SUMMARY
The possibility of alloimmunization in patients receiving protein replacement therapy depends on (at least) three risk factors, which are necessary concomitantly but insufficient alone. The first is the degree of structural difference between the therapeutic protein and the patient’s own endogenous protein, if expressed. Such differences depend on the nature of the disease mutation and the pre-mutation endogenous protein structure as well as on post-translational changes and sequence-engineered alterations in the therapeutic protein. Genetic variations in the recipients’ immune systems comprise the second set of risk determinants for deleterious immune responses. For example, the limited repertoire of MHC-class II proteins encoded by a given person’s collection of HLA genes may or may not be able to present “foreign” peptide(s) produced from the therapeutic protein on the surface of their antigen-presenting cells (APCs). The third (and least characterized) variable is the presence or absence of immunologic “danger signals” during the display of foreign-peptide/MHC-complexes on APCs. A choice between existing therapeutic proteins or the design of less immunogenic protein drugs may require prior definition of the first two of these variables. This leads then to the possibility of developing personalized therapies for disorders due to genetic deficiencies in endogenous proteins, such as haemophilia A and B.
Keywords: Haemophilia A, Factor VIII Inhibitor, Nonsynonymous-SNPs, HLA-class II, Antigen-presentation repertoire, pharmacogenetics
Infusion of factor VIII (FVIII) concentrates derived from plasma donations or recombinant preparations has allowed successful management of haemophilia A (HA) during the past several decades [1]. The effectiveness of this strategy has been tempered by the development of alloantibodies, termed “inhibitors”, which neutralize the activity of FVIII replacement proteins [2]. Inhibitors develop in 20% or more of patients with severe HA overall [3,4]. Although clinical strategies for the management of patients with inhibitory antibodies to FVIII have improved, these interventions are extremely expensive and not always successful. Alloimmunized patients experience high levels of morbidity and mortality and a reduced quality of life [5].
Studies carried out over the last two decades have greatly expanded our understanding of the factors that contribute to the development of inhibitors in HA patients or, in other words, to the immunogenicity of the FVIII protein(s) in therapeutic replacement products. The complex pathogenesis of inhibitor development involves several variables including product characteristics, treatment issues, and patient genetics (see for example [6,7]). The most well established genetic determinant of alloimmunization risk is the type of FVIII gene (F8) mutation causing HA. This highly heterogeneous variable contributes to the structural difference between a patient’s abnormal endogenous FVIII protein (if any is produced) and the exogenous FVIII replacement protein, which, in turn, affects the degree to which the infused wild-type FVIII molecules may be seen as foreign by his specific immune system. Additional differences between exogenous (infused) and endogenous (dysfunctional hemophilic) FVIII proteins may occur due to bi-allelic nonsynonymous (ns)-single-nucleotide polymorphisms (SNPs) within the F8 gene. A ns-SNP encodes an amino acid residue that is distinct from the residue at the corresponding site in another version of the same protein but, by definition, does not cause HA. Although phenotypically “silent” with respect to hemophilia causation, all F8 ns-SNPs arose originally as single-base substitution mutations, i.e. the same pathogenetic mechanism that gave rise to the highly heterogeneous collection of (individually rare) missense mutations, which, through variable disruptions of FVIII function, together comprise the most common overall type of hemophilic F8 abnormality. Many SNPs, including a subset of ns-SNPs, reflect genetic changes that have occurred since ancestral populations separated by migration, and hence some of them are strongly associated with particular racial groups and/or geographic areas. In recent studies, race-associated differences were found in the distribution of several ns-SNPs in F8 [8], suggesting that they may contribute to the clinically-noted higher incidence of inhibitor development in HA patients with some black African ancestry (for whom we shall use the term “black”) [9–13]. Black HA patients have been observed to develop inhibitors more often than “white” patients with European Caucasian ancestry. The genetic basis for this increased risk has not yet been elucidated fully and is the subject of current research. We now propose that these observations could lay the groundwork for personalized FVIII replacement strategies — whether through intravenous infusion, as is currently performed, or by future gene-based delivery methods — that could reduce the incidence of alloimmunization in both previously-untreated and previously-treated patients. The use of FVIII proteins with more closely matched amino acid sequences could, in principle, also improve the efficacy of immune-tolerance induction in patients with pre-existing inhibitors.
