Abstract
Background
This is a cross-sectional study designed to evaluate the histologic characteristics of graft injury in the presence of anti-angiotensin II type 1 receptor antibody (AT1R-Ab) and anti-endothelial cell antibody (AECA).
Methods
Non-HLA antibody testing was included in the posttransplant evaluation for 70 kidney recipients. Biopsies were performed for cause for 47 patients and as protocol for the remaining 23 patients. Biopsy-proven rejection was defined according to the Banff 2009-2013 criteria. AT1R-Ab was measured on an ELISA platform. Patients were divided into 3 groups based on AT1R-Ab levels (>17, 10-17, and <10 U/ml). AECA was evaluated using an endothelial cell crossmatch (ECXM) in patients whose HLA antibody level was insufficient to cause a positive flow cytometric crossmatch.
Results
AT1R-Ab levels were higher in patients diagnosed with antibody mediated rejection compared to those with no rejection (P = 0.004). Glomerulitis (g) and peritubular capillaritis (ptc) scores were independently correlated with increased AT1R-Ab concentrations in the presence or absence of HLA-DSA (P = 0.007 and 0.03 for g scores; p = 0.005 and 0.03 for ptc scores). Patients with a positive ECXM had higher AT1R-Ab levels compared to those with a negative ECXM (P = 0.005). Microcirculation inflammation (MCI = g + ptc score) was higher in patients with a positive ECXM and with AT1R-Ab >17 U/ml, although this did not reach statistical significance (P = 0.07).
Conclusions
The data show an association between non-HLA antibodies detected in the ECXM and AT1R ELISA and microvascular injury observed in antibody mediated rejection.
The histologic characteristics of kidney graft injury are examined in terms of the presence of anti-angiotensin II type 1 receptor antibody (AT1R-Ab) and anti-endothelial cell antibody (AECA). The presence of AECA or AT1R-Ab correlates with microvascular injury observed in antibody-mediated rejection.
Numerous reports have provided evidence for an association between angiotensin II type 1 receptor antibodies (AT1R-Ab)1-6 and/or endothelial cell specific antibodies (AECA)7,8 with the development of antibody mediated rejection and kidney allograft failure. Furthermore, mechanistic studies have shown that these non-HLA antibodies may directly contribute to allograft dysfunction.9,10 Despite these observations, testing for presence of non-HLA antibodies is often performed when donor-specific HLA antibodies (HLA-DSA) are not identified in the sera of patients who are experiencing allograft dysfunction.
Current guidelines for diagnosing antibody mediated rejection require the presence of donor-specific antibody (HLA or non-HLA) with evidence of renal microcirculation inflammation.11,12 The relevance of HLA-DSA in allograft damage has been substantiated by observed morphological changes in the biopsies at time of graft dysfunction, including evidence of complement activation.13,14 Targets of AT1R-Ab and some AECA may be polymorphic and are constitutively expressed on the vascular endothelium, and expression may be induced or increased during inflammatory events. Given that targets of AT1R-Ab and some AECA are expressed on the vascular endothelium,8 analysis of the phenotypic characteristics of biopsies in the presence of non-HLA antibodies would potentially provide further evidence linking them to allograft dysfunction.
In this study, we examined the histopathologic characteristics associated with allograft dysfunction in the presence of AT1R-Ab and AECA both alone and in the presence of HLA-DSA and determined if damage was exacerbated when both HLA and non-HLA antibodies were present together.
RESULTS
Characteristics of Study Population
Posttransplant biopsies, HLA-DSA, and AT1R-Ab assessments were performed for 70 patients who received a kidney transplant between 1988 and 2014. These tests were performed to investigate allograft dysfunction in 47 patients (67%) and as protocol in the remaining 23 patients (33%). The cohort was divided into 3 groups based on the AT1R-Ab levels [group 1, high level: >17 U/ml, n = 21 (30%); group 2, moderate level: 10-17 U/ml, n = 27 (38%); and group 3, low level: <10 U/ml, n = 22 (31%)].
The characteristics of the patients and their donors were comparable among the 3 groups (Table 1). The average estimated glomerular filtration rate (eGFR; ml/min/1.73 m2) was less than 60 ml/min/1.73 m2 in all 3 groups (48, 44, and 43 ml/min/1.73 m2, respectively; P = 0.7). The distribution of males and females among the 3 AT1R-Ab groups was skewed with fewer females in group 1 (AT1R-Ab >17 U/ml) and the converse in group 3 (AT1R-Ab <10 U/ml), and the difference approached statistical significance (P = 0.07). The number of patients who were treated with an angiotensin receptor blocker (ARB; losartan or valsartan) for their hypertension was higher in group 1 (48%) compared to group 2 (26%) and group 3 (23%), although this did not reach statistical significance (P = 0.2).
TABLE 1.
