Abstract
Inbreeding adversely affects fitness, whereas heterozygosity often augments it. Therefore, mechanisms to avoid inbreeding and increase genetic distance between mates should be advantageous in species where adult relatives reside together. Here we investigate mate choice for genetic dissimilarity in chimpanzees, a species in which many females avoid inbreeding through dispersal, but where promiscuous mating and sexual coercion can limit choice when related adults reside together. We take advantage of incomplete female dispersal in Gombe National Park, Tanzania to compare mate choice for genetic dissimilarity among immigrant and natal females in two communities using pairwise relatedness measures in 135 genotyped chimpanzees. As expected, natal females were more related to adult males in their community than were immigrant females. However, among 62 breeding events, natal females were not more related to the sires of their offspring than immigrant females, despite four instances of close inbreeding. Moreover, females were generally less related to the sires of their offspring than to non-sires. These results demonstrate that chimpanzees may be capable of detecting relatedness and selecting mates on the basis of genetic distance.
Keywords: inbreeding avoidance, mate choice, relatedness, kin recognition
1. Introduction
Inbreeding depression, a reduction of fitness in the offspring of closely related mates, is a widely documented phenomenon thought to result largely from the homozygous expression of recessive deleterious alleles [1–3]. Inbreeding can adversely affect fitness through numerous pathways, notably via early death or reproductive disadvantage [3]. In contrast, fitness is often positively correlated with increased heterozygosity, whether genome wide or at specific loci [4,5]. Selection should, therefore, favour the evolution of mechanisms to avoid inbreeding and promote mate selection of genetically different individuals, especially in species that live at high population densities or in social groups, and where the risk of encountering relatives is high [6,7].
Several potential mechanisms exist for inbreeding avoidance and mate choice. In many animals, the likelihood of close inbreeding is reduced via dispersal of individuals of one sex before breeding [8–11], but in some cases, close relatives continue to reside together as adults. In such instances, there is increasing evidence of behavioural avoidance of inbreeding where closely related pairs avoid mating altogether (e.g. elephants [12]; white-faced capuchins [13]) or tend only to mate when the likelihood of conception is low (e.g. macaques [14]). Kin recognition in such cases may be based on prior association and/or phenotype matching [6,15]. In other species, where relatives reside together as adults, mating among relatives sometimes occurs but offspring born to closely related parents are rare (e.g. blue tit [16]; hihi [17]). Post-copulatory mechanisms may account for this disparity, as illustrated by laboratory studies where females mate in short succession with a related and an unrelated male, but most offspring are fathered by the unrelated mate (e.g. field crickets [18]; agile antechinus [19]; house mice [20]).
A growing number of studies have also found evidence for mate choice for overall genetic dissimilarity in wild populations where the opportunity to encounter relatives is high. In highly philopatric banner-tailed kangaroo rats [21], Cunningham's skinks [22] and bushtail possums [23], breeding pairs are generally more distantly related than non-breeding pairs. Similarly, in greater sac-winged bats [24] and several bird species (e.g. reed buntings [25], red-backed fairy wrens [26], white-rumped swallows [27]), females breed with extra-pair or group males when those individuals are less related to them than their social mate or when their social mate is genetically similar. However, this phenomenon does not seem to be a general trend across species, even when the chance of inbreeding is high (e.g. Atlantic salmon [28], American pika [29], white-toothed shrews [30]), and recent meta-analyses in birds found no indication that relatedness differed between females and their social mate or their extra-pair mate [31,32]. Moreover, in some species, breeding pairs are more related than non-breeding pairs (e.g. pigeons [33]). While the mechanisms are unknown, choice for genetic dissimilarity probably depends on processes similar to those employed to avoid inbreeding.
In species in which females invest heavily in each offspring through gestation/incubation, lactation/provisioning and/or parental care, each offspring represents a significant proportion of a female's lifetime reproductive output such that the selection of a genetically different sire should be particularly important. If, in such species, the opportunity to mate with relatives is also elevated (e.g. through philopatry, promiscuity and/or sociality), there should be strong selection for multiple mechanisms that reduce close inbreeding and promote mating between unrelated pairs.
Chimpanzees (Pan troglodytes) have several features that are likely to favour the evolution of strong mate choice mechanisms for genetic dissimilarity. They have a very slow reproductive schedule, investing heavily in a single offspring at a time, and only giving birth approximately every 5 years [34]. Although most females disperse to other social groups before breeding, some remain in their natal group with their male relatives [35]. Long lifespans also result in males and some females residing as adults in the same social group as their opposite-sexed parents. Chimpanzees are highly promiscuous; during a female's sexual cycle, she mates multiply with most or all of the males in her community [36]. Preliminary evidence from both captive and wild populations suggests inbred offspring suffer high mortality [37,38]. Under these conditions, inbreeding should not be tolerated [7]. However, among eastern chimpanzees (Pan troglodytes schweinfurthii), males use sexual coercion to influence female mating patterns, probably restricting female choice [39–41]. The socioecology and mating patterns of chimpanzees thus put them at risk of conceiving with a close relative and potentially limit female choice for dissimilar mates. Therefore, mechanisms that reduce the chances of inbreeding and favour genetically dissimilar mates should be particularly important in this species.
Behavioural avoidance of mating with close relatives has been documented in chimpanzees but is more complete among some classes of relatives, identified by pedigree information, than others. Mother–son mating is rare across sites but has occasionally been observed [42–45], and the only offspring that has been identified as resulting from closely related parents in any study population came from a mother–son pairing [38]. Avoidance of mating between maternal siblings also occurs in some populations [42,45] but not others [46]. Such avoidance of maternal relatives is consistent with kin recognition by prior association [6]. The degree to which mating between paternal relatives occurs is less well known. In the only study to examine this, mating frequency between father–daughter dyads was surprisingly high, after accounting for male age and other known correlates of mating, and was primarily driven by male behaviour [47]. Daughters resisted the mating attempts of fathers at higher rates than those of unrelated males. There is some evidence for preferential interaction between males and their infant and juvenile offspring in this species [48,49], but the degree to which preferential interaction and/or association persists past juvenility is uncertain. Therefore, some form of phenotype matching may be involved in the identification of fathers in adulthood. In another study, dyadic relatedness calculated from microsatellite data was negatively related to mating frequency but mating did occur in most dyads [41]. Taken together, these patterns suggest that there is some recognition of relatedness which influences mating patterns in this species but that mating tactics may constrain choice for unrelated partners. As yet, the consequences of these patterns for the parentage of infants have not been examined. That is, the extent to which inbreeding is successfully avoided or genetic dissimilarity is favoured is not yet known.
Here we take advantage of differing demographic characteristics in two eastern chimpanzee communities in Gombe National Park, Tanzania to investigate patterns of mate selection and the factors influencing these by examining the parentage of 64 infants. The two communities (Kasekela and Mitumba) differ in size, number of adult males and the proportion of females that disperse. The larger Kasekela community is unusual in that about 50% of females breed in their natal community [50], whereas in Mitumba, 83% of females have dispersed, and the community currently contains only one natal female. First, we ask how effectively inbreeding is avoided by examining the incidence of moderate and close inbreeding based on known pedigree information. Second, we test our expectation based on sex-biased dispersal patterns that natal females are more closely related to resident males, and therefore at higher risk of inbreeding than immigrant females. Third, we examine the extent to which natal females are able to avoid inbreeding by testing whether they are more closely related than immigrant females are to the sires of their offspring. Last, we test the hypothesis that, because of the general advantages of heterozygosity, there will be overall mate choice for genetic dissimilarity whereby breeding dyads are less related than non-breeding dyads.