The completion of the Human Genome Project and two generations of the International HapMap Project [14,15] have established that single-nucleotide substitutions constitute the most abundant type of genetic variation, occurring approximately once in every 100 to 300 bases [16]. These substitutions include variants with rare minor alleles found in <1% of the population(s) studied as well as polymorphisms (i.e., SNPs), which are found in 1% or more of the sampled population(s). These observations have raised the expectation in both the popular press [17] and the scientific literature [18] that pharmacogenetic approaches to the diagnosis and treatment of disease (also referred to as “personalized medicine”) could soon become a reality.
Initial pharmacogenetic approaches have focused on drug metabolizing enzymes [19–21] and transporters [22] that effect the disposition of small molecule drugs. In August 2007, the FDA announced the potential benefits of genetic testing for managing warfarin; thus, warfarin became the first drug with pharmacogenetic information included in the product label This was considered by many as a breakthrough that confirmed the utility of personalized medicine [23], however, its utility is now being questioned. There has been a steady increase in the use of pharmacogenetic data to enhance risk/benefit ratios; the product inserts of over a dozen drugs now carry pharmacogenetic information. However, progress is particularly lacking in the application of pharmacogenetics in the development and use of protein therapeutics [24]. It would be logical for drug development approaches to integrate pharmacogenetic information about both the protein-drug and its protein-target; nevertheless, given the complexity of biological systems, the selection of appropriate biomarkers and the study design remain a daunting task.
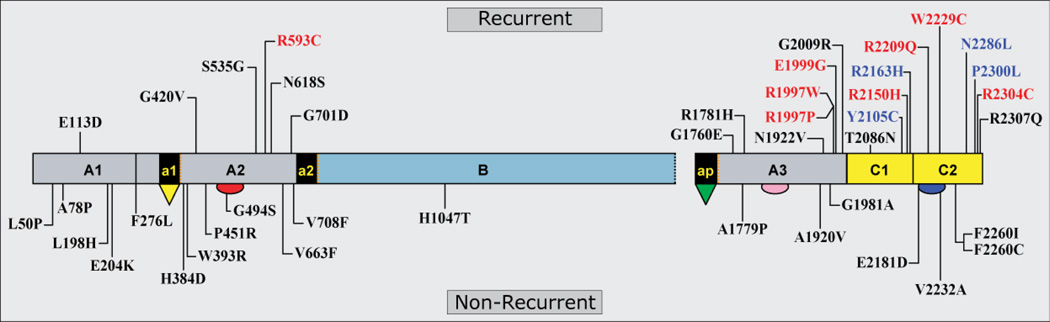
Can ns-SNPs in the F8 genes of HA patients be predictors (biomarkers) of immunogenicity for wild-type FVIII replacement proteins via the amino acid substitutions encoded in their endogenous (albeit nonfunctional or dysfunctional) FVIII proteins? Allelically mismatched ns-SNPs create structural differences between the endogenous and infused FVIII proteins that are comparable in scale to differences created by missense mutations, which comprise the most common type of HA-causing F8 abnormality. Although typically less than 5–10% of all HA patients with missense mutations become alloimmunized to replacement products, this low incidence partly reflects the fact that patients with mild HA require treatment infrequently and that the use of DDAVP is preferred to FVIII infusions whenever possible. The HAMSTeRS (Haemophilia A Mutations, Structure, Test and Resource Site) database (http://hadb.org.uk/) [25] lists inhibitor development in 15–50% of subjects with five highly recurrent missense mutations (Arg593Cys, Tyr2105Cys, Arg2150His, Trp2229Cys, or Pro2300Leu) [26–29]. These recurrent mutations, together with greater than 50 non-recurrent or less frequently recurring missense mutations identified in inhibitor patients, most of which are also reported in HAMSTeRS (Figure 1), provide evidence that wild-type FVIII proteins can be immunogenic even when infused in patients who express and circulate dysfunctional FVIII proteins with sequences that differ from the infused FVIII by as little as a single amino acid residue. Despite this knowledge, ns-SNPs are only now beginning to be rigorously sought and appropriately evaluated in studies of the FVIII immune response.
Figure 1.
Missense FVIII proteins expressed by HA patients with inhibitors (based on reference 25). This illustration of FVIII designates the location of 42 different F8 missense mutations, all of which were identified in at least one patient who developed an inhibitor during treatment. In these individuals, the replacement FVIII molecule differed from the endogenous mutant FVIII protein at only one amino acid residue (if recipient and therapeutic haplotypes matched), yet this difference was sufficient to induce alloimmunization. The bottom half of the figure shows 19 non-recurrent, “private” mutations that have been found in only one individual, while the top part shows 23 recurrent mutations that have arisen independently in more than one unrelated family. Among the recurrent mutations, those shown in black indicate that only a single individual with this mutation developed an inhibitor. Those shown in blue indicate that more than one individual developed an inhibitor. Those shown in red indicate that more than one individual developed an inhibitor and that at least one of these alloimmunized patients also developed an autoantibody (presumably through epitope spreading) against endogenous FVIII resulting in an undetectable FVIII:C level.
