Abstract
New skeletal elements of the recently described endemic giant anseriform Garganornis ballmanni Meijer, 2014 are presented, coming from the type-area of the Gargano and from Scontrone, southern and central Italy, respectively. The new remains represent the first bird remains found at Scontrone so far, and another shared element between these two localities, both part of the Apulia-Abruzzi Palaeobioprovince. The presence of a very reduced carpometacarpus confirms its flightlessness, only previously supposed on the basis of the very large size, while the morphologies of tarsometatarsus and posterior phalanges clearly indicate the adaptation of G. ballmanni to a terrestrial, non-aquatic, lifestyle. Its very large body size is similar to that observed in different, heavily modified, insular waterfowl and has been normally interpreted as the response to the absence of terrestrial predators and a protection from the aerial ones. The presence of a carpal knob in the proximal carpometacarpus also indicates a fighting behaviour for this large terrestrial bird species.
Keywords: fossil bird, Anseriformes, flightlessness, insular gigantism, Miocene, Italy
1. Introduction
Since Darwin's and Wallace's works, the study of islands has been a powerful tool in many evolutionary studies, and birds are a key group in understanding the biological changes in island environments. Birds on islands often display dramatic size increases, allometric variations and, in some cases, flightlessness. The latter is known in different bird group like ibises, cormorants, rails, pigeons, parrots, passerines and waterfowl [1–4].
The waterfowl, crown-group Anseriformes, includes Anhimidae (South American screamers), Anseranatinae (Australasian magpie-geese) and Anatidae with a worldwide distribution [5]. The fossil record of Anatidae is very rich and comprises several endemic species, which evolved flightlessness in insular conditions, both on remote oceanic islands [4,6–8] as well as in intermediate-type islands, such as those of the Mediterranean Sea [9–11]. Most of the insular anatids overall show slight reduction of the wings towards flightlessness [7,12–14], but in some cases, they were extremely adapted to a terrestrial lifestyle with a great increase in body size, such as the Hawaiian moa-nalos Chelychelynechen and Thambetochen [4] and New Zealand's Cnemiornis [15]. Among the giant insular species of Anseriformes was Garganornis ballmanni, a species recently described from the Neogene fissure-filling deposits of the Gargano (southern Italy) on the basis of a single distal tibiotarsus [16].
Here we describe new skeletal elements of G. ballmanni from Gargano and Scontrone, southern and central Italy, respectively (figure 1), the main localities of the so-called Apulia-Abruzzi Palaeobioprovince [17], which during the Miocene was characterized by insular conditions and a highly endemic fauna. These new remains allow us to clarify the peculiar adaptations of Garganornis to a terrestrial lifestyle and confirm its flightlessness, which was only previously suggested [16]. In addition, G. ballmanni represents the first fossil bird found at Scontrone, and a new terrestrial taxon shared between Scontrone and Gargano, showing that it was widespread in the Apulia-Abruzzi Palaeobioprovince.
Figure 1.
Map of Italy with the location of Gargano and Scontrone.
2. Material and methods
2.1. Study areas
The Gargano area (Foggia, southern Italy) during the Late Miocene was an insular domain that was part of the Apulia-Abruzzi Palaeobioprovince [17]. In the sediment filling the karst network, exposed in the quarries exploiting the Mesozoic Calcare di Bari near the town of Apricena, a large amount of vertebrate remains has been found, demonstrating the high diversity of the fauna inhabiting this insular domain [18]. The Scontrone fossil locality is situated on Monte Civita, close to the village of Scontrone (L'Aquila, central Italy). The fossil remains have been found in the lower part of the Scontrone Calcarenite, marginal marine carbonates representing the early stage of the Tortonian transgression, in an interval represented by coastal sandbars [19]. Gargano and Scontrone represent the most important fossil localities of this palaeobioprovince [17], which was characterized by very peculiar vertebrate associations with highly endemic macro- and micromammals, reptiles and birds [16–18,20–25], all of them grouped in the so-called Mikrotia fauna [18,26]. It is worth mentioning that the fossil vertebrate association of this palaeobioprovince is also characterized by the absence of terrestrial predators, with the exception of the very peculiar hedgehog Deinogalerix [25], the otter Paralutra [27] and a crocodile [28], the latter two known from very few remains.
Despite the various common taxa of mammals and reptiles shared between Gargano and Scontrone, not even one taxon of the rich bird assemblage of Gargano has been found at Scontrone or in other localities of the palaeobioprovince until now [17]. This is most probably due to the taphonomic characteristics of the Scontrone deposits [29] and to the very hard sediments embedding the bones, which make it hard to find and collect the smaller and more fragile bones during excavations.