2. Methods
2.1. Study site
Gombe National Park, in western Tanzania, has an area of 35 km2 and contains three chimpanzee communities. The chimpanzees of the Kasekela and Mitumba communities have been habituated and observed almost daily since 1966 and 1995, respectively [51], and the dispersal patterns of all females born into or entering these communities are known. Individuals in the Kalande community are known genetically from faecal sampling [52]. All individuals surviving beyond 2 years of age and alive between 1995 and 2012 in Kasekela and 2004 and 2012 in Mitumba have been genotyped. Within these periods, the Kasekela community included 12–25 adult females (age 12+ years) and 10–12 adolescent and adult males (age 12+ years), and the Mitumba community included five to nine adult females and two to five adolescent and adult males.
Since the 1990s, the park has been isolated from other forested areas and is primarily surrounded by anthropogenic landscapes, potentially limiting dispersal opportunities [53]. However, healthy females occasionally disappear from the park shortly after sexual maturity and females of unknown provenance sometimes immigrate into communities in the park. In the last 5 years, two immigrant females have been determined to originate from outside the park; they were never observed in Mitumba or Kasekela and their genotypes did not match any known chimpanzee, including those genetically sampled in the Kalande community. A chimpanzee community of at least 20 individuals is known to reside approximately 12 km north of the park (D. Mjungu 2016, personal communication). Although this community is likely to be a potential source and destination for dispersing females, it is highly unlikely that offspring born within the park are sired by males outside the park, because extra-group paternities are rare in chimpanzees [38,54–57] and, in this case, would require significant travel across risky landscapes by within-park females.
2.2. Genotyping, paternity and relatedness
Included in this study were 153 genotyped chimpanzees from the Kasekela, Mitumba and Kalande communities [38,52,56,58]. Most individuals (98.6%) were typed at 8–11 microsatellite loci, whereas two individuals were typed at 4–5 loci (electronic supplementary material, figure S1a). More information on sample collection, DNA extraction and microsatellite genotyping (primarily from faeces) is provided in the electronic supplementary material.
Paternities for 37 offspring born into the Kasekela community had previously been reported (including one set of non-identical twins (GLI, GLD) whose paternity was tested individually) [38,52,56,58]. For this study, we repeated the analysis for these 37 paternities and we also report paternities for 28 new individuals born into the Kasekela and Mitumba communities. This provides a total sample size of 65 offspring with known paternities: 52 in the Kasekela community and 13 in the Mitumba community (electronic supplementary material, figure S2). All infants had known mothers that were also genotyped, and we had good sampling of candidate males, with genotypes for a mean of 97.2% of candidate males from within the community, and 91.6% of males between the two habituated communities (KK and MT; electronic supplementary material, figure S2). Ten is the youngest age documented for a chimpanzee sire in the wild [59], whereas 11 is the youngest age of any sire at Gombe (2015, unpublished data). All males at least 9 years of age at the time of conception that were resident in the mother's community (either Kasekela or Mitumba) were genotyped for 57 conceptions (87.7%), and all males were sampled from both communities for 41 (63.1%) conceptions (electronic supplementary material, figure S2). Fathers were first identified using the exclusion principle and confirmed with likelihood methods using the program Cervus [60]. Detailed information on the paternity assignment procedure is included in the electronic supplementary material.
2.2.1. Pedigrees and inbreeding
We classified offspring as inbred if the father was related to the mother at the level of half-siblings (R = 0.25) or above based on known pedigrees. Using known pedigrees combined with genetic paternity information, we can detect any inbreeding between parent–offspring dyads, full siblings and maternal half-siblings for all genotyped offspring but can detect inbreeding between paternal siblings in only 53 of 65 offspring. Because extra-group paternity is rare in chimpanzees [38,54–57], we assumed that sires of offspring born to immigrant mothers were unrelated to the mother. Among 32 offspring born to natal mothers, 10 were born to parents where the father of both the mother and the sire was unknown, preventing us from determining if they were paternal siblings. Two mothers in Mitumba were present as adults at the time of habituation and their provenance is unknown (i.e. natal or immigrant); to be conservative, the offspring born to these mothers were also excluded from the determination of rates of inbreeding among paternal siblings (n = 2). A lack of generational depth in the pedigree prevented a systematic analysis of moderate inbreeding between other relatives related at the 0.25 level (i.e. grandmothers and grandsons, aunts and nephews, etc.) [61].
2.2.2. Pairwise relatedness measures
We used Co-ancestry to calculate pairwise relatedness [62]. In this programme, allele frequencies were calculated once from the same set of microsatellite genotypes (8–11 loci) of 135 individuals from the Kasekela and Mitumba communities (electronic supplementary material, figure S1c). Bias can result from using the same markers to determine paternity and calculate relatedness [63–65]; however, our microsatellite data could not be split into two subsets as recommended, because the full set is necessary to determine paternity. Nonetheless, the high degree of polymorphism and heterozygosity of our markers reduces the potential for bias [63] (electronic supplementary material, figure S1a,c) and we found that when we calculated R-values based on different allele frequency subsets as recommended by Wang [65], values were highly correlated with the full dataset (see electronic supplementary material).
To facilitate comparison with previous work on genetic relatedness in chimpanzees [54,55,66], we used the Queller and Goodnight pairwise relatedness estimator [67] to compare relatedness between classes of dyads. We found that estimates of pairwise relatedness from this method correlated highly with kinship coefficients calculated between dyads of known parentage confirmed through paternity analysis and pedigree information (n = 70) using the KINSHIP2 package in R (see electronic supplementary material, table S1 and figures S3,S4) [68]. These estimates were also correlated with six other pairwise estimators calculated in Co-ancestry [62] (see electronic supplementary material, table S1) including the more recent triadic likelihood (TL) method [69], which allows for inbreeding, weights loci based on their informativeness and accounts for typing errors. We repeated our analysis using the TL method and found similar results (see electronic supplementary material, figures S5–S7).
2.3. Definitions and inclusion criteria
2.3.1. Females and offspring
All females present in each community, aged 12 years or over and encountered in more than 10% of male focal follows (i.e. resident rather than peripheral community members [70]) were considered adults for relatedness analyses and were categorized as either natal (nKasekela = 15, nMitumba = 1) or immigrant (nKasekela = 20, nMitumba = 9) depending on their community of birth. Two females in Mitumba were of unknown provenance (they were present in the community when observations began) and were excluded from analyses in which females of different residence status were compared. Females that did not give birth (nKasekela = 8, nMitumba = 3) or have offspring genotyped (nKasekela = 3, nMitumba = 1) were excluded from analyses that required the use of breeding females. For the purposes of this study, the conception of twins was treated as a single event because they were sired by the same male (electronic supplementary material, figure S2). This leads to a total of 64 breeding events (51 in Kasekela and 13 in Mitumba).