In a recent study, we identified four common ns-SNPs in the F8 genes of a small number of healthy unrelated individuals sampled from seven distinct racial groups (Figure 2A) [8]. The alleles of these ns-SNPs existed as six naturally-occurring combinations (“haplotypes”) referred to as H1–H6 (Figure 2B) [13]. Thus, these six haplotypic variations of F8 — all of which are wild-type — were observed in the healthy male and female subjects studied. Figure 2B shows the distribution of these six F8 haplotypes, as well as the six wild-type FVIII proteins they encode, in healthy Americans with either white European, black African, or Chinese ancestry. The majority of the white individuals in this study (>90%) had haplotype H1 while the rest carried H2. The black population in this study showed far greater diversity, with haplotypes H1 to H5 being represented in approximately 35%, 37%, 22%, 4% and 1% of the sampled individuals, respectively. Figure 2B shows the prevalence of inhibitor development in a cohort of 78 black American HA patients as well as the specific background F8 haplotypes on which their mutations arose; the distribution of patient haplotypes was comparable to that observed in the healthy black population [13]. Two previously unknown F8 ns-SNPs (A1229C encoding Gln334Pro and G4007A encoding Arg1260Lys) (Figure 2A), which were also identified in this cohort of 78 total black HA patients, defined two additional F8 haplotypes referred to as H7 and H8 (Figure 2B).
Figure 2.
Nonsynonymous-SNPs defining distinct haplotypes for FVIII proteins. To date, the human F8 gene has been found to contain four common and two less common ns-SNPs whose allelic combinations encode eight distinct wild-type FVIII proteins, only two of which have the amino acid sequences found in recombinant FVIII molecules used clinically [8,13]. Panel A illustrates both F8, with its 26 exons and 25 introns indicated by red triangles and intervening lines, respectively, and the FVIII protein, with the A1, A2, and A3 domains shown in gray, the B domain in blue, and the C1 and C2 domains in yellow; the three acidic regions a1, a2, and ap are shown in black. A region in both the A2 domain (red oval) and the C2 domain (blue oval) are known to be immunodominant B-cell inhibitor epitopes. By sequencing all 26 exons of the F8 gene in 137 unrelated healthy persons from seven racial groups, four common ns-SNPs were identified: one in exon 10 (G1679A), two in exon 14 (A2554G and C3951G), and one in exon 25 (A6940G). These polymorphisms encode the following amino acid substitutions, respectively: histidine for arginine at position 484 (R484H), glycine for arginine at position 776 (R776G), glutamic acid for aspartic acid at position 1241 (D1241E), and valine for methionine at position 2238 (M2238V). The numbering systems used to designate the four ns-SNPs and the amino acid substitutions they encode are based on their nucleotide and residue locations, respectively, in the full-length F8 complementary DNA and the mature circulating form of FVIII. R484H and M2238V are components of the A2 and C2 domains, which are both known to be immunogenic regions of FVIII. Panel B shows eight structurally distinct wild-type FVIII proteins encoded by the naturally occurring allelic combinations (haplotypes) of the F8 nonsynonymous SNPs G1679A, A2554G, C3951G, A6940G, A1229C, and G4007A. Amino acid residues at positions 334 (Q or P), 484 (R or H), 776 (R or G), 1241 (D or E), 1260 (R or K), and 2238 (M or V) are shown. Indicated are the frequencies (f) of FVIII proteins H1–H6 in healthy persons — including some females providing two X-chromosomes for analysis — 86 white (fwhite), 67 black (fblack), and 10 Chinese (fChinese), and also a cohort of 78 black HA patients; FVIII proteins H7 and H8 are based on two novel ns-SNPs, each found in a single patient of this cohort. The last column shows the frequency of inhibitor development in each haplotype group among these black patients, i.e. the number of inhibitor patients divided by the total number of patients with any given haplotype (percentage). In Panel C, the two full-length recombinant FVIII proteins used in replacement therapy, Kogenate (same as Helixate) and Recombinate (same as Advate), contain the same amino acid sequences found in H1 (Q–R–R–D–R–M) and H2 (Q–R–R–E–R–M), respectively. The B-domain deleted recombinant FVIII protein, Refacto (same as Xyntha), does not contain the ns-SNP site differentiating Kogenate and Recombinate (D1241E).
