Abstract
Background
In 0.5–4% of pregnancies, the prospective mother sustains a primary infection with human cytomegalovirus (HCMV). An HCMV infection of the fetus in the first or second trimester can cause complex post-encephalitic impairment of the infant brain, leading to motor and mental retardation, cerebral palsy, epilepsy, retinal defects, and progressive hearing loss.
Methods
This review is based on pertinent publications from January 2000 to October 2016 that were retrieved by a selective search in PubMed employing the terms “cytomegalovirus and pregnancy” and “congenital cytomegalovirus.”
Results
85–90% of all neonates with HCMV infection are asymptomatic at birth. The main long-term sequela is hearing impairment, which develops in 8–15% of these affected children. Hygienic measures can lower the risk of primary HCMV infection in pregnancy by 50–85%. The first randomized and controlled trial (RCT) of passive immunization with an HCMV-specific hyperimmune globulin (HIG) preparation revealed a trend toward a lower risk of congenital transmission of the virus (30% versus 44% with placebo, p = 0.13). The effect of HIG was more marked in the initial non-randomized trial (15% versus 40%, p = 0.02). The RCT also showed HIG to be associated with a higher frequency of fetal growth retardation and premature birth (13% versus 2%, p = 0.06). Valaciclovir is a further, non-approved treatment option.
Conclusion
In the absence of an active vaccine against HCMV, counseling about hygienic measures may currently be the single most effective way to prevent congenital HCMV infection. Moreover, HCMV serologic testing is recommended in the guideline of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF). Further randomized trials of treatment with HIG and with valaciclovir are urgently needed so that the options for the prevention and treatment of congenital HCMV infection can be assessed.
In the 19th century, enlarged cells with intranuclear and cytoplasmic inclusion bodies (owl‘s eye appearance) were discovered by pathologists in the salivary glands, lungs, kidneys, and livers of stillborn infants for the first time. The human cytomegalovirus (HCMV), also known as human herpesvirus-5 (HHV-5) according to more recent nomenclature, is a double-stranded, enveloped DNA virus and a member of the Herpesviridae family.
Methods
This paper is based on a selective literature search. Data were derived from a search of the PubMed database for the period 1 January 2000 to 1 October 2016, using the search terms “cytomegalovirus and pregnancy“ or “congenital cytomegalovirus“. The following references go beyond that: 30, e1, e2, e10–e13, e18, e21, e28. Case reports were not included.
Natural routes of transmission
HCMV is transmitted by smear infection, i.e. the direct contact of mucous membranes with infectious body fluids, such as nasal secretions, saliva, tears, urine, genital secretions, or breast milk (1). After infection, the virus initially replicates in the epithelial cells at the site of entry, followed by hematogenous spread to numerous organs and cell types, including the cytotrophoblast cells of the placenta (1). After an acute viremic phase, in which nonspecific flu-like symptoms are only observed in 5 to 20% of immunocompetent persons, HCMV persists lifelong in infected individuals. The virus genome can be detected in stem cells (cluster of differentiation [CD] 34+), myeloid precursor cells and monocytes. Reactivation of the virus with genome detection can originate from terminally differentiated macrophages and dendritic cells along with a transient loss of CD8 T-cell control. Lymphoid progenitor cells, T and B cells as well as venous endothelial cells do not harbor HCMV genome (e1). Recently, the cellular receptor for the HCMV gHgLgO glycoprotein trimer was discovered on fibroblasts and epithelial cells (e2). Especially breastfed and congenitally infected young children often shed the virus in saliva, tears and urine asymptomatically over many years (1).
Epidemiology of congenital human cytomegalovirus infection
Subject to lifestyle, style of childcare and hygiene standards, the global seroprevalence of HCMV is 40 to 60% in Western industrialized countries and 80–100% in low-resource rural areas and developing countries (2, e3– e5). In Germany, where the seroprevalence is low (<50–70%), primary HCMV infection in pregnancy plays a more significant role in congenital HCMV infection than nonprimary HCMV infection, while in populations with high seroprevalence the latter is of greater importance (3, e4). With primary HCMV infection rarely causing mononucleosis-like and nonspecific symptoms in individuals with a healthy immune system, HCMV awareness is low among women, despite the worldwide presence of the virus (e6). The harmlessness of HCMV infection makes it dangerous in pregnancy: In women infected with HCMV not long before or during pregnancy (0.5–4% of pregnant women) (2, 3, e3, e5), there is an immediate risk of placental transmission to the fetus after viremia. The mother-to-fetus transmission rate increases with gestational age. The mean transmission rates are approximately 9% for primary HCMV infection within 3 to 12 weeks prior to conception (4), 31% for infection within 3 weeks before and after conception, respectively (4), 30% for infection in the first, 38% in the second and 72% in the third trimester of pregnancy (5). In pregnant women, the likelihood of being HCMV-seropositive before conception correlates negatively with socioeconomic status and is 92%, 47% and 34% in expectant mothers with low, middle and high socioeconomic status, respectively (e7). In German children, the HCMV seroprevalence increases independent of socioeconomic status from 22% at age 1–2 years to 32% at age 14–17 years (6). Before conception, HCMV immunoglobulin(Ig)G-seropositive pregnant women have a low transmission risk of approximately 0.5 to 2% (e4, e5, e8) and their infected newborns are rarely symptomatic (e5, e9). However, the exact transmission rate for recurrent maternal HCMV infection in unknown due to a lack of prospective epidemiological studies (3). In industrialized countries, congenital HCMV infection is seen in approximately 0.4 to 1.2% of live births, mainly due to primary, but to some extent also to non-primary infection in pregnancy (figure 1) (2, 3, 7, e3– e5, e8, e10, e11). With an expected incidence of approximately 0.2–0.5% of live births in Germany (e10, e11), congenital HCMV infection is more common than other congenital syndromes such as Down syndrome or neural tube defects, each with an incidence of 0.1 to 0.2% of live births (e12, e13), but it has attracted far less media attention. Up to one-fifth (17–20% [(7, e5]) of HCMV-infected newborns experience late neurological sequelae of the infection, such as delayed cognitive, motor or language development, as a result of hearing loss (12.6% [8]); in some cases, these are only picked up at the start of schooling (2, 7).