2.2. Methods
The fossil material is stored in the Dipartimento di Scienze della Terra of the Firenze University, Firenze, Italy (DSTF), the Museo di Geologia e Paleontologia of the Torino University (MGPT-PU), the Naturmuseum of Augsburg, Augsburg, Germany (NMA), the Naturalis Biodiversity Center, Leiden, the Netherlands (RGM) and the Centro di Documentazione Paleontologico Hoplitomeryx, Scontrone, Italy (SCT). The fossil specimens have been compared with the recent bird skeletons stored in the Laboratoire de Géologie de Lyon, Université Claude-Bernard Lyon 1, Lyon, France (FSL), the Dipartimento di Scienze della Terra of the Torino University, Torino, Italy (MPOC), the Natural History Museum, London and Tring, UK (NHMUK) and in the Ditsong National Museum of Natural History, Pretoria, South Africa (TM). The osteological terminology follows Baumel & Witmer [30]. The circumference of tibiotarsi of selected fossil Anseriformes at the National Museum of Natural History in Washington D.C., USA were measured by wrapping a thin strap of paper around the thinnest portion of the tibiotarsus and measuring the minimum circumference with sliding calipers calibrated to the nearest 0.05 mm. Body mass was then estimated using the least-squares regression estimates of Iwaniuk et al. [31].
3. Systematic palaeontology
Anseriformes Wagler, 1831
Anatidae Vigors, 1825
Garganornis ballmanni Meijer, 2014
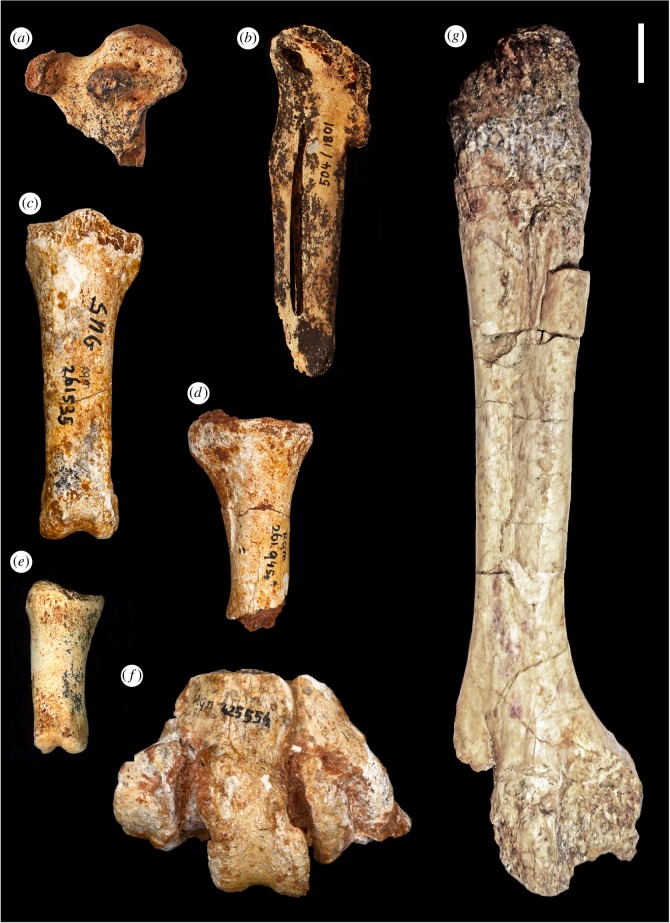
Figure 2.
Bones of Garganornis ballmanni Meijer, 2014 from the Late Miocene of Gargano (a–f) and Scontrone (g), Italy. (a) Right carpometacarpus DSTF-GA 49, ventral view; (b) left carpometacarpus NMA 504/1801, ventral view; (c) proximal pedal phalanx RGM 261535, dorsal view; (d) proximal pedal phalanx RGM 261945, dorsal view; (e) mesial pedal phalanx MGPT-PU 135536, dorsal view; (f) left tarsometatarsus RGM 425554, dorsal view; (g) right tarsometatarsus SCT 23, dorsal view. Scale bar represents 1 cm.
Figure 4.
Reconstruction of Garganornis ballmanni Meijer, 2014 based on the newly described fossil remains. This reconstruction is based on a generic Western Palaearctic Goose with short and robust tarsometatarsus, short toes and very short wings according to the known elements of Garganornis ballmanni. Illustration made by Stefano Maugeri.
Holotype: distal left tibiotarsus RGM 443307 from the Late Miocene of Gargano (Posticchia 5), southern Italy.
New material from Gargano: right carpometacarpus DSTF-GA 49 (F15), proximal end; left carpometacarpus NMA 504/1801 (Fina 2), lacking proximal end; right tibiotarsus DSTF-GA 77 (F15), damaged proximal end; left tarsometatarsus RGM 425554 (San Giovannino), distal end; trochlea metatarsi III of a right tarsometatarsus RGM 425943 (Chirò 14A); indeterminate pedal phalanges MGPT-PU 135356 (unnamed fissure), RGM 261535 (Pizzicoli 12), RGM 261945 (unnamed fissure).
New material from Scontrone: right tarsometatarsus, SCT 23, almost complete with proximal and distal ends damaged and missing trochlea metatarsi IV.