The recombinant FVIII products currently used for HA replacement therapy correspond to either haplotype H1 or H2, both of which are common in white populations with European ancestry [13]. As a result, white patients — all (or most) of whom will have an H1 or H2 background haplotype — treated with the currently available recombinant products can receive a matched (or more accurately a “least mismatched”) FVIII protein, i.e. one that differs from their defective endogenous FVIII protein (if any is produced) only at the sites encoded by their HA-causing F8 mutations. Patients infused with plasma-derived products may also be receiving FVIII proteins that are matched to a greater or lesser extent to their endogenous FVIII sequence, depending on their background haplotypes and the haplotypes of the donors who contributed to the plasma pool [13]. Our earlier study, in contrast, indicated that approximately one in four African American HA patients have a background haplotype other than H1 or H2. The currently available recombinant FVIII proteins are thus mismatched at one or more of the sites encoded by ns-SNPs, in addition to the site corresponding to the hemophilic F8 mutation (Figure 2). Although D1241E, the ns-SNP site that differentiates haplotypes H1 and H2, is removed in B-domain deleted FVIII (Figure 2C), an additional amino acid sequence mismatch exists between the endogenous dysfunctional FVIII proteins in patients and this recombinant product at its non-naturally occurring B-domain junction [30]. This newly manufactured junction site is “foreign” to all patients but will likely only be immunogenic in a subset of patients.
Considered together, our findings provide one plausible mechanistic explanation for reports that black HA patients are approximately twice as likely as white HA patients to produce inhibitors against therapeutic FVIII proteins [9–12]. Moreover, our findings are consistent with the principle that there is a greater risk of developing anti-drug antibodies where the sequences of endogenous and infused proteins differ at one or more sites other than those causing HA. Specifically, we found that approximately 20% of African American HA patients carrying the H1 or H2 variation of F8, who therefore received matched (or least-mismatched) infusions with recombinant FVIII, developed inhibitors; this is equivalent to the overall rate observed in prior studies that have been comprised predominantly of white HA patients [31]. On the other hand, black HA patients with the H3 or H4 haplotype, which are mismatched with all currently available recombinant FVIII products, and likely with most of the FVIII contained in plasma-derived FVIII products in the U.S. (which are typically derived from a predominantly white blood donor population), developed inhibitors at about three times the rate of black patients with the H1 or H2 haplotype [13].
As described above, differences between the infused and endogenous FVIII in patients may arise naturally, due to ns-SNPs and/or the causal F8 mutation, or from structural alterations of recombinant products, e.g. due to distinct post-translational modifications, or to sequence engineering for increasing protein expression yield [30] or prolonging protein half-life in patients’ circulation [32–34]. Thus, patients infused with “mismatched” FVIII may be exposed to neo-epitopes that can cause immune responses. HA patients with major F8 gene deletions or premature stop codons will obviously have the greatest degree of mismatch between their endogenous FVIII and a therapeutic FVIII product. The most common defect causing HA is the intron-22 inversion (I22-inv) that occurs in approximately 40% of all severely affected patients [35]. An intron-22 inverted F8 allele cannot be transcribed into a full-length mRNA since the promoter region and the adjacent gene region containing exons 1–22 has been inverted [36]. In I22-inv patients, exons 1 through 22 are transcribed as a polyadenylated fusion transcript in which two (or more) unrelated 3’-exons have replaced F8 exons 23 through 26. However, intron 22 of the F8 locus also contains two nested genes, F8A and F8B, which are controlled by a CpG-island containing bidirectional promoter [37,38]. In I22-inv patients, transcription and translation of the F8B gene would be predicted to generate a polypeptide encoded by exons 23–26; this putative protein is referred to as F8B. If a partial FVIII protein encoded by the mRNA containing exons 1–22 is expressed, along with the F8B protein, then one would expect that I22-inv patients would be more likely to tolerate infused FVIII, unless “mismatched” amino acid sequences, e.g. at the exon−22/−23 junction region, were recognized as foreign by their immune systems. This hypothesis is consistent with clinical observations indicating that only about one in five patients with the I22-inv develop inhibitory antibodies [39], whereas inhibitor frequencies of close to 90% have been observed in patients with large deletions involving multiple exons, whose plasma also tests antigenically negative for FVIII-cross-reactive material (“CRM–”) [28]. However, tolerance could be broken (or not achieved in the first place) if I22-inv patients were infused with a FVIII protein containing an immunogenic sequence variation, e.g. due to one or more mismatched ns-SNPs in their F8 gene.