2.3.2. Males
All males 12 years or older and present in the community during the study period were considered adults for relatedness analyses (nKasekela = 24, nMitumba = 9). All males were born in the community in which they resided as adults except one (BE) that immigrated into Kasekela with a female assumed to be his sister at approximately 4 years of age [36]. The dominance rank of each male on the estimated date of conception of each infant [36,71] was given by his Elo score, calculated from the occurrence of submissive pant grunts [72]. In Mitumba, rank was only known for the alpha male, preventing a systematic analysis of rank and relatedness in this community.
2.4. Analyses of pairwise relatedness
2.4.1. Female–male dyadic relatedness
To test for differences in the degree of genetic relatedness between natal and immigrant females with resident males, we calculated and compared average relatedness between all immigrant female–male dyads (n = 388) with average relatedness between all natal female–male dyads (n = 279) that overlapped as adults in the Kasekela community. This analysis was restricted to the Kasekela community, beause Mitumba had only one natal female (with no infant). Differences between natal female–male dyads and immigrant female–male dyads were tested for significance using a permutation procedure that compares the normalized difference between means from 10 000 random pairs of replicates from the pooled dataset using JMP v. 11 [73].
2.4.2. Relatedness of natal and immigrant breeding dyads
Relatedness between immigrant mothers and sires of 30 offspring (19 in Kasekela and 11 in Mitumba) was compared with relatedness between natal mothers and sires of 32 offspring (all in Kasekela) in two ways: (i) including both communities and (ii) restricting analysis to just Kasekela since, as above, Mitumba had only one natal female (with no infant). Differences between groups were tested using the same permutation procedure explained above.
2.4.3. Relatedness of breeding and non-breeding dyads
Relatedness between breeding and non-breeding dyads was examined by comparing the relatedness between the mother and sire of an infant (breeding dyads) with the relatedness between the mother and each other adult male present in the community at the conception of her infant (non-breeding dyads) [36,71]. We tested each community separately, using 50 offspring in the Kasekela community and 11 offspring in the Mitumba community. One offspring born to a transient mother in Kasekela (KAR) and two offspring born in Mitumba prior to 2004 (APL and LAM) were excluded from this analysis because not all candidate sires were genotyped. Differences between groups were first compared using the same permutation procedure explained above. To determine if any observed difference was due to female residence status, we also compared breeding and non-breeding dyads among natal and immigrant females separately.
Male rank and age influences the probability of paternity [56]. Therefore, to be confident that selection of breeding partners was influenced by genetic distance, in addition to other known factors, we also ran a linear mixed model using the lme4 package [74] in R [75] controlling for male rank and age. Relatedness was the outcome variable and dyad type (breeding or non-breeding), male rank and male age were included as fixed effects, and male and female ID were both included as random effects. To investigate if breeding patterns varied by community, we ran a second linear mixed model where relatedness was the outcome variable, and dyad type, community and the interaction term community × dyad type were included as fixed effects. Male ID and Female ID were also included as random effects.
3. Results
3.1. Paternity and inbreeding
The fathers identified for all 37 offspring reported previously were confirmed here, with one exception (DIA). Typing at additional microsatellite loci resolved paternity between two maternal half-brothers for DIA, which we update here (electronic supplementary material, figure S2). Detailed information on paternity likelihood is included in the electronic supplementary materials. All 65 offspring, including 28 for which paternity is newly reported here, were sired by within-community males except for one (KAR), whose mother (TT) resided primarily in the southern Kalande community but conceived during a visit to Kasekela (table 1). KAR was also determined to be inbred, a product of breeding between maternal siblings. TT emigrated before her maternal brother was born and the conception occurred when she revisited her natal community after a sudden decrease in the adult male population in her transfer community (see the electronic supplementary material) [52].
Table 1.
Paternities and related demographic information for 65 offspring.
| community | offspring | mother | sire | mother's residence status | new paternity | inbred | status in 2016 (age at death in years) |
|---|---|---|---|---|---|---|---|
| Kasekela | CN | CD | WL | natal | |||
| Kasekela | COC | CD | FD | natal | |||
| Kasekela | DIA | DL | FO | natal | Yd | ||
| Kasekela | DUK | DL | TN | natal | Y | ||
| Kasekela | FO | FF | WL | natal | |||
| Kasekela | FI | FF | FR | natal | mother–son | dead (0.9) | |
| Kasekela | FE | FF | EV | natal | |||
| Kasekela | FLI | FF | KS | natal | |||
| Kasekela | FU | FN | SL | natal | |||
| Kasekela | FND | FN | SL | natal | |||
| Kasekela | FAM | FN | SL | natal | |||
| Kasekela | FAD | FN | WL | natal | |||
| Kasekela | FFT | FN | WL | natal | Y | ||
| Kasekela | GGL | GA | FO | natal | Y | paternal sibs | alive |
| Kasekela | GAb1 | GA | SL | natal | Y | ||
| Kasekela | GAb2 | GA | AO | natal | Y | ||
| Kasekela | GLA | GLD | FU | natal | Y | ||
| Kasekela | GLIb1 | GLI | SL | natal | Y | ||
| Kasekela | GD | GM | AL | natal | |||
| Kasekela | GA | GM | WL | natal | |||
| Kasekela | GLI/GLDa | GM | FR | natal | |||
| Kasekela | GIM | GM | TB | natal | |||
| Kasekela | GIZ | GM | FE | natal | Y | paternal sibsc | dead (6.3) |
| Kasekela | SR | SA | BE | natal | |||
| Kasekela | SN | SA | AO | natal | |||
| Kasekela | SAM | SA | FR | natal | |||
| Kasekela | SIR | SA | AO | natal | |||
| Kasekela | SAF | SI | AO | natal | Y | ||
| Kasekela | SHA | SR | WL | natal | |||
| Kasekela | TOM | TG | KS | natal | |||
| Kasekela | TAB | TG | FE | natal | Y | ||
| Kasekela | KAR | TT | TN | natalb | Y | maternal sibs | dead (approx. 2b) |
| Kasekela | BRZ | BAH | KS | immigrant | |||
| Kasekela | BAS | BAH | TN | immigrant | Y | ||
| Kasekela | ERI | EZA | KS | immigrant | |||
| Kasekela | EZAb1 | EZA | TN | immigrant | Y | ||
| Kasekela | IPO | IMA | WL | immigrant | Y | ||
| Kasekela | JK | JF | AL | Immigrant | |||
| Kasekela | KEA | KP | WL | immigrant | |||
| Kasekela | MAM | MAK | GL | immigrant | |||
| Kasekela | NYO | NUR | FU | immigrant | Y | ||
| Kasekela | TG | PI | GB | immigrant | |||
| Kasekela | TN | PI | FR | immigrant | |||
| Kasekela | TZN | PI | FR | immigrant | |||
| Kasekela | SI | SW | WL | immigrant | |||
| Kasekela | SDB | SW | FR | immigrant | |||
| Kasekela | TOF | TTA | SL | immigrant | |||
| Kasekela | ZS | TZ | FR | immigrant | |||
| Kasekela | ZEL | TZ | KS | immigrant | |||
| Kasekela | ZIN | TZ | GL | immigrant | |||
| Kasekela | YAM | YD | WL | immigrant | |||
| Mitumba | APL | AP | VIN | immigrant | Y | ||
| Mitumba | AND | AP | EDG | immigrant | Y | ||
| Mitumba | ARI | AP | RUD | immigrant | Y | ||
| Mitumba | MAY | DB | RUD | immigrant | Y | ||
| Mitumba | FLW | FS | RUD | immigrant | Y | ||
| Mitumba | FAL | FS | EDG | immigrant | Y | ||
| Mitumba | FED | FS | EDG | immigrant | Y | ||
| Mitumba | KOM | KON | RUD | immigrant | Y | ||
| Mitumba | LAM | LUC | VIN | immigrant | Y | ||
| Mitumba | LTT | LUC | EDG | immigrant | Y | ||
| Mitumba | MIS | MGA | EDG | immigrant | Y | ||
| Mitumba | EDE | EVA | VIN | unknown | Y | ||
| Mitumba | LOS | LOR | RUD | unknown | Y |
aTwins, confirmed to have the same father, treated as one conception.
bTT is transient, born in KK, currently resides in Kalande with occasional visits to KK such that age at death for KAR is approximate.
cStrong but not conclusive evidence that offspring is inbred.
dPaternity previously reported as FE.