Measurements: DSTF-GA 49: proximal width, 27.7 mm; proximal depth, 10.5 mm. NMA 504/1801: total length as preserved, 53.6 mm. DSTF-GA 77: proximal width, 43.2 mm; proximal depth, 31.0 mm (estimated). SCT 23: total length of preserved specimen, 168.2 mm; distal width, 22.5 mm (estimated); width of shaft at mid length, 18.3 mm. RGM 425554: distal width, 47.5 mm; distal depth, 24.8 mm (estimated). MGPT-PU 135356: total length, 29.0 mm; proximal width, 13.4 mm; proximal depth, 11.6 mm; distal width, 9.1 mm; distal depth, 7.7 mm; width of shaft at mid length, 9.9 mm. RGM 261535: total length, 55.1 mm; proximal width, 19.6 mm; proximal depth, 17.7 mm; distal width, 13.3 mm; distal depth, 11.9 mm; width of shaft at mid length, 11.3 mm. RGM 261945: proximal width, 20.9 mm; proximal depth, 16.2 mm; width of shaft at mid length, 9.7 mm.
Chronological distribution: G. ballmanni is now reported from the Tortonian locality of Scontrone, at an age of about 9 Ma [19], to the Late Miocene fissure fillings of Gargano, for which an age between 6 and 5.5 Ma has been recently proposed [32]. Across the karst network system of the Gargano area, the remains of G. ballmanni have now been found in different fillings spread over the various chronological phases outlined by Masini et al. [18,23] from the Phase 1b to the Phase 3c. In particular, the chronological attribution to the Phase 2b of the recently collected pedal phalanx MGPT-PU 135356 has been made after study of an associated Mikrotia specimen, a rodent of key importance to assess the relative ages of the samples from the Gargano fissure-filling system [18].
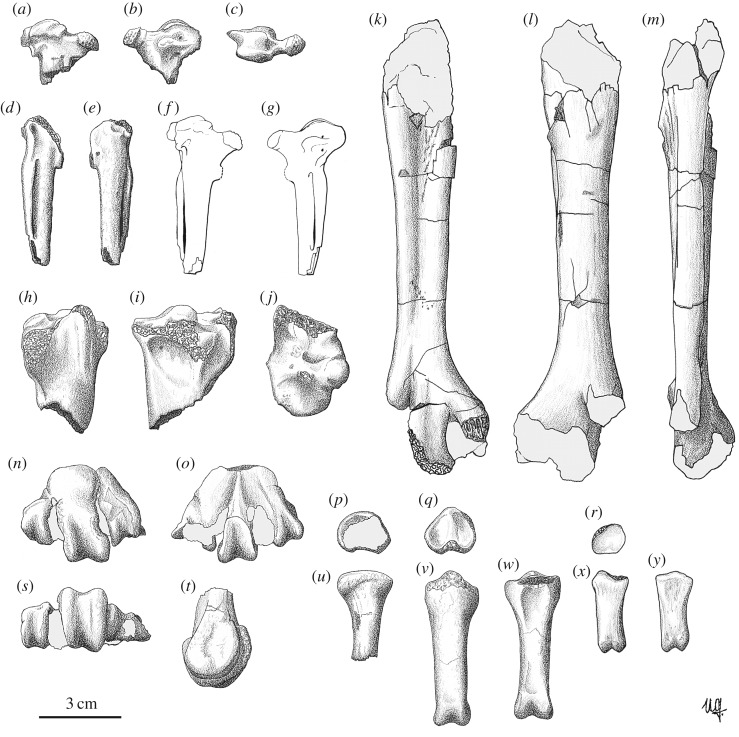
Comparative osteology: The proximal carpometacarpus DSTF-GA 49 (figures 2a and 3a–c) is very peculiar, with the two sides of the trochlea carpalis greatly extended laterally, rendering the proximal part of the bone very flat in respect to other Anseriformes. The presence of a processus extensorius parallel to the proximal trochlea and not tilted ventrally, a relatively wide and not pointed processus pisiformis, and a small knob dorsally to the fovea carpalis caudalis allows its attribution to the Anatidae. On the top of the processus extensorius of the proximal carpometacarpus DSTF-GA 49, we can observe a small bony outgrowth, similar in shape and size to that observed in other Anseriformes, in female Pezophaps solitaria, and in Goura cristata (Columbidae), but less developed than those of the male of P. solitaria, and surely not a spur as in Anhimidae, Plectropterus (Anatidae) and Vanellus (Charadriidae) [33]. Specimen NMA 504/1081 (figures 2b and 3d–e) lacks the proximal end, except the distal parts of the trochlea carpalis and the squared processus alularis. The shaft is very reduced in length with the spatium intermetacarpale very short, as demonstrated by the partially preserved synostosis metacarpalis distalis. Despite the distal end of the bone being incompletely preserved, the synostosis metacarpalis distalis is well developed, which indicates that the total length of the bone was not much longer than in the preserved specimen. The presence of a peculiar fossa on the ventral side of the carpometacarpus lateral to the processus pisiformis, which is also observed in other Anatidae although less evident, and other morphological details make it possible to graphically reconstruct the complete bone (figure 3f–g) and to confirm the relative shortness of the carpometacarpus. This is the first wing bone known for G. ballmanni.
Figure 3.