Structural differences between endogenous and therapeutic FVIII proteins meet the first and minimal requirement for eliciting an immune response, but such differences are not sufficient and thus may or may not be immunogenic in a given individual. Differences in the immune system from one person to another are also of critical importance. FVIII inhibitor responses are mediated by helper T cells [40]. Therefore, the limited collection of major-histocompatibility complex (MHC) genes (and alleles) in a given patient will determine whether a particular “mismatched” FVIII sequence can be presented by the restricted repertoire of MHC-class II molecules on their antigen-presenting cells (APCs). Additional genetic variations may influence events following antigen presentation, including avidity of interactions with T-cell receptors on responding T cells. These variations, plus the presence or absence of “danger signals” — and the co-stimulatory interactions they induce between APCs and T cells — will influence the evolution of the immune response, e.g. along tolerogenic or immunogenic pathways [41].
CD4+ T-cell epitopes are linear stretches of 9–14 amino acid residues that bind to a specific groove on the surface of an MHC-class II molecule. Foreign proteins (together with “self” proteins co-internalized from the vascular space) are broken down into peptides by enzymes in the MHC-class II compartment of APCs. Although large numbers of peptide fragments are released, only about 2% of all the fragments generated have permissive structures — based on their amino acid side chain and backbone conformation — that allow them to interact strongly with the residues comprising the binding groove of a given MHC molecule. A critical determinant of immunogenicity for a T-cell epitope is the strength of its binding to one or more MHC molecules. As alluded to above, the development of an antibody response also requires a secondary danger signal; in the case of infectious immunity this is provided by repetitive structural components of bacteria or viruses that are recognized by toll-like receptors as non-self and thus dangerous to self [41]. The nature of danger signals that may accompany intravenous infusions of FVIII and their role in inhibitor development is poorly understood and is a subject of current research. The MHC proteins, which in humans comprise the human leukocyte antigen (HLA) system, are extremely polymorphic [42]. Figure 3 illustrates this diversity in the structural DRB1 gene (DRB1), which is by far the most variable of the HLA-class II loci, using data from the U.S. National Marrow Donor Program [43,44]. It is not unreasonable to hypothesize that HA patients with certain HLA-class II alleles and/or haplotypes will be more susceptible to T-cell stimulation by particular variant sequences in therapeutic FVIII than patients with other alleles and/or haplotypes. Indeed, the identification of HLA-restricted T-cell responses against wild-type peptides, which correspond to endogenous FVIII regions with missense mutations in several mild HA patients [45–47], serves as proof-of-principle that even a single amino acid change can cause T-cell stimulation leading to inhibitor development when the patient is infused with FVIII that differs at this site. Some higher-risk HLA haplotypes (i.e., those encoding HLA-class II molecules that can effectively bind and present particular FVIII sequence variants) may be more prevalent in some racial/ethnic groups than in others. We are currently investigating associations of genetic variations with inhibitor prevalence. Cell-based assays are also being used to directly test the immunogenicity of FVIII sequence variations due to both hemophilic missense mutations and non-hemophilic ns-SNPs. It is hoped that the results of these and similar studies will identify immunodominant epitopes in FVIII and thus motivate the development of new therapeutic FVIII products targeted to particular populations. We expect that susceptible patient populations will increasingly be defined by the results of genetic tests that are more specific, i.e. predictive of potential immune responses. Such genetic markers will be more predictive of inhibitor risk in certain sub-populations than the general assumption of a higher inhibitor risk in black HA patients. Increasing knowledge of immune mechanisms leading to neutralizing antibodies will also suggest new therapeutic approaches targeted to specific at-risk sib-populations of HA patients. We anticipate that genotyping of HA patients will eventually include testing for the presence of particular SNPs in both F8 and immune regulatory genes, e.g. those encoding the HLA-class II antigen presentation repertoire, in addition to the HA-causing F8 mutation. Appropriate genetic testing will allow better matching of HA patients with FVIII products that are likely to be less immunogenic. The development of next-generation “personalized” approaches to hemophilia therapy should decrease the incidence of inhibitor responses in HA patients.