Of the 28 new infants, two were the products of inbreeding (R ≥ 0.25 as determined from pedigree data) and strong evidence points to a third case (table 1). KAR was the offspring of maternal half-siblings (as discussed above); GGL and probably GIZ (see the electronic supplementary material) were the offspring of paternal half-siblings.
Offspring conceived by close relatives (R = 0.5; n = 1) accounted for 1.5% of all genotyped infants and 3% of those born to natal females. Offspring conceived by half-siblings (R = 0.25; n = 3) account for 5% of all genotyped infants and 12% of those born to natal females. We currently lack depth of pedigree to identify most offspring born to other relative classes at the 0.25 level; however, in the instances where such relationships were known, no inbreeding was detected.
Three of the four inbred infants died prior to attaining sexual maturity; two died around or prior to 2 years (FI and KAR) and the third (GIZ) died at 6 years. Importantly, none of these infants, nor their mothers, were positive for simian immunodeficiency virus (SIVcpz) infection, which is known to increase infant mortality [76].
3.2. Female–male dyadic relatedness
In Kasekela, adult female–adult male dyads (n = 667) had a mean relatedness of −0.009 (s.d. = 0.198). In accordance with relatedness patterns expected from sex-biased dispersal, natal females were more closely related to the males of their community (n = 279 dyads, mean R = 0.022, s.d. = 0.216) than immigrant females were (n = 388 dyads, mean R = −0.031, s.d. = 0.183; p < 0.001; figure 1).
Figure 1.
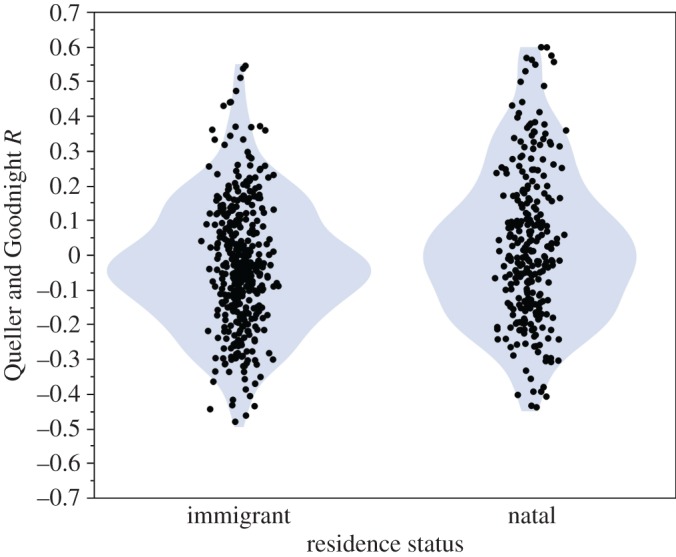
Relatedness between immigrant female–male dyads and natal female–male dyads in Kasekela. Natal female–male dyads are more closely related than are immigrant female–male dyads (p < 0.001).
3.3. Relatedness of natal and immigrant breeding dyads
Despite the fact that natal females were more closely related to resident males, natal females were not more closely related to the sires of their offspring (n = 32 dyads, mean R = −0.052) than were immigrant females (n = 30 dyads, mean R = −0.063; p = 0.81), including data from both communities. There was also no significant difference when analysis was restricted to dyads from the Kasekela community (natal breeding dyads; n = 32 dyads, mean R = −0.052; immigrant breeding dyads: n = 19 dyads, mean R = −0.101; p = 0.35).
3.4. Relatedness of breeding and non-breeding dyads
In the Kasekela community, breeding dyads including both natal and immigrant females (n = 50 dyads, mean R = −0.072) were significantly more distantly related than non-breeding dyads (n = 486 dyads, mean R = 0.000, p = 0.01; figure 2). This difference remained significant in a model that included the rank and age of the male (β = 0.07, t = 2.397, p = 0.017). A similar difference was found in the Mitumba community but did not reach significance (breeding dyads: n = 11 dyads, mean R = −0.017; non-breeding dyads: n = 16 dyads, mean R = 0.003; p = 0.81; figure 2). That the pattern generally applies to both communities is supported by the linear mixed model results, which shows that while the dyad effect remains significant when including both communities (β = 0.07, t = 2.40, p = 0.017), there is no significant effect of community (β = 0.04, t = 0.55, p = 0.58) or in the community × dyad type interaction (β = −0.05, t = −0.65, p = 0.51). To determine if the bias towards unrelated breeding partners was a general pattern or if it was driven by natal females avoiding close relatives, we compared dyads for immigrant and natal females in Kasekela separately (figure 3). For immigrant females, breeding dyads (n = 19, mean R = −0.101) were still significantly more distantly related than non-breeding dyads (n = 181 dyads, mean R = −0.018, p < 0.01) and, for natal females, there was a trend in the same direction (breeding dyads: n = 31, mean R = −0.049; non-breeding dyads: n = 305, mean R = 0.011, p = 0.10).
Figure 2.
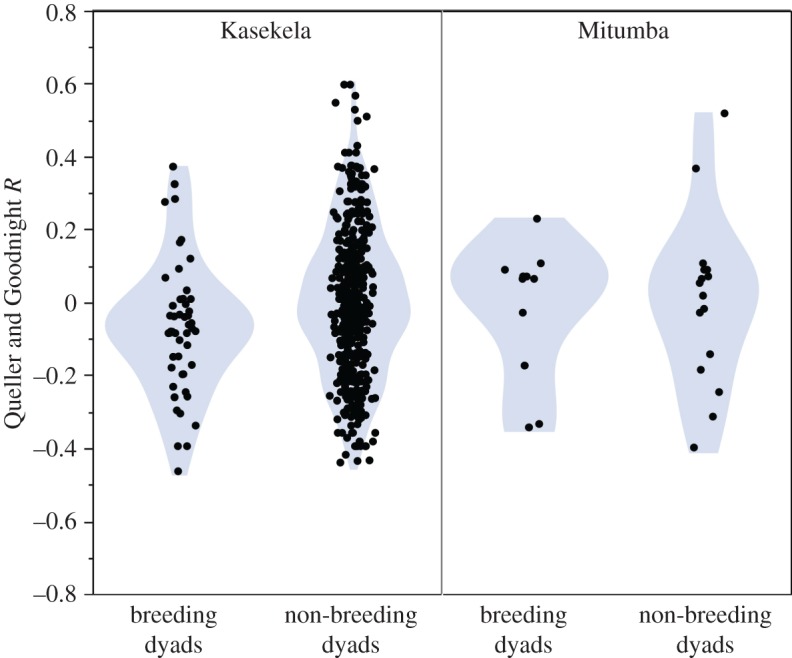
Relatedness between breeding and non-breeding dyads in Kasekela and Mitumba. In Kasekela, breeding dyads are less related than non-breeding dyads (p = 0.017).