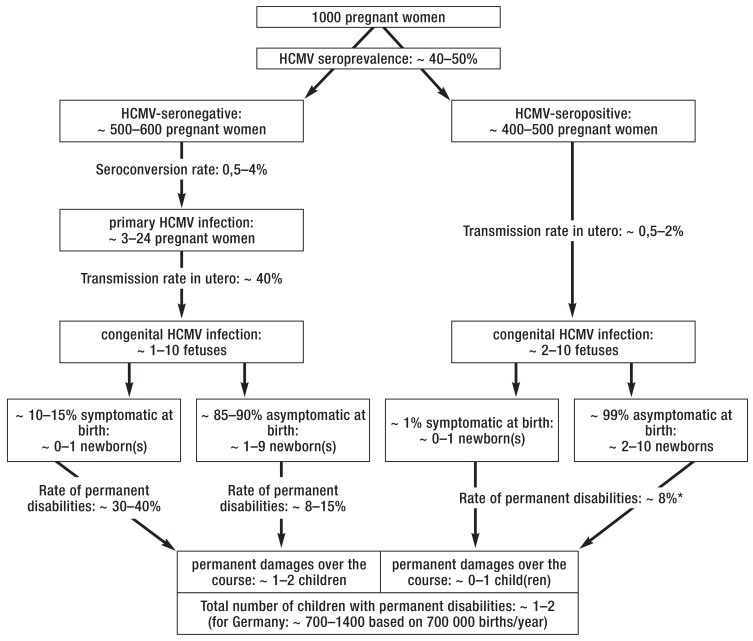
FIGURE 1.
Study-based projections on the epidemiology of congenital infection with the human cytomegalovirus (HCMV) in populations with high and low seroprevalence in absolute numbers (2, 3, 7, e3– e5, e8, e10, e11). The term “permanent disabilities“ includes symptoms that can only be detected with special devices, e.g. subclinical hearing deficit and cognitive late sequelae
* Rate of disabilities at age 2 years (e5)
Clinical presentation of congenital human cytomegalovirus infection
The clinical presentation of congenital HCMV infection varies widely, from asymptomatic infection (approx. 85–90% [e4]) to fetal hydrops, abortion and postnatal death (approx. 0.5% [7, e4]). The clinical signs and symptoms in between these extremes range from subclinical sensorineural losses to growth, mental and motor retardation, to central nervous system damages (microcephaly), with possible involvement of inner ear and retina, bone marrow and internal organs (hepatitis, pneumonia, enteritis) (box 1) (9). Due to the lack of clear definitions of these entities, the exact prevalence of mild and severe symptomatic HCMV infections in newborns is unknown. Children are most affected when the primary maternal infection occurs in the periconceptional period; with increasing gestational age, disease severity decreases (5, 12, 13). Ultrasonography and magnetic resonance imaging (MRI) may show characteristic changes in the fetal brain which vary with gestational age. Infection in the first trimester of pregnancy is frequently (16–49% [14]) associated with post-encephalitic atrophy of the developing brain, complex malformations of the cerebral convolutions, and microcephaly (efigure 1). These damages to the central nervous system are caused by cytopathic effects of HCMV on neuroepithelial stem cells and by local immune reaction (14). Other abnormal ultrasound findings that may be encountered in the effected fetus include hepatomegaly, ascites, pleural and pericardial effusion, hyperechogenic bowel (efigure 2), oligo- or polyhydramnios, or a diagnostically relevant thickening of the placenta (15, 16). Infection-related damage to the interface between decidua and placenta leads to local hypoxia which induces, by growth factor dysregulation, hyperproliferation of blood vessels and disruptions to the architecture of the villous trees (16). It is assumed that viral placentitis largely contributes to the cystic infarctions observed in the fetal brain and the postinflammatory periventricular gliosis, including calcifications (17).
BOX 1. Clinical symptoms and abnormal laboratory findings*.
-
Clinical symptoms
lenticulo-striatal vasculopathy (71–88%)
petechiae (41–76%)
icterus (67%)
microcephaly (30–53%)
growth retardation (36–50%)
hearing loss (26–42%)
chorioretinitis (4–20%)
seizures
migration disorders of the central nervous system
“blue-berry muffin spots“
pneumonia
enteritis
-
Abnormal laboratory findings
conjugated hyperbilirubinemia (81%)
elevated aspartate aminotransferase levels (76%)
thrombocytopenia (77–81%)
*of newborns with symptomatic congenital infection with the human cytomegalovirus (HCMV), ranked by frequency (10, 11, e14– e16)
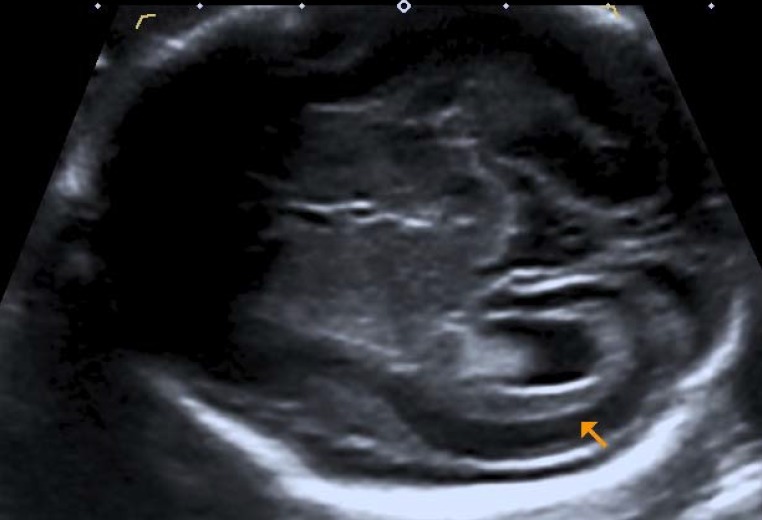
eFigure 1.
Prenatal ultrasound image of a fetus at 26 weeks’ gestation after asymptomatic maternal human cytomegalovirus (HCMV) primary infection during the first trimester. The fetal CNS is damaged by embryofetal HCMV encephalitis: mild extension of the intracerebral CSF spaces, inflammatory periventricular augmented echogenicity and early microcephaly.