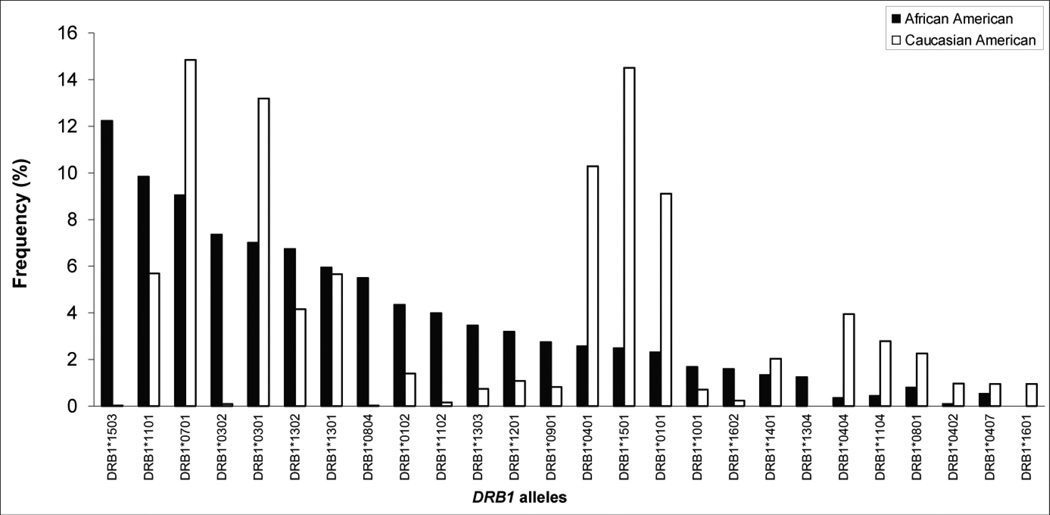
Figure 3.
Population-associated allele frequencies at the highly polymorphic DRB1 structural locus (DRB1). Data in this figure are redrawn from information provided in references 43 and 44. Black and white columns represent the 20 most frequent DRB1 alleles in African Americans (found in between 12.2% to 1.2% of that population) and the 20 most frequent DRB1 alleles in Caucasian Americans (found in between 14.9% to 0.7% of that population), respectively. Most of these alleles are not found exclusively in either population, e.g. DRB1*1503, the most common allele in the black population, is also found in approximately 0.2% of the white population. While these differences in allele frequencies may have arisen through known population genetic mechanisms, racial admixture cannot be excluded. The data show that some alleles are common in persons with either black African or white Caucasian genetic ancestry, whereas others are clearly more common in particular racial groups (e.g. DRB1*1503 in blacks). The 27 alleles shown here represent only a small subset of the more than 800 DRB1 alleles identified in the human population to date.
Acknowledgments
We thank Drs. Kenneth Mann, David Lillicrap, Georg Lemm, Yvette Latchman, and Glenn Pierce for critical reading of the manuscript and helpful suggestions. Support for this study included the ARRA-funded NIH/NHLBI grant 1RC2-HL101851 (TEH and KPP) and grants from the Bayer Hemophilia Awards Program (KPP and TEH) and the CSL Behring Foundation (KPP). We thank the following Community Advisory Board members who reviewed the manuscript and who are providing ethical oversight for studies associated with NIH-1RC2-HL101851: Dr. Louis Aledort (Chair), Ms. Toni Allen-Ellingson, Ms. Olita Fitzgerald, Dr. William Hobbs, and Dr. Yvette Latchman.
REFERENCES
- 1.Mannucci PM, Tuddenham EG. The hemophilias--from royal genes to gene therapy. N Engl J Med. 2001;344:1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 2.Dasgupta S, Navarrete AM, Delignat S, et al. Immune response against therapeutic factor VIII in hemophilia A patients--a survey of probable risk factors. Immunol Lett. 2007;110:23–28. doi: 10.1016/j.imlet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 3.de Biasi R, Rocino A, Papa ML, Salerno E, Mastrullo L, De Blasi D. Incidence of factor VIII inhibitor development in hemophilia A patients treated with less pure plasma derived concentrates. Thromb Haemost. 1994;71:544–547. [PubMed] [Google Scholar]
- 4.Ehrenforth S, Kreuz W, Scharrer I, et al. Incidence of development of factor VIII and factor IX inhibitors in haemophiliacs. Lancet. 1992;339:594–598. doi: 10.1016/0140-6736(92)90874-3. [DOI] [PubMed] [Google Scholar]
- 5.Gringeri A, Mantovani LG, Scalone L, Mannucci PM. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood. 2003;102:2358–2363. doi: 10.1182/blood-2003-03-0941. [DOI] [PubMed] [Google Scholar]
- 6.Lacroix-Desmazes S, Navarrete AM, Andre S, Bayry J, Kaveri SV, Dasgupta S. Dynamics of factor VIII interactions determine its immunologic fate in hemophilia A. Blood. 2008;112:240–249. doi: 10.1182/blood-2008-02-124941. [DOI] [PubMed] [Google Scholar]
- 7.Zhang AH, Skupsky J, Scott DW. Factor VIII inhibitors: risk factors and methods for prevention and immune modulation. Clin Rev Allergy Immunol. 2009;37:114–124. doi: 10.1007/s12016-009-8122-5. [DOI] [PubMed] [Google Scholar]
- 8.Viel KR, Machiah DK, Warren DM, et al. A sequence variation scan of the coagulation factor VIII (FVIII) structural gene and associations with plasma FVIII activity levels. Blood. 2007;109:3713–3724. doi: 10.1182/blood-2006-06-026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aledort LM, Dimichele DM. Inhibitors occur more frequently in African-American and Latino haemophiliacs. Haemophilia. 1998;4:68. doi: 10.1046/j.1365-2516.1998.0146c.x. [DOI] [PubMed] [Google Scholar]