Figure 3.
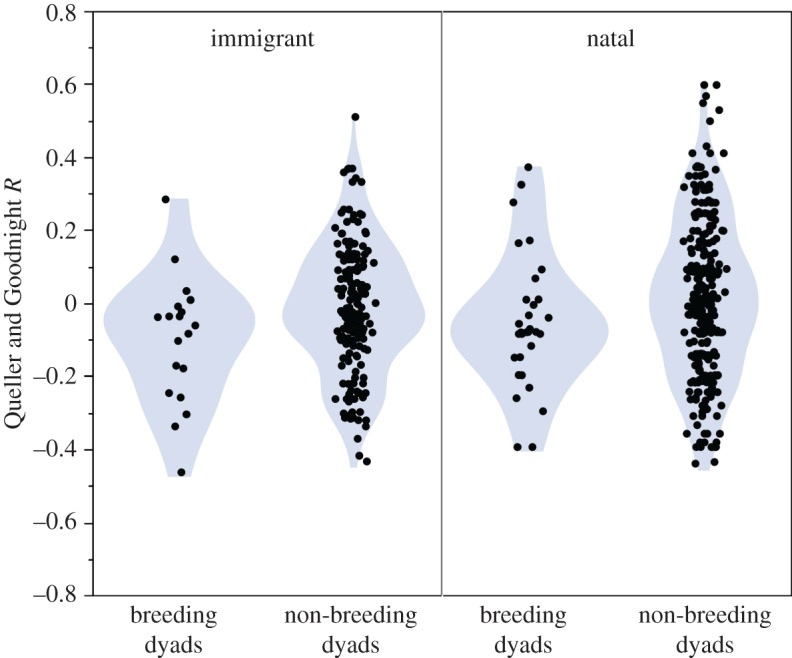
Relatedness between breeding and non-breeding dyads in Kasekela by residence status. Among immigrant females, breeding dyads are less related than non-breeding dyads (p < 0.01) and among natal females, there was a trend in the same direction (p = 0.10).
4. Discussion
Our results suggest that, as expected for a slowly reproducing species in which females invest heavily in each offspring and sometimes reside with close relatives, several mechanisms promote inbreeding avoidance and mate choice in chimpanzees. First, sex-biased dispersal reduced the relatedness between breeding adults in the community: immigrant females were more distantly related to the males in the community than were females that did not disperse. Second, although they often resided with close male relatives, natal females were not more closely related to the sires of their offspring than were immigrant females. Third, females in the Kasekela community were more distantly related to the sires of their offspring than to the other resident males. These latter two patterns provide evidence for the existence of mechanisms to detect genetic similarity among individuals and promote outbreeding.
Nonetheless, these systems are not perfect and we identified four cases of inbreeding; one between closely related parents and three between moderately related parents. Three of these occurred between relatives residing together. One resulted from a rare forced son–mother mating [38], whereas two occurred between females that did not disperse from their natal Kasekela community and their male paternal siblings. The fourth case occurred between maternal siblings under unusual circumstances in which the parents never resided together. We may have underestimated the prevalence of inbreeding in the population, because most infants that die prior to age two are not genotyped (19 of 23 offspring in Kasekela and 2 of 2 offspring in Mitumba); however, 15 of these infants were born to immigrant mothers and were unlikely to be inbred. Furthermore, we were only able to measure the extent of inbreeding between half-siblings and parents/offspring because, owing to the long lifespan of chimpanzees, our pedigree was not deep enough to accurately assess rates of other forms of inbreeding [61]. In the cases where we could identify grandparents/grandchildren and aunts/uncles/nieces/nephews, we found no evidence of inbreeding. Although the sample size is small, the outcome of the four cases of inbreeding is suggestive of inbreeding depression. Fifty per cent of inbred offspring died in the first 2 years and 75% died by age six, compared with an overall average infant mortality of 29% in the first 2 years and 40% in the first 6 years [77].
In support of selection for genetically dissimilar mates, we observed that breeding pairs were more distantly related than non-breeding pairs. Importantly, this result was not driven by natal females avoiding close relatives as breeding partners. Rather, we found a general bias among all females, including immigrant females, for unrelated mates. These patterns suggest that chimpanzees are able to detect degrees of genetic relatedness and breed with more distantly related individuals and do not simply avoid relatives with whom they have a history of association. While we found a strong effect of choice for genetic dissimilarity in Kasekela, the effect was diminished in the smaller Mitumba dataset, though when modelled together, no community difference was found. Nevertheless, it is possible that demographic differences between communities may influence patterns of mate choice. In small groups, more effective mate guarding by the alpha male may constrain female choice, whereas larger groups may provide more scope for alternative mating strategies such as consortships [56,78] and future work, with a larger sample, should clarify these effects. Mate choice for genetic dissimilarity is likely to confer fitness benefits on the resultant offspring and infant survival is, thus, expected to vary by the degree of relatedness between the parents. Here, we document high infant mortality in moderate and close inbreeding events, but future work should examine fitness effects across all degrees of relatedness.
An alternative explanation for the difference in relatedness between breeding and non-breeding pairs is that more genetically similar pairs do breed but that offspring born to these pairs suffer high infant mortality in the first 2 years and are, thus, generally not included in the genotyped sample. However, this explanation is unlikely, given that selection for dissimilar mates is more robust among immigrants, the class of females that faces the lowest risk of inbreeding. Moreover, the majority of non-genotyped infants were born to immigrant mothers and unlikely to be inbred. Nevertheless, when possible, efforts to genotype young infants should be undertaken to confirm the origin of this effect.
Our data indicate that genetic relatedness influences mate choice but cannot distinguish between female or male effort in such choice nor do they speak to the mechanisms involved in the detection of degrees of relatedness, both issues that merit further investigation. Choice of good genes is particularly important for mammalian females because of their investment in gestation and lactation [34,79,80]. Accordingly, female chimpanzees frequently do not respond to mating invitations from maternal male relatives and will often resist if a relative persists in a mating attempt [36,42,45]. However, males also contribute to inbreeding avoidance and, once past sexual maturity, rarely mate with their mothers and infrequently pursue maternal sisters [36,42,43,45].
Identifying the mechanisms that animals use to determine relatedness is an active area of research. In chimpanzees, prior association with the mother during the juvenile period is a likely mechanism for detection of maternal relatives [6,15,45] and it is striking that the only case of inbreeding between maternal siblings occurred in a pair that had no contact prior to adulthood. Pre-copulatory detection of paternal relatives or general genetic difference is more likely to depend on phenotype matching on the basis of such cues as odour similarity [81–84], or physical resemblance to known relatives [85,86]. In addition, because mating does occur between relatives, but infants rarely result, post-copulatory sperm selection or cryptic female choice like that detected in agile antechinuses and house mice and suspected to occur in mandrills may also be important [19,20,87].