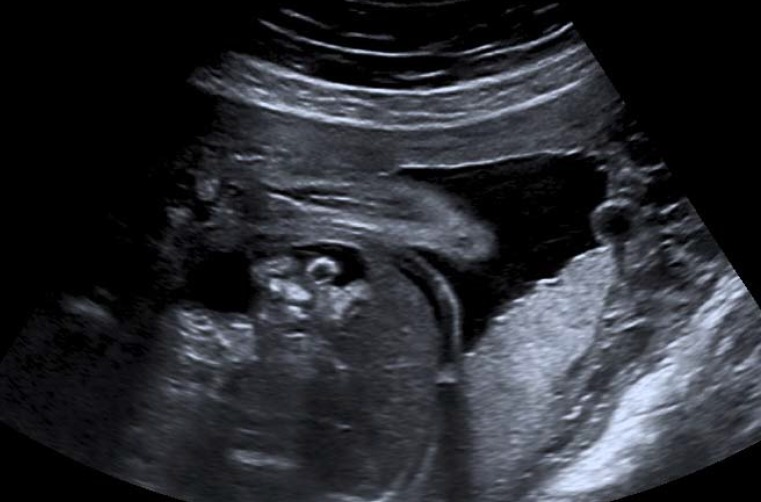
eFigure 2.
Ultrasound image of a fetus at 24 weeks’ gestation with fetal ascites and intestinal augmented echogenicity. The mother-to-fetus transmission of human cytomegalovirus (HCMV) following periconceptional maternal primary HCMV infection is confirmed by amniotic fluid testing.
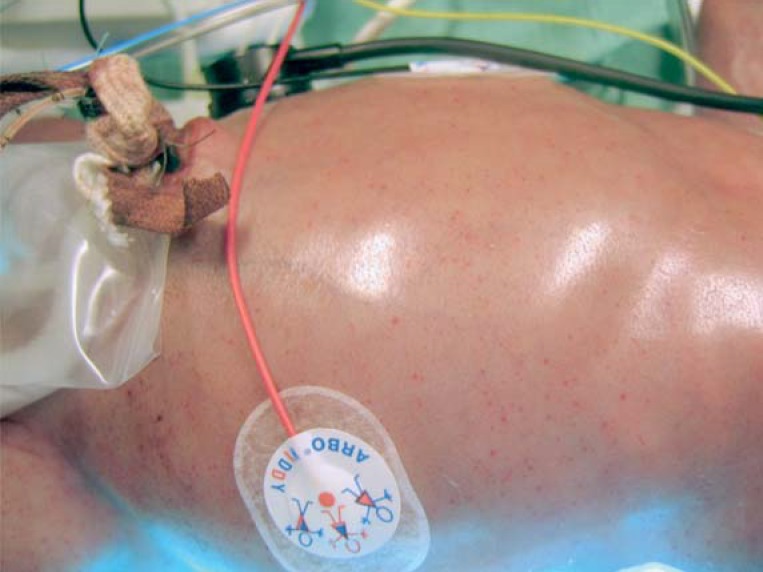
At birth, neonates with congenital infection may present with signs and symptoms of florid viral infection. Peripheral blood count abnormalities, such as lymphocytosis, neutropenia, thrombocytopenia, anemia, petechiae (Figure, Box 1), hepatitis with elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and/or direct hyperbilirubinemia, diffuse pneumonic infiltrates, fundus changes (retinitis), and hearing loss (18) are indicative of congenital infection. Typically, potentially progressive sensorineural hearing loss is present—the most common non-genetic cause of hearing loss (19). The progressive nature of the hearing loss is credited to a chronic productive HCMV infection of the labyrinth (e17, e18).
Figure: Petechiae in a newborn with congenital human cytomegalovirus infection requiring intensive care.
In contrast to symptomatic HCMV infection, in Europe approximately 85 to 90% of neonates with congenital infection are clinically asymptomatic and initially show normal development; in some affected children, late-onset sensorineural hearing loss or neurocognitive deficits are only diagnosed in the year before the start of schooling (approx. 3% [7, 20, e18]). More recent data from countries with a high prevalence of HCMV indicate that hearing loss is a major long-term complication in children (in approx. 10/1000 cases) with congenital HCMV infection resulting from recurrent HCMV infection in pregnant women (e4).
Protection of expectant mothers
The attention HCMV gets in prenatal care and the level of information provided to pregnant women is in stark contrast to these epidemiological data and the in some cases devastating effects of congenital HCMV infection (e6, e19). The value of HCMV screening as part of the prenatal program has become a subject of worldwide discussion (e20). Much of the controversy about the introduction of universal HCMV screening stems from the fact that neither an active vaccination option nor sufficiently evidence-based treatment options are currently available. The 2014 S2K guideline of the Association of Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF) for the diagnosis of viral infections in pregnancy (e11) recommends HCMV-IgG testing at the time a woman is found to be pregnant (Individual Health Service [IGeL]) and in case of professional or familial exposure to the virus during contact with children up to 3 years of age. Especially HCMV-IgG-negative pregnant women benefit from hygiene counseling on how to prevent HCMV-transmission (box 2); a significant preventative effect of hygiene counseling aimed at HCMV exposure prophylaxis was demonstrated (21, 22). In a French hospital, the rate of seroconversion during pregnancy was reduced by half from 0.4% to 0.2% (21). The risk of primary infection is particularly high in mothers with HCMV shedding asymptomatic children (seroconversion rate of up to 24% per year [23]). In a current controlled HCMV prevention study conducted in Italy, evaluating hygiene counseling in pregnant women caring for at least one infant/toddler, the French outcomes were exceeded with a seroconversion rate reduction from 7.6% to 1.2% (22).
BOX 2. Recommendations for exposure prophylaxis against human cytomegalovirus in pregnancy (20, e11, e21).
-
Do‘s
Kiss child on forehead or cheek; give hugs
-
Frequently wash hands with water and normal soap, especially
after changing nappies
feeding or bathing a child
after wiping off nasal secretion, tears or saliva
after touching toys
-
- Don’ts
Don’t kiss children on the mouth
Don’t put a pacifier in your own mouth
Don’t try food from a spoon or bottle of a child
Don’t eat left-overs from a child’s meal
Don’t use the same toothbrushes, dishes, cutlery, and towels as the child
Don’t touch or clean objects or clothing soiled with saliva, tears or urine without gloves (Be careful when removing gloves!)