- 10.Astermark J, Berntorp E, White GC, Kroner BL. The Malmo International Brother Study (MIBS): further support for genetic predisposition to inhibitor development in hemophilia patients. Haemophilia. 2001;7:267–272. doi: 10.1046/j.1365-2516.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 11.Bray GL, Gomperts ED, Courter S, et al. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. The Recombinate Study Group. Blood. 1994;83:2428–2435. [PubMed] [Google Scholar]
- 12.Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. Kogenate Previously Untreated Patient Study Group. N Engl J Med. 1993;328:453–459. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 13.Viel KR, Ameri A, Abshire TC, et al. Inhibitors of factor VIII in black patients with hemophilia. N Engl J Med. 2009;360:1618–1627. doi: 10.1056/NEJMoa075760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altshuler D, Brooks LD, Chakravarti A, Collins FS, Consortium IH. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazer KA, Ballinger DG, Cox DR, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruglyak L, Nickerson DA. Variation is the spice of life. Nat Genet. 2001;27:234–236. doi: 10.1038/85776. [DOI] [PubMed] [Google Scholar]
- 17.Collins FJ. Personalized medicine: A new approach to staying well. Boston Globe. 2005 [Google Scholar]
- 18.Guttmacher AE, McGuire AL, Ponder B, Stefansson K. Personalized genomic information: preparing for the future of genetic medicine. Nat Rev Genet. 11:161–165. doi: 10.1038/nrg2735. [DOI] [PubMed] [Google Scholar]
- 19.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 20.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 21.Salavaggione OE, Wang L, Wiepert M, Yee VC, Weinshilboum RM. Thiopurine S-methyltransferase pharmacogenetics: variant allele functional and comparative genomics. Pharmacogenet Genomics. 2005;15:801–815. doi: 10.1097/01.fpc.0000174788.69991.6b. [DOI] [PubMed] [Google Scholar]
- 22.Sadee W, Dai Z. Pharmacogenetics/genomics and personalized medicine. Hum Mol Genet. 2005;14(Spec No. 2):R207–R214. doi: 10.1093/hmg/ddi261. [DOI] [PubMed] [Google Scholar]
- 23.Kurnik D, Loebstein R, Halkin H, Gak E, Almog S. 10 years of oral anticoagulant pharmacogenomics: what difference will it make? A critical appraisal. Pharmacogenomics. 2009;10:1955–1965. doi: 10.2217/pgs.09.149. [DOI] [PubMed] [Google Scholar]
- 24.Krejsa C, Rogge M, Sadee W. Protein therapeutics: new applications for pharmacogenetics. Nat Rev Drug Discov. 2006;5:507–521. doi: 10.1038/nrd2039. [DOI] [PubMed] [Google Scholar]
- 25.Kemball-Cook G, Tuddenham EG, Wacey AI. The factor VIII Structure and Mutation Resource Site: HAMSTeRS version 4. Nucleic Acids Res. 1998;26:216–219. doi: 10.1093/nar/26.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodeve A. The incidence of inhibitor development according to specific mutations--and treatment? Blood Coagul Fibrinolysis. 2003;14(Suppl 1):S17–S21. doi: 10.1097/00001721-200306001-00005. [DOI] [PubMed] [Google Scholar]
- 27.Hay CR. Factor VIII inhibitors in mild and moderate-severity haemophilia A. Haemophilia. 1998;4:558–563. doi: 10.1046/j.1365-2516.1998.440558.x. [DOI] [PubMed] [Google Scholar]
- 28.Oldenburg J, Pavlova A. Genetic risk factors for inhibitors to factors VIII and IX. Haemophilia. 2006;12(Suppl 6):15–22. doi: 10.1111/j.1365-2516.2006.01361.x. [DOI] [PubMed] [Google Scholar]
- 29.Peerlinck K, Jacquemin MG, Arnout J, et al. Antifactor VIII antibody inhibiting allogeneic but not autologous factor VIII in patients with mild hemophilia A. Blood. 1999;93:2267–2273. [PubMed] [Google Scholar]
