangue et al., 1996; Sugawara et al., 2004; Poon et al., 2010). Cerebral ischemia leads to irreversible neuronal injury in the ischemic core area within minutes of onset (Lavrik et al., 2005; Uyttenboogaart et al., 2009; Murray et al., 2010; Jiang et al., 2016). While in the infarct penumbra area, reversible apoptosis is the main cell death mechanism, and is closely related to the final infarct area (Olsen et al., 1983; Dirnagl et al., 1999; Lo, 2008; Popp et al., 2009; Deng et al., 2016). Promisingly, reversible apoptosis provides multiple opportunities for therapeutic intervention in ischemic stroke (Nakka et al., 2008; Broughton et al., 2009). Consequently, we examined the effect of nicotiflorin on neuronal apoptosis in I/R rats. TUNEL staining was chosen as an indicator of apoptotic neurons. Our results show significantly decreased apoptosis in the nicotiflorin group. In addition, hematoxylin-eosin staining showed that nicotiflorin reduces pathological neuronal injury and the number of dead neurons induced by cerebral I/R. This is the first time the effect of nicotiflorin on neuronal apoptosis has been studied.
Apoptotic signal transduction mechanisms play a vital role (Chong et al., 2010; Wang et al., 2015). The JAK/STAT pathway is a critical human disease target (Ghoreschi et al., 2009; Babon et al., 2014; Tao et al., 2015; Chai et al., 2016). Within this family, JAK2/STAT3 is closely associated with ischemia-induced neuronal apoptosis and cancer cell apoptosis (Satriotomo et al., 2006; Xie et al., 2007; Du et al., 2012; Zhang et al., 2015). AG490, a potent JAK2 phosphorylation inhibitor, blocks post-ischemic JAK2 and STAT3 phosphorylation, and significantly decreases cerebral infarction, cell apoptosis, and neurological dysfunction (Satriotomo et al., 2006). We previously found that nicotiflorin is protective against brain injury and neuroinflammation by inhibiting STAT3 activation (Yu et al., 2013). Whether nicotiflorin prevents apoptosis and influences ischemic outcome via the JAK2/STAT3 pathway is not known. In the present study, we examined JAK2 and STAT3 phosphorylation in post-ischemic rat brain by western blot assay, in accordance with previous studies (Gorina et al., 2005; Satriotomo et al., 2006). Consistent with previous AG490 findings, we found nicotiflorin blocked post-ischemic JAK2 and STAT3 phosphorylation. Therefore, our results suggest that the protective effect of nicotiflorin against brain damage is related to the JAK2/STAT3 pathway.
To further understand the downstream anti-apoptotic mechanism of nicotiflorin in cerebral I/R injury, we also examined Bax, Bcl-2, and caspase-3 expression. Undoubtedly, both extrinsic and intrinsic pathways are responsible for activation of apoptosis (Gorina et al., 2005; Venderova and Park, 2012). Internally, cerebral ischemia increases intracellular calcium levels, activates calpains, and mediates cleavage of Bid to truncated Bid. Truncated Bid interacts with apoptotic proteins, such as Bax, which generally maintains balance with the anti-apoptotic protein, Bcl-2 (Hassan et al., 2014). Mitochondrial transition pores are opened and release cytochrome c or apoptosis-inducing factor to activate caspase-9, and subsequently caspase-3, which leads to nuclear DNA damage and apoptosis (Mattson and Kroemer, 2003; Mishra and Kumar, 2005; Taylor et al., 2008; Broughton et al., 2009). Our results show significantly increased expression of the main pro-apoptotic proteins, Bax and caspase-3, in the ipsilateral cortical penumbra. Encouragingly, nicotiflorin strongly inhibits their expression. Alternatively, the anti-apoptotic protein, Bcl-2, was significantly increased in the nicotiflorin group compared with the I/R group. These findings provide improved understanding of the cerebral protective mechanism of nicotiflorin.
In conclusion, nicotiflorin ameliorates cortical neuronal shape and structure, and also decreases the number of apoptotic neurons. Further, this protective mechanism involves the JAK2/STAT3 signaling pathway. Our findings offer further theoretical evidence for nicotiflorin as a potential therapeutic drug for ischemic stroke. Of course, other possible mechanisms need to be further studied.
Acknowledgments
We would like to thank Drug Discovery Research Center of Southwest Medical University of China for providing technical platform.