In conclusion, we provide evidence that chimpanzees breed with genetically dissimilar mates and that inbreeding is uncommon even where opposite sex adult relatives reside together. Chimpanzees are probably sensitive to genetic distance in choosing a mate, although post-copulatory processes may also contribute to observed patterns of outbreeding. Such mechanisms should optimize genetic diversity in the resultant offspring and increase their fitness.
Supplementary Material
Acknowledgements
We thank Tanzania National Parks, the Tanzania Wildlife Research Institute and the Tanzanian Commission for Science and Technology for granting us permission to work on this project in Gombe National Park; the Gombe Stream Research Center staff for data collection; and Dr Jane Goodall for granting us permission to work with the long-term dataset. We thank M. Ramirez for expert technical assistance. We thank S. Alberts, J. Tung, A. Lea and N. Snyder-Mackler for comments on earlier drafts.
Ethics
This research adhered to all the laws and guidelines of Tanzania and was approved by the Institutional Animal Care and Use Committees at the University of Minnesota and Duke University.
Data accessibility
Genotypes and pairwise relatedness data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.d7g4c [88].
Authors' contributions
K.K.W., E.W. and A.E.P. conceived of and designed the study. R.S.R., Y.L. and B.H.H. carried out molecular labotaratory work. E.W. conducted paternity analysis. K.K.W. carried out statistical analyses. K.K.W., E.W. and A.E.P. drafted the manuscript. All authors gave their final approval for publication.
Competing interests
We have no competing interests.
Funding
We thank the Jane Goodall Institute for funding data collection at Gombe. Additional funding was provided by National Science Foundation grant nos. DBS-9021946, SBR-9319909, BCS-0452315, IOS-LTREB-1052693 and DGE-1106401, and by National Institutes of Health grant nos. R01 AI 058715, R01 AI 120810, P30 AI 045008 and R00 HD 057992.
References
- 1.Charlesworth D, Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18, 237–268. (doi:10.1146/annurev.es.18.110187.001321) [Google Scholar]
- 2.Charlesworth B, Charlesworth D. 1999. The genetic basis of inbreeding depression. Genet. Res. 74, 329–340. (doi:10.1017/S0016672399004152) [DOI] [PubMed] [Google Scholar]
- 3.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241. (doi:10.1016/S0169-5347(02)02489-8) [Google Scholar]
- 4.Chapman JR, Nakagawa S, Coltman DW, Slate J, Sheldon BC. 2009. A quantitative review of heterozygosity-fitness correlations in animal populations. Mol. Ecol. 18, 2746–2765. (doi:10.1111/j.1365-294X.2009.04247.x) [DOI] [PubMed] [Google Scholar]
- 5.Szulkin M, Bierne N, David P. 2010. Heterozygosity-fitness correlations: a time for reappraisal. Evolution 64, 1202–1217. (doi:10.1111/j.1558-5646.2010.00966.x) [DOI] [PubMed] [Google Scholar]
- 6.Pusey AE, Wolf M. 1996. Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206. (doi:10.1016/0169-5347(96)10028-8) [DOI] [PubMed] [Google Scholar]
- 7.Kokko H, Ots I. 2006. When not to avoid inbreeding. Evolution 60, 467–475. (doi:10.1111/j.0014-3820.2006.tb01128.x) [PubMed] [Google Scholar]
- 8.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. (doi:10.1016/S0003-3472(80)80103-5) [Google Scholar]
- 9.Pusey AE. 1987. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol. Evol. 2, 295–299. (doi:10.1016/0169-5347(87)90081-4) [DOI] [PubMed] [Google Scholar]
- 10.Clutton-Brock T. 1989. Female transfer and inbreeding avoidance in social mammals. Nature 337, 70–72. (doi:10.1038/337070a0) [DOI] [PubMed] [Google Scholar]
- 11.Perrin N, Mazalov V. 1999. Dispersal and inbreeding avoidance. Am. Nat. 154, 282–292. (doi:10.1086/303236) [DOI] [PubMed] [Google Scholar]
- 12.Archie EA, Hollister-Smith JA, Poole JH, Lee PC, Moss CJ, Maldonado JE, Fleischer RC, Alberts SC. 2007. Behavioural inbreeding avoidance in wild African elephants. Mol. Ecol. 16, 4138–4148. (doi:10.1111/j.1365-294X.2007.03483.x) [DOI] [PubMed] [Google Scholar]
- 13.Muniz L, Perry S, Manson JH, Gilkenson H, Gros-Louis J, Vigilant L. 2006. Father–daughter inbreeding avoidance in a wild primate population. Curr. Biol. 16, 156–157. (doi:10.1016/j.cub.2006.02.055) [DOI] [PubMed] [Google Scholar]
- 14.Manson JH, Perry SE. 1993. Inbreeding avoidance in rhesus macaques: whose choice?. Am. J. Phys. Anthropol. 90, 335–344. (doi:10.1002/ajpa.1330900307) [DOI] [PubMed] [Google Scholar]
- 15.Mateo JM, Johnston RE. 2000. Kin recognition and the ‘armpit effect’: evidence of self-referent phenotype matching. Proc. R. Soc. Lond. B 267, 1444, 695–700. (doi:10.1098/rspb.2000.1058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foerster K, Valcu M, Johnsen A, Kempenaers B. 2006. A spatial genetic structure and effects of relatedness on mate choice in a wild bird population. Mol. Ecol. 15, 4555–4567. (doi:10.1111/j.1365-294X.2006.03091.x) [DOI] [PubMed] [Google Scholar]
- 17.Brekke P, Wang J, Bennett PM, Cassey P, Dawson DA, Horsburgh GJ, Ewen JG. 2011. Postcopulatory mechanisms of inbreeding avoidance in the island endemic hihi (Notiomystis cincta). Behav. Ecol. 23, 278–284. (doi:10.1093/beheco/arr183) [Google Scholar]
- 18.Simmons LW, Beveridge M, Wedell N, Tregenza T. 2006. Postcopulatory inbreeding avoidance by female crickets only revealed by molecular markers. Mol. Ecol. 15, 3817–3824. (doi:10.1111/j.1365-294X.2006.03035.x) [DOI] [PubMed] [Google Scholar]
- 19.Parrott ML, Ward SJ, Temple-Smith PD. 2007. Olfactory cues, genetic relatedness and female mate choice in the agile antechinus (Antechinus agilis). Behav. Ecol. Sociobiol. 61, 1075–1079. (doi:10.1007/s00265-006-0340-8) [Google Scholar]