Drawings of the bones of Garganornis ballmanni Meijer, 2014; all the bones are from the Late Miocene of Gargano except one (k–m) which is from the Late Miocene of Scontrone, Italy. (a–c) Right carpometacarpus DSTF-GA 49 in ventral (a), dorsal (b) and proximal (c) views; (d,e) left carpometacarpus NMA 504/1801 in ventral (d) and dorsal (e) views; (f,g) graphical reconstruction of the carpometacarpus of Garganornis ballmanni based on the specimens DSTF-GA 49 and NMA 504/1801 in ventral (f) and dorsal (g) views; (h–j) right tibiotarsus DSTF-GA 77 in cranial (h), caudal (i) and proximal (j) views; (k–m) right tarsometatarsus SCT 23 in dorsal (k), plantar (l) and lateral (m) views; (n,o,s,t) left tarsometatarsus RGM 425554 in dorsal (n), plantar (o), distal (s) and medial (t) views; (p,u) proximal pedal phalanx RGM 261945 in proximal (p) and dorsal (u) views; (q,v,w) proximal pedal phalanx RGM 261535 in proximal (q), dorsal (v) and plantar (w) views; (r,x,y) mesial pedal phalanx MGPT-PU 135536 in proximal (r), dorsal (x) and plantar (y) views. Scale bar represents 3 cm. Drawings made by Ursula B. Göhlich.
The proximal tibiotarsus DSTF-GA 77 (figure 3h–j) is highly damaged but shows a very distinct fossa retropatellaris and a deep fossa flexoria, which are compatible with the morphology of Anatidae. The holotype tibiotarsus RGM 443307, already described in detail by Meijer [16], clearly shows its affinities with the Anatidae, rather than with other Anseriformes.
The tarsometatarsus SCT 23 (figures 2g and 3k–m), although badly preserved, is morphologically similar to the biggest Anatidae, but ever bigger than the extant Cygnus cygnus and the fossil Cy. falconeri, the giant extinct taxon from Sicily and Malta [34]. In particular, the tarsometatarsus is short and robust with a central hypotarsus, deduced from the distal part of the hypotarsus that is preserved, the sulcus flexorius absent, a well-marked sulcus extensorius, and a wide distal end with the rounded trochlea metatarsi III. The proximal end including the hypotarsus is not preserved. However, as the tuberositas musculi tibialis cranialis is visible on the specimen, the greatest length of the complete bone can be expected to be not much greater than the total length of the specimen. The crista plantaris lateralis is well developed, more protruding plantarly than in Cygnus and Anser, but similar to Cereopsis; the sulcus extensorius is very deep, deeper than in Cygnus and in the other large Anseriformes, and extends distally to the mid-point of the bone. The distal part of the diaphysis of the two fossil specimens is flat just proximal to the trochleae, which is not common in the Anseriformes, but similar to that observed in Cereopsis. The three trochleae metatarsi, preserved in the RGM 425554 (figures 2f and 3n–q), are latero-medially aligned in distal view with the trochlea metatarsi II and not tilted medially as in most of the Anseriformes. In addition, the trochleae metatarsi II and IV are more equal in distal extent than in most of the Anseriformes, but similar to what is observed in the Anhimidae, in Cereopsis, and in some unpublished specimens of the extinct Cy. falconeri preserved in the NHMUK (M.P. 2014, personal observation). It is important to note here that the arrangement of the distal trochlea differs from that in other large-bodied taxa such as cranes (Gruidae) and storks (Ciconiidae). The general morphology of the distal end of SCT 23 and RGM 425554 are very well comparable one to each other in both the shape of the preserved trochleae and in the flat area proximally to the trochleae. The Gargano avifauna does not contain any other birds of this size, and we therefore think it likely that both specimens represent the same species.
The pedal phalanges (figures 2c–e and 3r–y) are similar to those of the biggest Anatidae [15,34], but stouter with no strong ligament impressions.
Remarks: The faunal composition of the Apulia-Abruzzi Palaeobioprovince has been extensively studied [16,18,20–22,24]. The largest avian taxa are the Accipitridae Garganoaetus freudenthali and the Tytonidae Tyto gigantea [20,21]. Despite their size, their bones are smaller than the bones here described. In addition, as part of a revision of the whole bird association coming from the Palaeobioprovince, ongoing by one of us (M.P.), all specimens have been checked and all of the Anatidae remains have been published [24]. None of them is comparable in size with G. ballmanni.
The morphology of the bones described here, although highly modified, fits best with that of the Anatidae and their size is well compatible with G. ballmanni. Despite them coming from different localities, we refer them to G. ballmanni.
The study of the newly discovered material allows us to clarify the overall morphology of G. ballmanni and its adaptations to a terrestrial lifestyle. The morphology of the tarsometatarsus is different from most of the extant and fossil Anseriformes concerning the proportionately deeper and distally more developed sulcus extensorius, the dorso-plantarly flattened distal part of the shaft, and the distally and latero-medially almost aligned trochlea metatarsi II and IV. These features recall the tarsometatarsus morphology of Anhimidae and Cereopsis, among the more terrestrially adapted taxa within the crown-group Anseriformes. In addition, the preserved pedal phalanges are stout and short in respect to those of the swimming geese and swans.