Footnotes
Funding: This study was financially supported by the Natural Science Foundation of Education Department of Sichuan Province of China, No. 14ZB0152; the Joint Research Program of Luzhou and Southwest Medical University, in China, No. 14JC0120.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by James R, Hindle A, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- Akbik F, Hirsch JA, Cougo-Pinto PT, Chandra RV, Simonsen CZ, Leslie-Mazwi T. The evolution of mechanical thrombectomy for acute Stroke. Curr Treat Options Cardiovasc Med. 2016;18:32. doi: 10.1007/s11936-016-0457-7. [DOI] [PubMed] [Google Scholar]
- ArunaDevi R, Ramteke VD, Kumar S, Shukla MK, Jaganathan S, Kumar D, Sharma AK, Tandan SK. Neuroprotective effect of s-methylisothiourea in transient focal cerebral ischemia in rat. Nitric Oxide. 2010;22:1–10. doi: 10.1016/j.niox.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 2014;462:1–13. doi: 10.1042/BJ20140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani P, Schellenberger E, Klohs J, Steinbrink J, Cordell R, Zille M, Muller J, Harhausen D, Hofstra L, Reutelingsperger C, Farr TD, Dirnagl U, Wunder A. Visualization of cell death in mice with focal cerebral ischemia using fluorescent annexin A5, propidium iodide, and TUNEL staining. J Cereb Blood Flow Metab. 2011;31:1311–1320. doi: 10.1038/jcbfm.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee TK, Das SK. Fifty years of stroke researches in India. Ann Indian Acad Neurol. 2016;19:1–8. doi: 10.4103/0972-2327.168631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Chai HT, Yip HK, Sun CK, Hsu SY, Leu S. AG490 suppresses EPO-mediated activation of JAK2-STAT but enhances blood flow recovery in rats with critical limb ischemia. J Inflamm (Lond) 2016;13:18. doi: 10.1186/s12950-016-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab. 1996;16:186–194. doi: 10.1097/00004647-199603000-00002. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3:153–165. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Koo JS, Jung KH, Kim EH, Sinn DI, Kim JM, Ko SY, Kim SJ, Song EC, Kim M, Roh JK. Peroxisome proliferator-activated receptor-gamma-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res. 2006;1093:208–218. doi: 10.1016/j.brainres.2006.03.114. [DOI] [PubMed] [Google Scholar]
- Deng YH, He HY, Yang LQ, Zhang PY. Dynamic changes in neuronal autophagy and apoptosis in the ischemic penumbra following permanent ischemic stroke. Neural Regen Res. 2016;11:1108–1114. doi: 10.4103/1673-5374.187045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Du W, Hong J, Wang YC, Zhang YJ, Wang P, Su WY, Lin YW, Lu R, Zou WP, Xiong H, Fang JY. Inhibition of JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via mitochondrial pathway. J Cell Mol Med. 2012;16:1878–1888. doi: 10.1111/j.1582-4934.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YH, Zhu ZH, Wu CX, Zhou GP. Effects of electroacupuncture at points selected by orthogonal experiment on the extracellular signal regulated kinase signal pathway in a rat model of cerebral ischenia-reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:5953–5958. [Google Scholar]
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N, Yuan X, Turenius CI, Tone B, Ambadipudi K, Snyder EY, Obenaus A, Ashwal S. Automated core-penumbra quantification in neonatal ischemic brain injury. J Cereb Blood Flow Metab. 2012;32:2161–2170. doi: 10.1038/jcbfm.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina R, Petegnief V, Chamorro A, Planas AM. AG490 prevents cell death after exposure of rat astrocytes to hydrogen peroxide or proinflammatory cytokines: involvement of the Jak2/STAT pathway. J Neurochem. 2005;92:505–518. doi: 10.1111/j.1471-4159.2004.02878.x. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Donnan GA, Gorelick PB, Hacke W, Cramer SC, Kaste M, Fisher M, Brainin M, Buchan AM, Lo EH, Skolnick BE, Furie KL, Hankey GJ, Kivipelto M, Morris J, Rothwell PM, Sacco RL, Smith SC, Jr, Wang Y, Bryer A, et al. Stroke: working toward a prioritized world agenda. Stroke. 2010;41:1084–1099. doi: 10.1161/STROKEAHA.110.586156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JM, Chen YB, Jonas EA. Multipolar functions of BCL-2 proteins link energetics to apoptosis. Trends Cell Biol. 2012;22:318–328. doi: 10.1016/j.tcb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014. 2014 doi: 10.1155/2014/150845. 150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hou SK, Hao LN, Wei J, Zhou YJ. Propofol pretreatment combined with umbilical blood mesenchymal stem cell transplantation improves cerebral ischemia-reperfusion injuries. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:2810–2816. [Google Scholar]
- Huang JL, Fu ST, Jiang YY, Cao YB, Guo ML, Wang Y, Xu Z. Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmacol Biochem Behav. 2007;86:741–748. doi: 10.1016/j.pbb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Jiang CJ, Wang ZJ, Zhao YJ, Zhang ZY, Tao JJ, Ma JY. Erythropoietin reduces apoptosis of brain tissue cells in rats after cerebral ischemia/reperfusion injury: a characteristic analysis using magnetic resonance imaging. Neural Regen Res. 2016;11:1450–1455. doi: 10.4103/1673-5374.191219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Liu L, Zhang S, Nanda A, Li G. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013;6:834–851. doi: 10.1007/s12265-013-9508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvansakul M, Hinds MG. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis. 2013;4:e909. doi: 10.1038/cddis.2013.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118:265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Wadley VG. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314:41–51. doi: 10.1001/jama.2015.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Li R, Guo M, Zhang G, Xu X, Li Q. Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells. J Ethnopharmacol. 2006;107:143–150. doi: 10.1016/j.jep.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, Yang GY. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–156. doi: 10.1016/j.pneurobio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Neurological diseases: Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Mishra NC, Kumar S. Apoptosis: a mitochondrial perspective on cell death. Indian J Exp Biol. 2005;43:25–34. [PubMed] [Google Scholar]