- 20.Firman RC, Simmons LW. 2008. Polyandry facilitates postcopulatory inbreeding avoidance in house mice. Evolution 62, 603–611. (doi:10.1111/j.1558-5646.2007.00307.x) [DOI] [PubMed] [Google Scholar]
- 21.Waser PM, De Woody JA. 2006. Multiple paternity in a philopatric rodent: the interaction of competition and choice. Behav. Ecol. 17, 971–978. (doi:10.1093/beheco/arl034) [Google Scholar]
- 22.Stow AJ, Sunnucks P. 2004. Inbreeding avoidance in Cunningham's skinks (Egernia cunninghami) in natural and fragmented habitat. Mol. Ecol. 13, 443–447. (doi:10.1046/j.1365-294X.2003.02060.x) [DOI] [PubMed] [Google Scholar]
- 23.Blyton MDJ, Shaw RE, Peakall R, Lindenmayer DB, Banks SC. 2016. The role of relatedness in mate choice by an arboreal marsupial in the presence of fine-scale genetic structure. Behav. Ecol. Sociobiol. 70, 313–321. (doi:10.1007/s00265-015-2049-z) [Google Scholar]
- 24.Nagy M, Heckel G, Voigt CC, Mayer F. 2007. Female-biased dispersal and patrilocal kin groups in a mammal with resource-defence polygyny. Proc. R. Soc. B 274, 3019–3025. (doi:10.1098/rspb.2007.1008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suter SM, Keiser M, Feignoux R, Meyer DR. 2007. Reed bunting females increase fitness through extra-pair mating with genetically dissimilar males. Proc. R. Soc. B 274, 2865–2871. (doi:10.1098/rspb.2007.0799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varian-Ramos CW, Webster MS. 2012. Extrapair copulations reduce inbreeding for female red-back fairy-wrens. Anim. Behav. 83, 857–864. (doi:10.1016/j.anbehav.2012.01.010) [Google Scholar]
- 27.Ferretti V, Massoni V, Bulit F, Winkler DW, Lovette IJ. 2011. Heterozygosity and fitness benefits of extrapair mate choice in white-rumped swallows (Tachycineta leucorrhoa). Behav. Ecol. 22, 1178–1186. (doi:10.1093/beheco/arr103) [Google Scholar]
- 28.Landry C, Garant D, Duchesne P, Bernatchez L. 2001. ‘Good genes as heterozygosity’: The major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar). Proc. R. Soc. Lond. B 268, 1279–1285. (doi:10.1098/rspb.2001.1659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peacock MM, Smith AT. 1997. Nonrandom mating in pikas, Ochotona princeps: Evidence for inbreeding between individuals of intermediate relatedness. Mol. Ecol. 6, 801–811. (doi:10.1111/j.1365-294X.1997.tb00134.x) [PubMed] [Google Scholar]
- 30.Duarte LC, Bouteiller C, Fontanillas P, Petit E, Perrin N. 2003. Inbreeding in the greater white-toothed shrew, Crocidura russula. Evolution 57, 638–645. (doi:10.1111/j.0014-3820.2003.tb01555.x) [DOI] [PubMed] [Google Scholar]
- 31.Akçay E, Roughgarden J. 2007. Extra-pair paternity in birds: review of the genetic benefits. Evol. Ecol. Res. 9, 855–868. [Google Scholar]
- 32.Hsu Y, Schroeder J, Winney I, Burke T, Nakagawa S. 2015. Are extra-pair males different from cuckolded males? A case study and a meta-analytic examination. Mol. Ecol. 24, 1558–1557 (doi:10.1111/mec.13124) [DOI] [PubMed] [Google Scholar]
- 33.Jacob G, Prevot A, Baudry E. 2016. Feral pigeons (Columbia livia) prefer genetically similar mates despite inbreeding depression. PLoS ONE 11, e0162451 (doi:10.1371/journal.pone.0162451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson ME. 2013. Reproductive ecology of female chimpanzees. Am. J. Primatol. 75, 222–237. (doi:10.1002/ajp.22084) [DOI] [PubMed] [Google Scholar]
- 35.Pusey AE, Schroepfer-Walker KK. 2013. Female–female competition in chimpanzees. Phil. Trans. R. Soc. B 368, 20130077 (doi:10.1098/rstb.2013.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodall J. 1986. The Chimpanzees of Gombe. Cambridge, MA: Belknap Press. [Google Scholar]
- 37.Ralls K, Ballou J. 1982. Effects of inbreeding on infant mortality in captive primates. Int. J. Primatol. 3, 491–505. (doi:10.1007/BF02693747) [Google Scholar]
- 38.Constable JL, Ashley MV, Goodall J, Pusey AE. 2001. Noninvasive paternity assignment in Gombe chimpanzees. Mol. Ecol. 10, 1279–1300. (doi:10.1046/j.1365-294X.2001.01262.x) [DOI] [PubMed] [Google Scholar]
- 39.Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW. 2007. Male coercion and the costs of promiscuous mating for female chimpanzees. Proc. R. Soc. B 274, 1009–1014. (doi:10.1098/rspb.2006.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller MN, Thompson ME, Kahlenberg SM, Wrangham RW. 2011. Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behav. Ecol. Sociobiol. 65, 921–933. (doi:10.1007/s00265-010-1093-y) [Google Scholar]
- 41.Feldblum J, Wroblewski EE, Rudicell R, Hahn B, Paiva T, Cetinkaya-Rundel M, Pusey AE, Gilby I. 2014. Sexually coercive male chimpanzees sire more offspring. Curr. Biol. 23, 2855–2860. (doi:10.1016/j.cub.2014.10.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Lawick-Goodall J. 1968. The behaviour of free-living chimpanzees in the Gombe stream reserve. Anim. Behav. Monogr. 1, 161–311. (doi:10.1016/S0066-1856(68)80003-2) [Google Scholar]
- 43.Pusey AE. 2005. Inbreeding avoidance in primates. In Inbreeding, incest and the incest taboo (eds Wolf A, Durham W), pp. 61–75. Stanford, CA: Stanford University Press. [Google Scholar]
- 44.Takahata Y. 1990. Adult males' social relations with adult females. In The chimpanzees of the Mahale mountains (ed. Nishida T.), pp. 133–148. Tokyo, Japan: University of Tokyo Press. [Google Scholar]
- 45.Pusey AE. 1980. Inbreeding avoidance in chimpanzees. Anim. Behav. 28, 543–552. (doi:10.1016/S0003-3472(80)80063-7) [Google Scholar]
- 46.Stumpf RM, Thompson ME, Muller MN, Wrangham RW. 2009. The context of female dispersal in Kanyawara chimpanzees. Behaviour 146, 629–656. (doi:10.1163/156853909X413853) [Google Scholar]
- 47.Wroblewski EE. 2010. Paternity and father-offspring relationships in wild chimpanzees, Pan troglodytes schweinfurthii. PhD dissertation, University of Minnesota.