Thus, the morphological characteristics of G. ballmanni reveal its adaptation to a terrestrial, non-aquatic, lifestyle and suggest that it probably lived in open or arid environments, as regularly observed in other strongly modified island Anseriformes [4,15].
4. Discussion
4.1. Taxonomic affinities
The analysis of the holotype tibiotarsus of G. ballmanni revealed its very large size, significantly bigger than Cy. olor. Some characters of the tibiotarsus are also seen in extinct lineages of Palaeogene and Neogene birds, such as Dromornithidae, Gastornithidae and Presbyornithidae. In particular, G. ballmanni shares with Gastornithidae a tibiotarsus with the wide fossa intercondylaris and the circular opening of the distal side of the canalis extensorius, but differs in lacking the strong sulcus m. fibularis and in having the distal end of canalis extensorius not positioned more medially as in Gastornithidae. However, the presence of these old taxa in the Neogene of southern Italy is unlikely, as there is no evidence of survival of Palaeogene faunal elements in the Apulia-Abruzzi Palaeobioprovince. Alternatively, and more likely, the peculiar morphologies shown by the holotype of G. ballmanni are the result of a convergent evolution of this species within an insular environment, where mammalian carnivores were extremely rare if not absent [25,27]. This theory is also supported by the morphology of the newly described bones, which are much more similar to Anatidae than to Gastornithidae and other stem-groups of Anseriformes. It is also worth noting that the carpometacarpus of G. ballmanni shows some similarity with the one of Gastornis sp. from Louvois (France), while it is very different from the one from Cernay (France) [35]. As with the holotype tibiotarsus [16], this similarity does not reflect any phylogenetic relationships between Garganornis and Gastornis, but rather indicates convergent evolution. The morphology of the new fossil remains, although strongly modified and incompletely preserved, combined with that of the holotype of G. ballmanni, confirms its attribution to the family Anatidae, the only crown-group Anseriformes recorded in the European Neogene.
The strong morphological modifications of G. ballmanni and the small number and poor preservation of the fossil remains do not allow for a hypothesis regarding its nearest continental non-endemic relatives among the Anatidae and the phylogenetic affinities of this endemic taxon.
4.2. Flightlessness and body mass estimation
In the original description of G. ballmanni, Meijer [16, p. 22] hypothesized that it was flightless on the basis of its very large size, compared with flying Anatidae. The newly described material, in particular the modified carpometacarpus, confirms this hypothesis and permits us to reconstruct the life appearance of G. ballmanni (figure 4). In fact, the estimated length of the carpometacarpus of G. ballmanni is proportionately much shorter than that of the extant large-bodied flying Anseriformes, and too shortened to allow the species to fly (figure 3f–g). In addition, the flattening of the proximal end and the lateral projection of the trochlea carpalis are unique features of G. ballmanni possibly related to a modification of the carpometacarpus following the loss of its flying ability. In fact, the carpal trochleae articulate the carpometacarpus with the ulna and the carpal bones and drive the extension and flexion of the wings. Their weakness in shape would indicate less movement of the wrist joint, as observed in the carpometacarpus of other flightless species [15,31,36]. In most of the flightless Anseriformes, the major wing modifications occur in the mid-wing elements, radius and ulna, rather than in the carpometacarpus [11]. The great reduction of the carpometacarpus, which is common in flightless Rallidae and Columbidae [2,4,37], was only recorded in the highly modified Chendytes [12], Cnemiornis [15], Thambetochen [4] and in the recently described Shiriyanetta hasegawai [7].
Flightlessness is most frequently interpreted as an adaptive response to the absence or reduced diversity of terrestrial predators. However, in the case of G. ballmanni, this taxon was still exposed to aerial predators, which were very abundant in the area during the Late Miocene [20,21]. The presence of large-bodied aerial predators, such as the eagle Garganoaetus freudenthali and the barn owl T. gigantea, may be one of the main reasons behind the great increase of the body size of G. ballmanni, together with the general tendency of insular big-sized Anatidae to become terrestrial herbivores. This is in contrast with insular flightless small-sized Anatidae which remain aquatic and show a reduction in body size [6,12]. On the basis of the minimum circumference of the holotype tibiotarsus (60.2 mm), Meijer [16, p. 22] already estimated the body mass of G. ballmanni at 22.3 kg, larger than any living anseriform. It should be noted that the minimum circumference of the holotype is not the minimum circumference of the bone itself, as the minimum circumference is located more proximally on the shaft. Adjusting for this, Meijer [16] instead gave a range estimate of 15.3–22.3 kg, at which G. ballmanni is still heavier than extant anatids. In fact, all the measurements of the bones of G. ballmanni that we could compare with recent and fossil big-sized Anatidae revealed its very large size, indicating that the real body mass is probably much closer to the maximum suggested by Meijer [16, p. 22]. Unfortunately, the newly described elements do not allow a new estimation, as the tarsometatarsus is involved in different functions in addition to the locomotion, such as feeding and perching, and therefore not a good indicator of body mass [31], and the wing elements are never considered in such analysis, in particular, those of flightless birds.