- Murphy J. Pharmacological treatment of acute ischemic stroke. Crit Care Nurs Q. 2003;26:276–282. doi: 10.1097/00002727-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Murray V, Norrving B, Sandercock PA, Terent A, Wardlaw JM, Wester P. The molecular basis of thrombolysis and its clinical application in stroke. J Intern Med. 2010;267:191–208. doi: 10.1111/j.1365-2796.2009.02205.x. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Aihara M, Chen YN, Araie M, Tomita-Yokotani K, Iwashina T. Neuroprotective effects of flavonoids on hypoxia-, glutamate-, and oxidative stress-induced retinal ganglion cell death. Mol Vis. 2011;17:1784–1793. [PMC free article] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- Nita DA, Nita V, Spulber S, Moldovan M, Popa DP, Zagrean AM, Zagrean L. Oxidative damage following cerebral ischemia depends on reperfusion-a biochemical study in rat. J Cell Mol Med. 2001;5:163–170. doi: 10.1111/j.1582-4934.2001.tb00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14:332–341. doi: 10.1161/01.str.14.3.332. [DOI] [PubMed] [Google Scholar]
- Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010;17:381–397. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 2009;4:e4764. doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 2006;98:1353–1368. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Noshita N, Kim GW, Saito A, Hayashi T, Narasimhan P, Maier CM, Chan PH. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx. 2004;1:17–25. doi: 10.1602/neurorx.1.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5:8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Cheng M, Wang SC, Lv W, Hu HQ, Li CF, Cao BZ. JAK2/STAT3 pathway mediating inflammatory responses in heatstroke-induced rats. Int J Clin Exp Pathol. 2015;8:6732–6739. [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Troy CM, Jean YY. Caspases: therapeutic targets in neurologic disease. Neurotherapeutics. 2015;12:42–48. doi: 10.1007/s13311-014-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenboogaart M, De Keyser J, Luijckx GJ. Thrombolysis for acute ischemic stroke. Curr Top Med Chem. 2009;9:1285–1290. doi: 10.2174/156802609789869655. [DOI] [PubMed] [Google Scholar]
- Venderova K, Park DS. Programmed cell death in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a009365. doi: 10.1101/cshperspect.a009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DB, Kinoshita C, Kinoshita Y, Morrison RS. p53 and mitochondrial function in neurons. Biochim Biophys Acta. 2014;1842:1186–1197. doi: 10.1016/j.bbadis.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Yang ZT, Liu C, He YH, Zhao SS. L-carnosine inhibits neuronal cell apoptosis through signal transducer and activator of transcription 3 signaling pathway after acute focal cerebral ischemia. Brain Res. 2013;1507:125–133. doi: 10.1016/j.brainres.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Wang PF, Xiong XY, Chen J, Wang YC, Duan W, Yang QW. Function and mechanism of toll-like receptors in cerebral ischemic tolerance: from preconditioning to treatment. J Neuroinflammation. 2015;12:80. doi: 10.1186/s12974-015-0301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie HF, Xu RX, Wei JP, Jiang XD, Liu ZH. P-JAK2 and P-STAT3 protein expression and cell apoptosis following focal cerebral ischemia-reperfusion injury in rats. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:208–211. 218. [PubMed] [Google Scholar]
- Xu M, Zhang HL. Death and survival of neuronal and astrocytic cells in ischemic brain injury: a role of autophagy. Acta Pharmacol Sin. 2011;32:1089–1099. doi: 10.1038/aps.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Chen C, Wang LF, Kuang X, Liu K, Zhang H, Du JR. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-kappaB and STAT3 in transient focal stroke. PLoS One. 2013;8:e55839. doi: 10.1371/journal.pone.0055839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidat OO, Lazzaro MA, Liebeskind DS, Janjua N, Wechsler L, Nogueira RG, Edgell RC, Kalia JS, Badruddin A, English J, Yavagal D, Kirmani JF, Alexandrov AV, Khatri P. Revascularization grading in endovascular acute ischemic stroke therapy. Neurology. 2012;79:S110–116. doi: 10.1212/WNL.0b013e3182695916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevallos J, Santiago F, Gonzalez J, Rodriguez A, Pericchi L, Rodriguez-Mercado R, Nobo U. Burden of stroke in puerto rico. Int J Stroke. 2015;10:117–119. doi: 10.1111/ijs.12350. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Kuang S, Wang Y, Sun XX, Gu Y, Hu LH, Yu Q. Bigelovin inhibits STAT3 signaling by inactivating JAK2 and induces apoptosis in human cancer cells. Acta Pharmacol Sin. 2015;36:507–516. doi: 10.1038/aps.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]