Don’t have unprotected sex with a human cytomegalovirus (HCMV)-seropositive partner
After the introduction of the rubella vaccine for active immunization against the disease, a tremendous reduction in the incidence of congenital rubella syndrome (CRS) was observed (e22). In the light of this success, the development on a HCMV vaccine was given high priority (24). However, HCMV strains display such a degree of genetic polymorphism that despite the intensive efforts in this field, the efficacy of the experimental vaccines has been limited (24, e23– e25).
Diagnosis and treatment of congenital human cytomegalovirus infection
Prenatal investigations
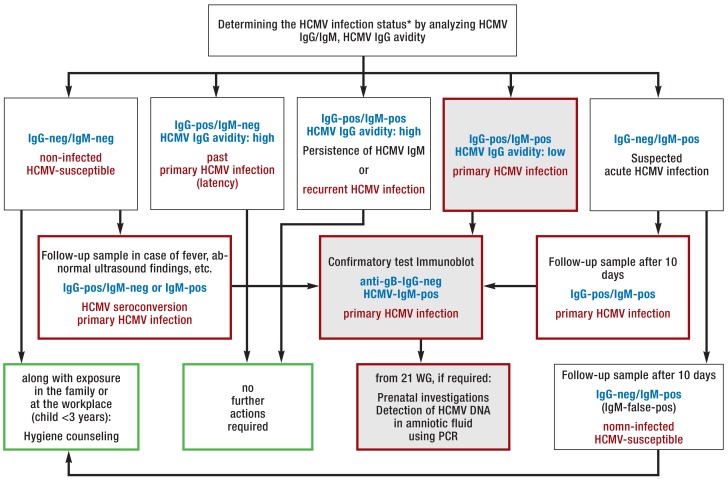
Primary HCMV infection of a pregnant woman and her fetus is asymptomatic in approximately 80% of cases (13, e5, e26). Suspicious prenatal ultrasound findings, e.g. a microcephalic fetus, or birth of a child with typical symptoms, should trigger an HCMV workup. The current AWMF guideline (e11) recommends to collect a serum reference sample at the start of pregnancy and to store it for two years. With this, HCMV-IgG seroconversion can be diagnosed in initially HCMV IgG-seronegative pregnant women. The AWMF guideline on viral infections in pregnancy (e11) contains a detailed diagnostic algorithm for this (figure 2). When no serum reference sample is available, HCMV-specific IgG avidity can be measured to differentiate between acute primary HCMV infection (low avidity index) and an infection which occurred more than 3 months ago (high avidity index) (25). In the literature, the time from primary HCMV infection to seroconversion to transmission with detection of the virus in the amniotic fluid, after the infected fetus excretes the virus via the kidneys, or in the fetal blood is reported to be 4 to 8 weeks (9, 26).
Figure 2.
Laboratory diagnostic procedure (from [e11]: Courtesy of the German Association for the Control of Virus Diseases (Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten) and the German Society for Virology) (Deutsche Gesellschaft für Virologie) to determine the human cytomegalovirus (HCMV) infection status in pregnant women with suspected primary HCMV infection, combined with possible actions.
Blue font: Result constellation; red font: interpretation; green border: actions; red border: further investigations required; gray background: constellation of results typical for primary HCMV infection.
* Note: The HCMV serostatus is determined at the start of pregnancy (HCMV screening), solely based on HCMV immunoglobulin (Ig)G testing. For more detailed information refer to the S2K guideline of the Association of the Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften) on the laboratory testing recommended to assess viral infections relevant in pregnancy (e11).
DNA, deoxyribonucleic acid; false-pos, false positive; neg, negative; PCR, polymerase chain reaction; pos, positive; WG, weeks’ gestation
Postnatal investigations
In neonates, suitable materials for routine direct viral testing (polymerase chain reaction [PCR], short-term culture) include urine, saliva and, to some extent, blood. Testing should be performed within the first two weeks after delivery (e11). In neonates with virologically confirmed congenital HCMV infection, regular hearing tests, assessments of age-appropriate cognitive development and inspection of the fundus should be performed—in addition to the initial check for the symptoms listed in Box 1—to ensure early identification of potential late-onset defects (20, 27, 28). Some authors describe a correlation between the level of viral load in the blood/urine of the fetus or newborn and the presence of neurological late-onset deficits (26, 29, e27).
Prenatal treatment options
In Europe, a HCMV-specific hyperimmunoglobulin (HCMV HIG) is available, containing neutralizing anti-HCMV IgG antibodies which can block the entry of free virons into target cells and boost viral phagocytosis (30, e28). In mainly non-randomized studies, the efficacy of HCMV HIG treatment in the prevention and treatment of placental HCMV transmission and resulting fetal damage was evaluated. In the as yet only available randomized controlled trial (RCT) evaluating mother-to-fetus HCMV transmission, Revello et al. (31) found no significant reduction in congenital HCMV transmission cases (44% placebo group versus 30% active group; p = 0.13). This is in contrast to the initial non-randomized study of Nigro et al. (32). According to Rawlinson et al. (e29), a combined analysis of the studies of Revello et al. (31) and Nigro et al. (32) showed a trend towards efficacy in Forest plots. A systematic review evaluating the efficacy of HIG prophylaxis in pregnancy (n = 583) found in all included studies a reduction in HCMV transmission rate and clinical symptoms among infected neonates; however, the results did not reach statistical significance (33) (table).