The large size of G. ballmanni becomes especially apparent in comparison with other insular wildfowls, as it is estimated to be one of the biggest Anseriformes to have ever existed (table 1). In particular, within the Mediterranean region, the extinct Maltese Swan Cy. falconeri also attained large size, and its size is estimated from 15.8 to 16.38 kg (mean of 16.09 kg) by Northcote [34]. The Hawaiian Islands were home to the large-bodied and flightless moa-nalo, but their estimated body sizes are well below that of G. ballmanni (table 1). The estimates for the New Zealand Geese Cnemiornis range from 8.03 to 15.55 kg (mean of 12.18 kg) for Cn. gracilis, and from 14.5 to 20.36 kg (mean of 17.46 kg) for Cn. calcitrans [8].
Table 1.
Body mass estimates of Garganornis ballmanni Meijer, 2014 in comparison with other extinct insular Anseriformes.
| species | location | age | body mass (kg) | source |
|---|---|---|---|---|
| Garganornis ballmanni | Gargano, Italy | Miocene | 22.3 | [16] |
| Cnemiornis calcitrans | New Zealand | Holocene | 17.46 (14.5–20.36) | [8] |
| Cygnus falconeri | Malta | Pleistocene | 16.09 | [32] |
| Cnemiornis gracilis | New Zealand | Holocene | 12.18 (8.03–15.55) | [8] |
| Thambetochen chauliodous | Hawaiian Islands | Holocene | 7.9 | this study |
| Thambetochen xanion | Hawaiian Islands | Holocene | 6.4 | this study |
| Ptaiochen pau | Hawaiian Islands | Holocene | 5.9 | this study |
4.3. Fighting adaptation
In addition to its reduced length, the proximal end of the carpometacarpus of G. ballmanni shows evident morphological modifications of the processus extensorius. On the external side of the processus extensorius of the proximal carpometacarpus DSTF-GA 49, a small carpal knob is present (figure 2a). It is of small size and there is no evidence that it could have become a spur, as in Anhimidae, but it is similar to those observed in Cygnus, Anser and other Anatidae [38], including Cy. falconeri (M.P. 2014, personal observation based on undescribed material at NHMUK). The carpal spurs or knobs, when present, are almost exclusively located on the processus extensorius and are always used as weapons [33]. Many Anseriformes display wing spurs or knobs in connection with fight behaviour, such as Anhimidae, Tachyeres, Chloephaga, Alopochen, Cereopsis and other Anatidae [38]. The presence of this knob in G. ballmanni should therefore be correlated with wing fight behaviour.
5. Conclusion
The data presented here confirm the taxonomic validity of G. ballmanni as a strongly modified species of crown-group Anatidae endemic to the Late Miocene Apulia-Abruzzi Palaeobioprovince of central-southern Italy, on the basis of fossil remains found in the Gargano area and at Scontrone.
The newly described material, although not perfectly preserved, gives new osteological details of the morphology of G. ballmanni, which confirms its flightlessness and its extreme adaptation to a terrestrial, non-aquatic, lifestyle. Furthermore, we observed evidence for wing fighting behaviour.
Garganornis ballmanni also demonstrates the strong endemic character of the Late Miocene Apulia-Abruzzi Palaeobioprovince, already suggested by the highly modified mammals and birds [17]. The Mediterranean Sea thus confirms its role as centre of speciation and its islands can also be confirmed to be an intermediate type of island, with faunal composition halfway between oceanic and continental ones, characterized by a very impoverished mammal fauna with high degree of endemism and no terrestrial carnivores, and by a diversified bird fauna with highly modified taxa [11].
Acknowledgements
We thank Federico Masini and Lorenzo Rook (DSTF), Cecile Mourer-Chauviré (FSL), Sandra Chapman and Judith White (NHMUK), Michael Rummel (NMA), Lars van den Hoek Ostende and Reinier van Zelst (RGM) and Greg Davies (TM) for providing access to the collections under their care. Trevor Worthy provided input on body mass estimates of Cnemiornis, and Megan Spitzer took measurements of tibiotarsus circumferences of selected Hawaiian taxa. We also thank Simone Colombero (MGPT) for the analysis of the Mikrotia remains to assess the relative age of the pedal phalanx MGPT-PU 135356, Matthijs Freudenthal who found the specimens preserved at RGM, particularly RGM 425554, and signalled them to M.P., Paul Mazza and Marco Rustioni who discovered the tarsometatarsus SCT 23, and Stefano Maugeri for the pictorial reconstruction of G. ballmanni. A special thank you to Cécile Mourer-Chauviré, Claudia P. Tambussi and an anonymous reviewer for their comments, which greatly improve the manuscript.
Data accessibility
Our paper has no associated data or other supplementary material, other than that presented in the manuscript.
Authors' contributions
M.P. identified and interpreted the fossil remains, prepared the illustrations and drafted the manuscript, H.J.M.M. performed the analysis of body mass, M.A.R. prepared and cleaned the Scontrone remains and U.B.G. made the drawings. All authors discussed the results and commented on the manuscript at all stages.