- 30.Eriksson RK, Fenge C, Lindner-Olsson E, et al. The manufacturing process for B-domain deleted recombinant factor VIII. Semin Hematol. 2001;38:24–31. doi: 10.1016/s0037-1963(01)90105-2. [DOI] [PubMed] [Google Scholar]
- 31.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9:418–435. doi: 10.1046/j.1365-2516.2003.00780.x. [DOI] [PubMed] [Google Scholar]
- 32.Mei B, Pan C, Jiang H, et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116:270–279. doi: 10.1182/blood-2009-11-254755. [DOI] [PubMed] [Google Scholar]
- 33.Schulte S. Use of albumin fusion technology to prolong the half-life of recombinant factor VIIa. Thromb Res. 2008;122(Suppl 4):S14–S19. doi: 10.1016/S0049-3848(08)70029-X. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Ishii-Watabe A, Tada M, et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol. 2010;184:1968–1976. doi: 10.4049/jimmunol.0903296. [DOI] [PubMed] [Google Scholar]
- 35.Graw J, Brackmann HH, Oldenburg J, Schneppenheim R, Spannagl M, Schwaab R. Haemophilia A from mutation analysis to new therapies. Nat Rev Genet. 2005;6:488–501. doi: 10.1038/nrg1617. [DOI] [PubMed] [Google Scholar]
- 36.Lakich D, Kazazian HH, Jr, Antonarakis SE, Gitschier J. Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nat Genet. 1993;5:236–241. doi: 10.1038/ng1193-236. [DOI] [PubMed] [Google Scholar]
- 37.Levinson B, Kenwrick S, Gamel P, Fisher K, Gitschier J. Evidence for a third transcript from the human factor VIII gene. Genomics. 1992;14:585–589. doi: 10.1016/s0888-7543(05)80155-7. [DOI] [PubMed] [Google Scholar]
- 38.Levinson B, Kenwrick S, Lakich D, Hammonds G, Jr, Gitschier J. A transcribed gene in an intron of the human factor VIII gene. Genomics. 1990;7:1–11. doi: 10.1016/0888-7543(90)90512-s. [DOI] [PubMed] [Google Scholar]
- 39.Antonarakis SE, Rossiter JP, Young M, et al. Factor VIII gene inversions in severe hemophilia A results of an international consortium study. Blood. 1995;86:2206–2212. [PubMed] [Google Scholar]
- 40.Qian J, Collins M, Sharpe AH, Hoyer LW. Prevention and treatment of factor VIII inhibitors in murine hemophilia A. Blood. 2000;95:1324–1329. [PubMed] [Google Scholar]
- 41.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 42.Geraghty DE, Daza R, Williams LM, Vu Q, Ishitani A. Genetics of the immune response: identifying immune variation within the MHC and throughout the genome. Immunol Rev. 2002;190:69–85. doi: 10.1034/j.1600-065x.2002.19006.x. [DOI] [PubMed] [Google Scholar]
- 43.Mack SJ, Tu B, Lazaro A, et al. HLA-A, -B, -C, and -DRB1 allele and haplotype frequencies distinguish Eastern European Americans from the general European American population. Tissue Antigens. 2009;73:17–32. doi: 10.1111/j.1399-0039.2008.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu B, Mack SJ, Lazaro A, et al. HLA-A, -B, -C, -DRB1 allele and haplotype frequencies in an African American population. Tissue Antigens. 2007;69:73–85. doi: 10.1111/j.1399-0039.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- 45.Ettinger RA, James EA, Kwok WW, Thompson AR, Pratt KP. Lineages of human T-cell clones, including T helper 17/T helper 1 cells, isolated at different stages of anti-factor VIII immune responses. Blood. 2009;114:1423–1428. doi: 10.1182/blood-2009-01-200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ettinger RA, James EA, Kwok WW, Thompson AR, Pratt KP. HLA-DR-restricted T-cell responses to factor VIII epitopes in a mild haemophilia A family with missense substitution A2201P. Haemophilia. 2010;16:44–55. doi: 10.1111/j.1365-2516.2008.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James EA, Kwok WW, Ettinger RA, Thompson AR, Pratt KP. T-cell responses over time in a mild hemophilia A inhibitor subject: epitope identification and transient immunogenicity of the corresponding self-peptide. J Thromb Haemost. 2007;5:2399–2407. doi: 10.1111/j.1538-7836.2007.02762.x. [DOI] [PubMed] [Google Scholar]