Table. Controlled studies on the prophylaxis of mother-to-fetus transmission of human cytomegalovirus or on the prevention of symptomatic congenital infection with human cytomegalovirus or its sequelae.
| Author/year (source) | Design | N | IV dose (PEIU/kg)*1 | Follow-up (years from birth) | Endpoint | Results | |
| HCMV HIG | control group | ||||||
| Revello, et al. 2014 (31) |
prospective, randomized, double-blind (RCT) |
123 | 100 q4w 3–6 doses |
0 | Proportion of infected fetuses/children |
18/61 (30%), p = 0.13 3/10 symptomatic 8 abortions*2 |
27/62 (44%) 4/17 symptomatic 11 abortions*2 |
| Nigro, et al. 2005 (32) |
prospective, non-randomized | 84 | 100 q4w 2–7 doses |
2 | Proportion of infected live births |
6/37 (16%), p = 0.02 0 symptomatic*3 |
19/47 (40%) 3 symptomatic*3 |
| Nigro, et al. 2005 (32) |
prospective, non-randomized | 45 | 200 q2–6w 1–3 doses*4 |
2 | Proportion of children with symptomat. infection |
1/31 (3%)*5, p<0.001 | 7/14 (50%)*5 |
| Nigro, et al. 2012 (34) |
retrospective | 64 | 200 q2–4w 1–4 doses |
1–5 | Proportion of children with sequelae |
4/32 (13%)*5, p<0.001 | 27/32 (85%)*5 |
| Nigro, et al. 2012 (35) |
prospective, non-randomized | 16*6 | 200 q4w 1–3 doses |
2–8 | Proportion of children with sequelae |
1/9 (11%)*5, p<0.001 | 8/8 (100%)*5 including 1 stillbirth |
| Visentin, et al. 2012 (36) |
prospective, non-randomized | 68 | 200 1 dose |
1 | Proportion of children with sequelae |
4/31 (13%), p<0.01 | 16/37 (43%) |
HCMV, human cytomegalovirus; HIG, hyperimmunoglobulin product; IV, intravenous; qXw, every X weeks; N, number of pregnant participants with primary infection; PEIU, units based on the reference standard of the Paul Ehrlich Institute; RCT, randomized controlled trial; symptomat., symptomatic
*1 units based on the reference standard of the Paul Ehrlich Institute; *2 With 18-times detection of HCMV in amniotic fluid, presumably altogether 18 abortions were induced, a further spontaneous abortion occurred in the control group; *3 signs and symptoms including neurological late-onset deficits; *4 In 9 women, additional 400 PEIU were injected into the amniotic cavity or umbilical cord; 8 out of the 9 children of these women were asymptomatic at birth and during the follow-up period; *5 These studies observed normalization of sonographic fetal abnormalities and catching-up on growth deficits (altogether in 30 out of 38 fetuses in the HCMV HIG groups and in 8 of 32 fetuses in the control groups); *6 Including 3 women with HCMV reactivation or reinfection (1 in the active treatment group—normal development— and 2 in the control group—both children symptomatic), and 2 women with twin births.
With regard to adverse events, the RCT by Revello et al. (31) found a trend towards increased prematurity und fetal growth retardation in the active group (13% versus 2% in the placebo group; p = 0.06), which is contrary to the results of the non-controlled study of Nigro et al. (37), reporting higher birth weights and longer pregnancy durations after repeated HCMV HIG administration. Revello et al. (31) reported birth defects or symptoms at birth in 13/48 patients (27.1%) in the active group and 9/47 patients (19.2%) in the placebo group. The validity of this secondary endpoint is very limited because fetuses with HCMV detection during amniocentesis were not included in the analysis. Apparently, these pregnancies were terminated prematurely (active group n = 8; placebo group n = 10), but no detailed information was provided (31).
Another prenatal off-label treatment option is the oral administration of valacyclovir in pregnant women with moderately symptomatic HCMV-infected fetuses. A recently published, non-randomized and non-controlled study (38) demonstrated a better outcome in newborns of women treated with high-dose valacyclovir (82% asymptomatic HCMV-infected newborns) compared to the untreated historic control group (43% asymptomatic HCMV-infected newborns). Pregnant women with severely symptomatic HCMV-infected fetuses were excluded from the study, making the interpretation of these results more difficult. Reported adverse events include transient headache (2/41; 4.81%) and a clinically not relevant increase (< 40 IU/L) in AST and ALT levels (38).
Postnatal treatment options
For the postnatal antiviral treatment of symptomatic neonates, intravenous ganciclovir (GCV) and its oral prodrug valganciclovir (VGCV) are available for the off-label use.
Intravenous GCV in a dose of 6 mg/kg body weight twice daily for 6 weeks significantly reduced the incidence of HCMV-related hearing loss at age 6 months (0% vs. 42%; p<0.001) and 12 months (21% vs. 61%; p = 0.002) compared with placebo (10). In addition, Oliver et al. demonstrated in their RCT significant positive differences in neurological development—as assessed by the number of delayed developmental milestones (social contact, fine motor skills, language, and gross motor skills) after 12 months—in 71 infants with congenital HCMV infection (10.06 versus 17.14; p = 0.007) (39). However, both hearing loss after 12 months (21% [10]) and developmental delays were also observed in the active group (39). The oral continuation of GCV treatment with VGCV for another 10 months resulted in superior hearing compared to GCV alone (no hearing loss in children with initially normal hearing vs. 35%; p = 0.001 [40, e30]). A randomized, placebo-controlled trial evaluating VGCV treatment in newborns with symptomatic HCMV infection (11) showed that in the long-term cohort (6-month treatment) the results achieved with regard to hearing loss, the study’s primary outcome, were not superior to those achieved in the cohort with 6-week treatment. However, with regard to the secondary outcomes, hearing after 24 months (normal hearing or improved hearing: 78% vs. 63%; p = 0.04) and the items related to the language component (85 vs.73; p = 0.004) and to the receptive communication component (7.3 versus 5.2; p = 0.003) of the developmental neurological examination, using the Bayley III Test, at age 24 months, the participants of the 6-month treatment group had significantly better scores compared to children of the control group with only 6 weeks of VGCV treatment. During the first 6 weeks, neutropenia was observed in only 20% of the newborns with VGCV treatment, compared to 63% in the pilot study with GCV (10). Since both active substances are myelotoxic and have not been approved for this indication, the decision to use or not to use them has to be made on an individual basis. The options for symptomatic treatment of severe late sequelae of congenital HCMV infection include neurorehabilitation and hearing aids or cochlear implants (e31). A systematic review evaluating hearing loss in children with congenital HCMV infection recommends to perform hearing screening at least until age 6 years (8). Systematic postnatal HCMV screening could be used to identify infected newborns who would then receive antiviral treatment (8, e32).