Competing interests
We have no competing interests.
Funding
The research was supported by University of Torino grant ex-60% 2015 and 2016.
References
- 1.Cheke A, Hume JP. 2008. Lost land of the dodo. The Ecological History of Mauritius, Réunion, and Rodrigues. New Haven, CT: Yale University Press. [Google Scholar]
- 2.Feduccia AJ. 1996. The origin and evolution of birds. New Haven, CT: Yale University Press. [Google Scholar]
- 3.Newton I. 2003. The speciation and biogeography of birds. London, UK: Elsevier. [Google Scholar]
- 4.Olson SL, James HF. 1991. Description of thirty-two new species of birds from the Hawaiian Islands: part I. Non-passeriformes. AOU Ornithol. Monogr. 45, 1–88. (doi:10.2307/40166794) [Google Scholar]
- 5.Dickinson EC, Remsen JVJ. 2013. The Howard & Moore complete checklist of the birds of the world, 4th edn Eastbourne, UK: Aves Press. [Google Scholar]
- 6.Livezey BC. 1989. Phylogenetic relationships and incipient flightlessness of the extinct Auckland Island Merganser. Wilson Bull. 101, 410–435. [Google Scholar]
- 7.Watanabe J, Matsuoka H. 2015. Flightless diving duck (Aves, Anatidae) from the Pleistocene of Shiriya, northeast Japan. J. Vert. Paleontol. 35, e994745 (doi:10.1080/02724634.2014.994745) [Google Scholar]
- 8.Worthy TH, Holdaway RN. 2002. The lost world of the moa: prehistoric life of New Zealand. Christchurch, New Zealand: Canterbury University Press. [Google Scholar]
- 9.Alcover JA, Florit F, Mourer-Chauviré C, Weesie PDM. 1992. The avifaunas of the isolated Mediterranean island during the Middle and the Late Pleistocene. Sci. Ser. Nat. Hist. Mus. Los Angeles County 36, 273–283. [Google Scholar]
- 10.Mayr G, Pavia M. 2014. On the true affinities of Chenornis graculoides Portis, 1884, and Anas lignitifila Portis, 1884—an albatross and an unusual duck from the Miocene of Italy. J. Vert. Paleontol. 34, 914–923. (doi:10.1080/02724634.2013.821076) [Google Scholar]
- 11.Pavia M. 2008. The evolution dynamics of the Strigiformes in the Mediterranean islands with the description of Aegolius martae n. sp. (Aves, Strigidae). Quat. Int. 182, 80–89. (doi:10.1016/j.quaint.2007.05.018) [Google Scholar]
- 12.Livezey BC. 1990. Evolutionary morphology of flightlessness in the Auckland Islands teal. Condor 92, 639–673. (doi:10.2307/1368685) [Google Scholar]
- 13.Livezey BC. 1993. Morphology and flightlessness in Chendytes, fossil seaducks (Anatidae, Mergini) of coastal California. J. Vert. Paleontol. 13, 185–199. (doi:10.1080/02724634.1993.10011500) [Google Scholar]
- 14.Livezey BC, Humphrey PS. 1986. Flightlessness in steamer-ducks (Anatidaea: Tachyeres): its morphological bases and probable evolution. Evolution 40, 540–558. (doi:10.2307/2408576) [DOI] [PubMed] [Google Scholar]
- 15.Worthy TH, Holdaway RN, Sorenson MD, Cooper AC. 1997. Description of the first complete skeleton of the extinct New Zealand goose Cnemiornis calcitrans (Aves: Anatidae) and a reassessment of the relationships of Cnemiornis. J. Zool. 243, 695–723. (doi:10.1111/j.1469-7998.1997.tb01971.x) [Google Scholar]
- 16.Meijer HJM. 2014. A peculiar anseriform (Aves: Anseriformes) from the Miocene of Gargano (Italy). C. R. Palevol. 13, 19–26. (doi:10.1016/j.crpv.2013.08.001) [Google Scholar]
- 17.Masini F, Petruso D, Bonfiglio L, Mangano G. 2008. Origination and extinction patterns of mammals in three central Western Mediterranean islands from the Late Miocene to Quaternary. Quat. Int. 182, 63–79. (doi:10.1016/j.quaint.2007.09.020) [Google Scholar]
- 18.Masini F, Rinaldi PM, Petruso D, Surdi G. 2010. The Gargano Terre Rosse insular faunas: an overview. Riv. Ital. Paleontol. Stratigrafia 116, 421–435. [Google Scholar]
- 19.Patacca E, Scandone P, Carnevale G. 2013. The Miocene vertebrate-bearing deposits of Scontrone (Abruzzo, Central Italy): stratigraphic and paleoenvironmental analysis. Geobios 46, 5–23. (doi:10.1016/j.geobios.2012.11.001) [Google Scholar]
- 20.Ballmann P. 1973. Fossile Vögel aus dem Neogen der Halbinsel Gargano (Italien). Scr. Geol. 17, 1–57. [Google Scholar]