- 48.Lehmann J, Fickenscher G, Boesch C. 2006. Kin biased investment in wild chimpanzees. Behaviour 143, 931–955. (doi:10.1163/156853906778623635) [Google Scholar]
- 49.Murray CM, Stanton MA, Lonsdorf EV, Wroblewski EE, Pusey AE. 2016. Chimpanzee fathers bias their behavior towards their offspring. R. Soc. open sci. 3, 160441 (doi:10.1098/rsos.160441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pusey AE, Williams J, Goodall J. 1997. The influence of dominance rank on the reproductive success of female chimpanzees. Science 277, 828–831. (doi:10.1126/science.277.5327.828) [DOI] [PubMed] [Google Scholar]
- 51.Wilson ML. 2012. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In Long-term field studies of primates (eds Kappeler PM, Watts DP), pp. 357–384. Berlin, Germany: Springer. [Google Scholar]
- 52.Rudicell RS, et al. 2010. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathog. 6, e1001116 (doi:10.1371/journal.ppat.1001116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J. 2007. The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conserv. Biol. 21, 623–634. (doi:10.1111/j.1523-1739.2007.00704.x) [DOI] [PubMed] [Google Scholar]
- 54.Vigilant L, Hofreiter M, Siedel H, Boesch C. 2001. Paternity and relatedness in wild chimpanzee communities. Proc. Natl Acad. Sci. USA 98, 12 890–12 895. (doi:10.1073/pnas.231320498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inoue E, Inoue-Murayama M, Vigilant L, Takenaka O, Nishida T. 2008. Relatedness in wild chimpanzees: Influence of paternity, male philopatry, and demographic factors. Am. J. Phys. Anthropol. 137, 256–262. (doi:10.1002/ajpa.20865) [DOI] [PubMed] [Google Scholar]
- 56.Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885. (doi:10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newton-Fisher NE, Emery Thompson M, Reynolds V, Boesch C, Vigilant L. 2010. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am. J. Phys. Anthropol. 142, 417–428. (doi:10.1002/ajpa.21241) [DOI] [PubMed] [Google Scholar]
- 58.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373–381. (doi:10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boesch C, Kohou G, Néné H, Vigilant L. 2006. Male competition and paternity in wild chimpanzees of the Tai Forest. Am. J. Phys. Anthropol. 130, 103–115. (doi:10.1002/ajpa.20341) [DOI] [PubMed] [Google Scholar]
- 60.Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. (doi:10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 61.Marshall TC, Coltman DW, Pemberton JM, Slate J, Spalton JA, Guinness FE, Smith JA, Pilkington JG, Clutton-Brock TH. 2002. Estimating the prevalence of inbreeding from incomplete pedigrees. Proc. R. Soc. Lond. B 269, 1533–1539. (doi:10.1098/rspb.2002.2035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J. 2011. COANCESTRY: a program for simulating, estimating and analyzing relatedness and inbreeding coefficients. Mol. Ecol. Resour. 11, 141–145. (doi:10.1111/j.1755-0998.2010.02885.x) [DOI] [PubMed] [Google Scholar]
- 63.Wetzel DP, Westneat DF. 2009. Heterozygosity and extra-pair paternity: biased tests from the use of shared markers. Mol. Ecol. 18, 2010–2021. (doi:10.1111/j.1365-294X.2009.04114.x) [DOI] [PubMed] [Google Scholar]
- 64.Wang JL. 2010. Do marker-based paternity assignments favour heterozygous and unrelated males? Mol. Ecol. 19, 1898–1913. (doi:10.1111/j.1365-294X.2010.04601.x) [DOI] [PubMed] [Google Scholar]
- 65.Wang JL. 2014. Marker-based estimates of relatedness and inbreeding coefficients: an assessment of current methods. J. Evol. Biol. 27, 518–530. (doi:10.1111/jeb.12315) [DOI] [PubMed] [Google Scholar]
- 66.Lukas D, Reynolds V, Boesch C, Vigilant L. 2005. To what extent does living in a group mean living with kin? Mol. Ecol. 14, 2181–2196. (doi:10.1111/j.1365-294X.2005.02560.x) [DOI] [PubMed] [Google Scholar]
- 67.Queller D, Goodnight K. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–275. (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 68.Sinnwell JP, Therneau TM, Schaid DJ. 2014. The kinship2 R package for pedigree data. Hum. Hered. 78, 91–93. (doi:10.1159/000363105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J. 2007. Triadic IBD coefficients and applications to estimating pairwise relatedness. Genet. Res. 89, 135–153. (doi:10.1017/S0016672307008798) [DOI] [PubMed] [Google Scholar]
- 70.Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. 2002. Female competition and male territorial behaviour influence female chimpanzees' ranging patterns. Anim. Behav. 63, 347–360. (doi:10.1006/anbe.2001.1916) [Google Scholar]
- 71.Wallis J. 1997. A survey of reproductive parameters in the free-ranging chimpanzees of Gombe National Park. J. Reprod. Fertil. 109, 297–307. (doi:10.1530/jrf.0.1090297) [DOI] [PubMed] [Google Scholar]
- 72.Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. 2011. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 82, 911–921. (doi:10.1016/j.anbehav.2011.07.016) [Google Scholar]
- 73.SAS Institute Inc. 2013 JMP®, Version 11. SAS Institute Inc., Cary, NC, 1989–2007.
- 74.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. (doi:10.18637/jss.v067.i01) [Google Scholar]
- 75.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 76.Keele BF, et al. 2009. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460, 515–519. (doi:10.1038/nature08200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bronikowski AM, et al. 2016. Female and male life tables of seven wild primates. Scientific Data 3, 160006 (doi:10.1038/sdata.2016.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tutin CEG. 1979. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 6, 29–38. (doi:10.1007/BF00293242) [Google Scholar]
- 79.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 1871–1971. Chicago, IL: Aldine. [Google Scholar]
- 80.Emlen S, Oring L. 1977. Ecology, sexual selection and the evolution of mating systems. Science 19, 215–223. (doi:10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 81.Havlicek J, Roberts SC. 2009. MHC-correlated mate choice in humans: A review. Psychoneuroendocrinology 34, 497–512. (doi:10.1016/j.psyneuen.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 82.Charpentier MJE, Crawford JC, Boulet M, Drea CM. 2010. Message ‘scent’: lemurs detect the genetic relatedness and quality of conspecifics via olfactory cues. Anim. Behav. 80, 101–108. (doi:10.1016/j.anbehav.2010.04.005) [Google Scholar]
- 83.Setchell JM, Huchard E. 2010. The hidden benefits of sex: evidence for MHC-associated mate choice in primate societies. Bioessays 32, 940–948. (doi:10.1002/bies.201000066) [DOI] [PubMed] [Google Scholar]
- 84.Setchell JM, Vaglio S, Abbott KM, Moggi-Cecchi J, Boscaro F, Pieraccini G, Knapp LA. 2011. Odour signals major histocompatibility complex genotype in an Old World monkey. Proc. R. Soc. B 278, 274–280. (doi:10.1098/rspb.2010.0571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parr L, de Waal FB. 1999. Visual kin recognition in chimpanzees. Nature 399, 647–648. (doi:10.1038/21345) [DOI] [PubMed] [Google Scholar]
- 86.Parr LA, Heintz M, Lonsdorf EV, Wroblewski EE. 2010. Visual kin recognition in nonhuman primates (Pan troglodytes and Macaca mulatta): Inbreeding avoidance or male distinctiveness? J. Comp. Psychol. 124, 343–350. (doi:10.1037/a0020545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Setchell JM, Abbott KM, Gonzalez JP, Knapp LA. 2013. Testing for postcopulatory selection for major histocompatibility complex genotype in a semi free-ranging primate population. Am. J. Primatol. 75, 1021–1031. (doi:10.1002/ajp.22166) [DOI] [PubMed] [Google Scholar]
- 88.Walker KK, Rudicell RS, Li Y, Hahn BH, Wroblewski E, Pusey AE. 2017. Data from: Chimpanzees breed with genetically dissimilar mates. Dryad Digital Repository. (doi:10.5061/dryad.d7g4c) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genotypes and pairwise relatedness data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.d7g4c [88].