Conclusion for clinical practice
Despite the comparatively high prevalence of the condition, only a minority of pregnant women is aware of the prenatal risk posed by HCMV to the fetus (e33, e6). The AWMF guidelines recommend that at the time a pregnancy is confirmed the HCMV IgG serostatus should be tested (e11) to enable individual risk assessment and prevention. Counseling of HCMV IgG-seronegative pregnant women with regard to preventive hygiene measures makes the most significant contribution to the prevention of mother-to-fetus HCMV transmission (21, 22), without the risks associated with drug therapy. Only when a maternal primary HCMV infection is confirmed in the first trimester, a passive off-label immunization with HCMV HIG to prevent HCMV transmission should be taken into consideration in the context of a study. However, antiviral treatment with valacyclovir after mother-to-fetus transmission may be discussed on an individual basis (38). The affected pregnant woman should be comprehensively informed about the experimental nature of the treatment, especially if the treatment is offered outside of current study protocols, and give her written consent to the use of the drug in the legal framework of an individual treatment attempt (compassionate use). It should be added that in case the treatment costs are not covered by the health insurance, the pregnant woman has to pay the significant treatment costs herself.
Key Messages.
Human cytomegalovirus (HCMV) is worldwide the most common pathogen causing teratogenic congenital infections.
The current guidelines of the Association of the Scientific Medical Societies (AWMF) in Germany recommend to determine the HCMV immunoglobulin (Ig)G serostatus at the time a pregnancy is confirmed.
Especially HCMV-seronegative pregnant women having contact with children (<3 years) benefit from hygiene counseling which is currently the most effective prevention against congenital HCMV infections.
In pregnant women with primary HCMV infection during the first trimester, the option of a currently not evidence-based treatment with HCMV hyperimmunoglobulin or antivirals, according to protocols of ongoing studies, may be considered in individual cases based on the individual risk constellation.
In newborns with clinical signs and symptoms of congenital HCMV infection or a history indicative of possible HCMV infection, a HCMV polymerase chain reaction or a HCMV viral culture of urine or an oral mucosal swab should be performed.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement.
Dr. Buxmann, Prof. Meyer-Wittkopf and Prof. Hamprecht are members of the Scientific Advisory Board of the online platform ICON (Initiative for the Prevention of Congenital HCMV Infections) and have received reimbursements of travel costs, congress and lecture fees related to their work in this role from Biotest AG, Dreieich, Germany.
In addition, Dr. Buxmann and Prof. Hamprecht have received research funding (third-party funding) from Biotest AG, Dreieich, Germany.
Prof. Hamprecht has also received reimbursement of continuing education and travel costs as well as lecture fees from Abbott, Roche and Siemens. He has made all lecture fees available to the Tübinger HCMV Congenital Study via a third-party funding account of the University Hospital Tübingen (UKT).
Prof. Meyer-Wittkopf also received fees for being an author/co-author of a publication and for expert opinions related to this topic.
Prof. Friese is coordinating principal investigator of a pan-European clinical study on the treatment of pregnant women with HCMV-specific hyperimmunoglobulin and has received research funding for this from the sponsor of the study, Biotest AG.
References
- 1.Britt W, et al. Arvin A, Campadelli-Fiume G, Mocarski E, editors. Virus entry into host, establishment of infection, spread in host, mechanisms of tissue damage. Cambridge: Cambridge University Press. 2007;41:1–88. [PubMed] [Google Scholar]
- 2.Ludwig A, Hengel A. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Eurosurveillance. 2009;14:1–7. [PubMed] [Google Scholar]
- 3.Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol. 2015;204:263–271. doi: 10.1007/s00430-015-0399-9. [DOI] [PubMed] [Google Scholar]
- 4.Revello MG, Zavattoni M, Furione M, Lilleri D, Gorini G, Gerna G. Diagnosis and outcome of preconceptional and periconceptional primary human cytomegalovirus infections. J Infect Dis. 2002;186:553–557. doi: 10.1086/341831. [DOI] [PubMed] [Google Scholar]
- 5.Enders G, Daiminger A, Bäder U, Exler S, Enders M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol. 2011;52:244–246. doi: 10.1016/j.jcv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Voigt S, Schaffrath Rosario A, Mankertz A. Cytomegalovirus seroprevalence among children and adolescents in Germany: data from the German Health Interview and Examination Survey for Children and Adolescents (KiGGS), 2003-2006. Open Forum Infect Dis. 2015;3 doi: 10.1093/ofid/ofv193. ofv193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 8.Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134:972–982. doi: 10.1542/peds.2014-1173. [DOI] [PubMed] [Google Scholar]
- 9.Jones CA. Congenital cytomegalovirus infection. Curr Probl Pediatr Adolesc Health Care. 2003;33:70–93. doi: 10.1067/mps.2003.3. [DOI] [PubMed] [Google Scholar]
- 10.Kimberlin DW, Lin CY, Sánchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143:16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 11.Kimberlin DW, Jester PM, Sánchez PJ, et al. National institute of allergy and infectious diseases collaborative antiviral study group Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372:933–943. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol. 2006;35:216–220. doi: 10.1016/j.jcv.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Picone O, Vauloup-Fellous C, Cordier AG, et al. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn. 2013;33:751–758. doi: 10.1002/pd.4118. [DOI] [PubMed] [Google Scholar]
- 14.Gabrielli L, Bonasoni MP, Santini D, et al. Congenital cytomegalovirus infection: patterns of fetal brain damage. Clin Microbiol Infect. 2012;18:E419–E427. doi: 10.1111/j.1469-0691.2012.03983.x. [DOI] [PubMed] [Google Scholar]