- 21.Ballmann P. 1976. Fossile Vögel aus dem Neogen der Halbinsel Gargano (Italien). Zweiter Teil. Scr. Geol. 38, 1–59. [Google Scholar]
- 22.Göhlich UB, Pavia M. 2008. A new species of Palaeortyx (Aves: Galliformes: Phasianidae) from the Neogene of Gargano, Italy. Oryctos 7, 95–108. [Google Scholar]
- 23.Masini F, Rinaldi PM, Savorelli A, Pavia M. 2013. A new small mammal assemblage from the M013 Terre Rosse fissure filling (Gargano, South-Eastern Italy). Geobios 46, 49–61. (doi:10.1016/j.geobios.2012.10.003) [Google Scholar]
- 24.Pavia M. 2013. The Anatidae and Scolopacidae (Aves: Anseriformes, Charadriiformes) from the Late Neogene of Gargano, Italy. Geobios 46, 43–48. (doi:10.1016/j.geobios.2012.10.013) [Google Scholar]
- 25.Villier B, van den Hoek Ostende LW, de Vos J, Pavia M. 2013. New discoveries on the giant hedgehog Deinogalerix from the Miocene of Gargano (Apulia, Italy). Geobios 46, 63–75. (doi:10.1016/j.geobios.2012.10.001) [Google Scholar]
- 26.Freudenthal M, van den Hoek Ostende LW, Martín-Suárez E. 2013. When and how did the Mikrotia fauna reach Gargano (Apulia, Italy)? Geobios 46, 105–109. (doi:10.1016/j.geobios.2012.10.004) [Google Scholar]
- 27.Villier B, Pavia M, Rook L. 2011. New remains of Paralutra garganensis Willemsen, 1983 (Mustelidae, Lutrinae) from the Late Miocene ‘Terre Rosse’ of Gargano (Apulia, Italy). Boll. Soc. Paleontol. Ital. 50, 135–143. (doi:10.4435/BSPI.2011.13) [Google Scholar]
- 28.Delfino M, Rossi MA. 2013. Fossil crocodylid remains from Scontrone (Tortonian, Southern Italy) and the Late Neogene Mediterranean biogeography of crocodilian. Geobios 46, 25–31. (doi:10.1016/j.geobios.2012.10.006) [Google Scholar]
- 29.Mazza PAP. 2015. Scontrone (central Italy), signs of a 9-million-year-old tragedy. Lethaia 48, 387–404. (doi:10.1111/let.12114) [Google Scholar]
- 30.Baumel JJ, Witmer LM. 1993. Osteologia. In Handbook of avian anatomy: Nomina Anatomica Avium (ed. Baumel JJ.), pp. 45–132, 2nd edn. Cambridge, MA: Nuttall Ornithological Club. [Google Scholar]
- 31.Iwaniuk AN, Nelson JE, James HF, Olson SL. 2009. A comparative test of the correlated evolution of flightlessness and relative brain size in birds. J. Zool. 263, 317–327. (doi:10.1017/s0952836904005308) [Google Scholar]
- 32.Savorelli A, Colombero S, Masini F. 2016. Apatodemus degiulii n. gen et sp. (Rodentia, Muridae), a hitherto undescribed endemite from the Terre Rosse of Gargano (Late Miocene, Southeastern Italy). Palaeontographica Abt. A Paleozool. Stratigr. 306, 25–49. [Google Scholar]
- 33.Hume JP, Steel L. 2013. Fight club: a unique weapon in the wing of the solitaire, Pezophaps solitaria (Aves: Columbidae), an extinct flightless bird from Rodrigues, Mascarene Islands. Biol. J. Linn. Soc. 110, 32–44. (doi:10.1111/bij.12087) [Google Scholar]
- 34.Northcote ME. 1982. Size, form and habits of the extinct Maltese swan Cygnus falconeri. Ibis 124, 148–158. (doi:10.1111/j.1474-919x.1982.tb03753.x) [Google Scholar]
- 35.Mourer-Chauviré C, Bourdon E. 2016. The Gastornis (Aves, Gastornithidae) from the Late Paleocene of Louvois (Marne, France). Swiss J. Palaeontol. 135, 327–341. (doi:10.1007/s13358-015-0097-7) [Google Scholar]
- 36.Worthy TH, Mitri M, Handley WD, Lee MSY, Anderson A. 2015. Osteology supports a stem-Galliform affinity for the giant extinct flightless birds Sylviornis neocaledoniae (Sylviornithidae, Galloanseres). PLoS ONE 11, e0150871 (doi:10.1371/journal.pone.0150871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson SL. 1973. Evolution of the rails of the South Atlantic islands (Aves: Rallidae). Smithson. Contrib. Zool. 152, 1–53. (doi:10.5479/si.00810282.152) [Google Scholar]
- 38.Woolfenden GE. 1961. Postcranial osteology of the waterfowl. Bull. Fla State Mus. 6, 1–129. (doi:10.5962/bhl.title.104997) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our paper has no associated data or other supplementary material, other than that presented in the manuscript.