- 15.La Torre R, Nigro G, Mazzocco M, Best AM, Adler SP. Placental enlargement in women with primary maternal cytomegalovirus infection is associated with fetal and neonatal disease. Clin Infect Dis. 2006;43:994–1000. doi: 10.1086/507634. [DOI] [PubMed] [Google Scholar]
- 16.Pereira L, Petitt M, Fong A, et al. Intrauterine growth restriction caused by underlying congenital cytomegalovirus infection. J Infect Dis. 2014;209:1573–1584. doi: 10.1093/infdis/jiu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler SP, Nigro G, Pereira L. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin Perinatol. 2007;31:10–18. doi: 10.1053/j.semperi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Goderis J, Keymeulen A, Smets K, et al. Hearing in children with congenital cytomegalovirus infection: results of a longitudinal study. J Pediatr. 2016;172:110–115. doi: 10.1016/j.jpeds.2016.01.024. e2. [DOI] [PubMed] [Google Scholar]
- 19.Karltorp E, Hellström S, Lewensohn-Fuchs I, Carlsson-Hansén E, Carlsson PI, Engman ML. Congenital cytomegalovirus infection—a common cause of hearing loss of unknown aetiology. Acta Paediatr. 2012;101:e357–e362. doi: 10.1111/j.1651-2227.2012.02711.x. [DOI] [PubMed] [Google Scholar]
- 20.Coll O, Benoist G, Ville Y, Weismann LE, Botet F. the WAPM Perinatal Infections Working Group: Guidelines on CMV congenital infection. J Perinat Med. 2009;37:433–445. doi: 10.1515/JPM.2009.127. [DOI] [PubMed] [Google Scholar]
- 21.Vauloup-Fellous C, Picone O, Cordier AG, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J Clin Virol. 2009;46:49–53. doi: 10.1016/j.jcv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Revello MG, Tibaldi C, Masuelli G, et al. Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine. 2015;2:1205–1210. doi: 10.1016/j.ebiom.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol. 2010;20:311–326. doi: 10.1002/rmv.659. [DOI] [PubMed] [Google Scholar]
- 24.Fu TM, An Z, Wang D. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine. 2014;32:2525–2533. doi: 10.1016/j.vaccine.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 25.Leruez-Ville M, Sellier Y, Salomon LJ, Stirnemann JJ, Jacquemard F, Ville Y. Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: a retrospective cohort. Clin Infect Dis. 2013;56:1428–1435. doi: 10.1093/cid/cit059. [DOI] [PubMed] [Google Scholar]
- 26.Leruez-Ville M, Stirnemann J, Sellier Y, et al. Feasibility of predicting the outcome of fetal infection with cytomegalovirus at the time of prenatal diagnosis. Am J Obstet Gynecol. 2016;342:e1–e9. doi: 10.1016/j.ajog.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi RS, Fernandez-Alvarez JR, Rabe H. Management of congenital cytomegalovirus infection: an evidence-based approach. Acta Paediatr. 2010;99:509–515. doi: 10.1111/j.1651-2227.2009.01655.x. [DOI] [PubMed] [Google Scholar]
- 28.Kadambari S, Williams EJ, Luck S, Griffiths PD, Sharland M. Evidence based management guidelines for the detection and treatment of congenital CMV. Early Hum Dev. 2011;87:723–728. doi: 10.1016/j.earlhumdev.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Lanari M, Lazzarotto T, Venturi V, et al. Neonatal cytomegalovirus load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns. J Pediatr. 2006;117:e76–e82. doi: 10.1542/peds.2005-0629. [DOI] [PubMed] [Google Scholar]
- 30.Cui X, Lee R, Adler SP, McVoy MA. Antibody inhibition of human cytomegalovirus spreadin epithelial cell cultures. J Virol Methods. 2013;192:44–50. doi: 10.1016/j.jviromet.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 32.Nigro Adler SP, La Torre R, Best AM. Congenital Cytomegalovirus Collaborating Group: Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton ST, van Zuylen W, Shand A, et al. Prevention of congenital cytomegalovirus complications by maternal and neonatal treatments: a systematic review. Rev Med Virol. 2014;24:420–433. doi: 10.1002/rmv.1814. [DOI] [PubMed] [Google Scholar]
- 34.Nigro G, Adler SP, Parruti G, et al. Immunoglobulin therapy of fetal cytomegalovirus infection occurring in the first half of pregnancy—a case-control study of the outcome in children. J Infect Dis. 2012;205:215–227. doi: 10.1093/infdis/jir718. [DOI] [PubMed] [Google Scholar]
- 35.Nigro G, Adler SP, Gatta E, et al. Fetal hyperechogenic bowel may indicate congenital cytomegalovirus disease responsive to immunoglobulin therapy. J Matern Fetal Neonatal Med. 2012;25:2202–2205. doi: 10.3109/14767058.2012.684111. [DOI] [PubMed] [Google Scholar]
- 36.Visentin S, Manara R, Milanese L, et al. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin Infect Dis. 2012;55:497–503. doi: 10.1093/cid/cis423. [DOI] [PubMed] [Google Scholar]
- 37.Nigro G, Capretti I, Manganello AM, Best AM, Adler SP. Primary maternal cytomegalovirus infections during pregnancy: association of CMV hyperimmune globulin with gestational age at birth and birth weight. J Matern Fetal Neonatal Med. 2015;28:168–171. doi: 10.3109/14767058.2014.907265. [DOI] [PubMed] [Google Scholar]
- 38.Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1–462e10. doi: 10.1016/j.ajog.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Oliver SE, Cloud GA, Sánchez PJ, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol. 2009;46:22–26. doi: 10.1016/j.jcv.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bilavsky E, Shahar-Nissan K, Pardo J, Attias J, Amir J. Hearing outcome of infants with congenital cytomegalovirus and hearing impairment. Arch Dis Child. 2016;101:433–438. doi: 10.1136/archdischild-2015-309154. [DOI] [PubMed] [Google Scholar]
- E1.Dupont L, Reeves MB. Cytomegalovirus latency and reactivation: recent insights into an age old problem. Rev Med Virol. 2016;26:75–89. doi: 10.1002/rmv.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Kabanova A, Marcandalli J, Zhou T, et al. Platelet-derived growth factor-a receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.82. 16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46:6–10. doi: 10.1016/j.jcv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- E4.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The „silent“ global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Hughes BL, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine (SMFM): Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5–B11. doi: 10.1016/j.ajog.2016.02.042. [DOI] [PubMed] [Google Scholar]
- E6.Jeon J, Victor M, Adler SP, et al. Knowledge and awareness of congenital cytomegalovirus among women. Infect Dis Obstet Gynecol. 2006: 80383 doi: 10.1155/IDOG/2006/80383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Enders G, Daiminger A, Lindemann L, et al. Cytomegalovirus (CMV) seroprevalence in pregnant women, bone marrow donors and adolescents in Germany, 1996-2010. Med Microbiol Immunol. 2012;201:303–309. doi: 10.1007/s00430-012-0232-7. [DOI] [PubMed] [Google Scholar]
- E8.Wang C, Zhang X, Bialek S, Cannon MJ. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis. 2011;52:e11–e13. doi: 10.1093/cid/ciq085. [DOI] [PubMed] [Google Scholar]
- E9.Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA. 2003;289:1008–1011. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- E10.Voigt S, Brune W. (Robert Koch-Institut): Die konnatale Cytomegalie: Ein unterschätztes Gesundheitsrisiko. 2008. www.bfr.bund.de/cm/343/die_konnatale_cytomegalie_ein_unterschaetztes_gesundheitsrisiko.pdf (last accessed on 10 March 2016) [Google Scholar]
- E11.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) Labordiagnostik schwangerschaftsrelevanter Virusinfektionen. S2k-Leitlinie. AWMF Registernummer 0093/001. Zytomegalie (Verantwortlicher Autor: Klaus Hamprecht): 179-203. www.awmf.org/uploads/tx_szleitlinien/093-001l_S2k_Labordiagnostik_schwangerschaftsrelevanter_Virusinfektionen_2014-05.pdf (last accessed on 1 October 2016) [Google Scholar]
- E12.Olbertz D, Voigt M, Straube S, et al. [Congenital malformations—a systematic cohort study from Mecklenburg-Western Pomerania (Germany)] Z Geburtshilfe Neonatol. 2010;214:243–248. doi: 10.1055/s-0030-1267187. [DOI] [PubMed] [Google Scholar]
- E13.Herrmann W, Obeid R. The mandatory fortification of staple foods with folic acid: a current controversy in Germany. Dtsch Arztebl Int. 2011;108:249–254. doi: 10.3238/arztebl.2011.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Gabrielli L, Bonasoni MP, Santini D, et al. Human fetal inner ear involvement in congenital cytomegalovirus infection. Acta Neuropathol Commun. 2013;1 doi: 10.1186/2051-5960-1-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E15.Ross SA, Boppana SB. Congenital cytomegalovirus infection: outcome and diagnosis. Semin Pediatr Infect Dis. 2005;16:44–49. doi: 10.1053/j.spid.2004.09.011. [DOI] [PubMed] [Google Scholar]
- E16.Engman ML, Lewensohn-Fuchs I, Mosskin M, Malm G. Congenital cytomegalovirus infection: the impact of cerebral cortical malformations. Acta Paediatr. 2010;99:1344–1349. doi: 10.1111/j.1651-2227.2010.01852.x. [DOI] [PubMed] [Google Scholar]
- E17.Bauer PW, Parizi-Robinson M, Roland PS, Yegappan S. Cytomegalovirus in the perilymphatic fluid. Laryngoscope. 2005;115:223–225. doi: 10.1097/01.mlg.0000154722.55044.fc. [DOI] [PubMed] [Google Scholar]
- E18.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135:60–64. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- E19.Korver AM, de Vries JJ, de Jong JW, Dekker FW, Vossen AC, Oudesluys-Murphy AM. Awareness of congenital cytomegalovirus among doctors in the Netherlands. J Clin Virol. 2009;46:11–15. doi: 10.1016/j.jcv.2009.09.006. [DOI] [PubMed] [Google Scholar]
- E20.Walker SP, Palma-Dias R, Wood EM, Shekleton P, Giles ML. Cytomegalovirus in pregnancy: to screen or not to screen. BMC Pregnancy Childbirth. 2013;13 doi: 10.1186/1471-2393-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5 doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E22.van Lier A, McDonald SA, Bouwknegt M, et al. Disease burden of 32 infectious diseases in the Netherlands, 2007-2011. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153106. e0153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Pass RF, Anderson B. Mother-to-child transmission of cytomegalovirus and prevention of congenital infection. J Pediatric Infect Dis Soc. 2014;(1):2–6. doi: 10.1093/jpids/piu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Plachter B. Prospects of a vaccine for the prevention of congenital cytomegalovirus disease. Med Microbiol Immunol. 2016;205:537–547. doi: 10.1007/s00430-016-0472-z. [DOI] [PubMed] [Google Scholar]
- E26.Hui L, Wood G. Perinatal outcome after maternal primary cytomegalovirus infection in the first trimester: a practical update and counseling aid. Prenat Diagn. 2015;35:1–7. doi: 10.1002/pd.4497. [DOI] [PubMed] [Google Scholar]
- E27.Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol. 2011;21:240–255. doi: 10.1002/rmv.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Andreoni KA, Wang X, Huong SM, Huang ES. Human CMV-IVIG (CytoGam®) neutralizes human cytomegalovirus (HCMV) infectivity and prevents intracellular signal transduction after HCMV exposure. Transpl Infect Dis. 2001;3:25–30. doi: 10.1034/j.1399-3062.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- E29.Rawlinson WD, Hamilton ST, van Zuylen WJ. Update on treatment of cytomegalovirus infection in pregnancy and of the newborn with congenital cytomegalovirus. Curr Opin Infect Dis. 2016;29:615–624. doi: 10.1097/QCO.0000000000000317. [DOI] [PubMed] [Google Scholar]
- E30.Amir J, Wolf DG, Levy I. Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur J Pediatr. 2010;169:1061–1067. doi: 10.1007/s00431-010-1176-9. [DOI] [PubMed] [Google Scholar]
- E31.Ramirez Inscoe JM, Nikolopoulos TP. Cochlear implantation in children deafened by cytomegalovirus: speech perception and speech intelligibility outcomes. Otol Neurotol. 2004;25:479–482. doi: 10.1097/00129492-200407000-00014. [DOI] [PubMed] [Google Scholar]
- E32.Harrison GJ. Current controversies in diagnosis, management, and prevention of congenital cytomegalovirus: updates for the pediatric practitioner. Pediatr Ann. 2015;44:e115–e125. doi: 10.3928/00904481-20150512-11. [DOI] [PubMed] [Google Scholar]
- E33.Ross DS, Victor M, Sumartojo E, Cannon MJ. Women’s knowledge of congenital cytomegalovirus: results from the 2005 HealthStyles survey. J Womens Health (Larchmt) 2008;17:849–858. doi: 10.1089/jwh.2007.0523. [DOI] [PubMed] [Google Scholar]