Patient and donor demographics
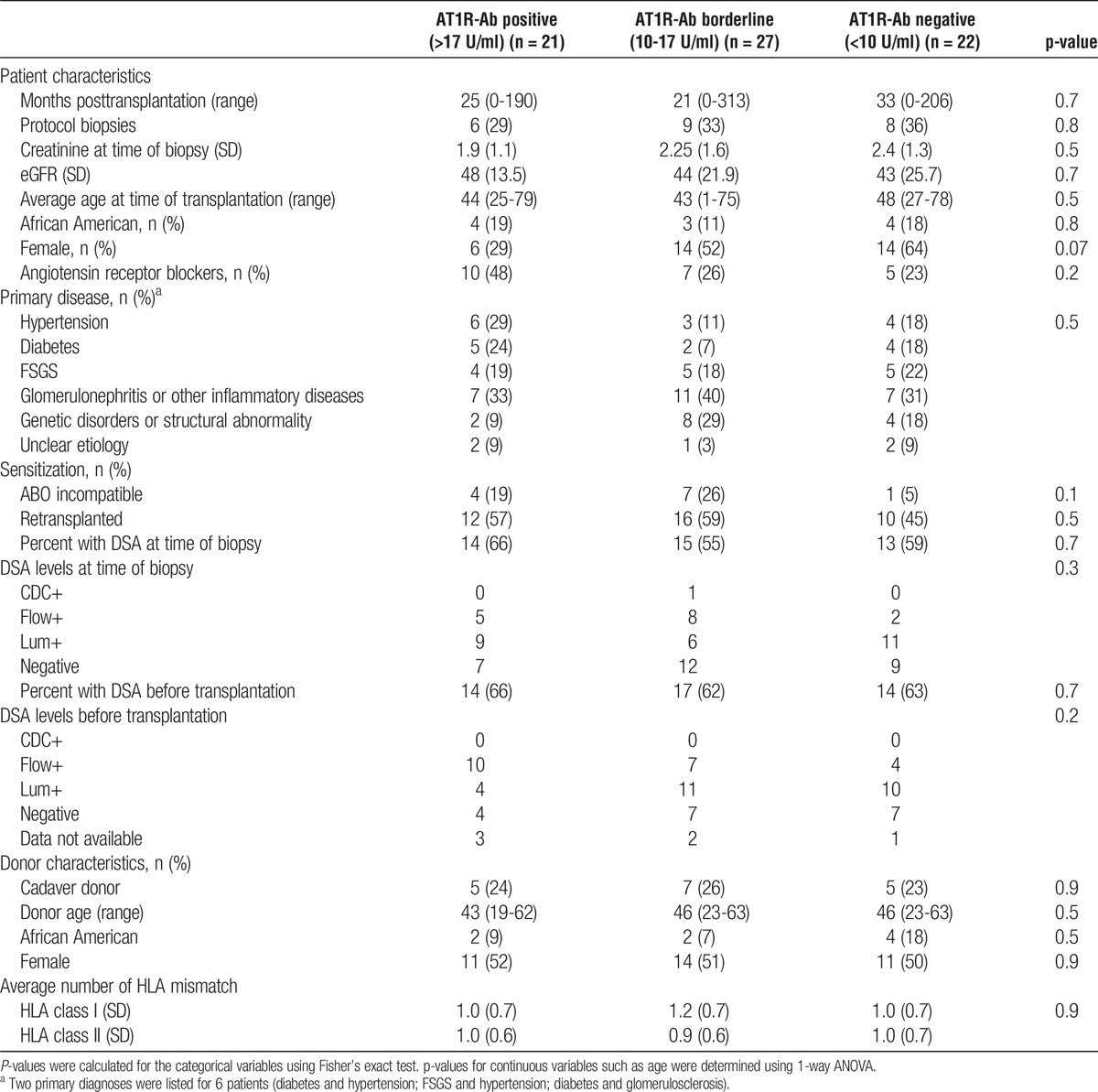
There was no significant difference among the 3 AT1R-Ab groups in the distribution of patients with known increased risks for rejection, such as African-Americans (P = 0.8) and recipients who had a previous transplant (P = 0.5). Among the 12 patients who were transplanted with an ABO incompatible donor, the ABO titer ranged between 0 and 32 at time of allograft dysfunction. The distribution of patients who underwent desensitization at time of transplantation due to HLA-DSA incompatibility (HLAi) or ABO incompatibility (ABOi) did not differ significantly between the 3 groups (P = 0.2 for HLAi and P = 0.1 for ABOi).
HLA-DSA is a well-recognized risk factor for graft dysfunction due to antibody mediated rejection (AMR). At the time of suspected allograft dysfunction, there was no significant difference between the number of patients who had HLA-DSA (P = 0.7) or in the strength of their antibodies (P = 0.3) among the 3 AT1R-Ab levels. The strength of HLA-DSA before transplantation was also not significantly different between the 3 groups (P = 0.7).
Overall, the data in Table 1 show that factors associated with an increased risk of acute rejection were not statistically different among the 3 AT1R-Ab levels.
Rejection in the 3 AT1R-Ab Groups
Analysis of the biopsies performed at time of antibody testing indicated that 31 (44%) of the patients had antibody mediated rejection (AMR); 1 of whom had both antibody and cell mediated rejection. Seven patients (10%) had only cell mediated rejection (CMR) and 30 patients (42.8%) had no rejection. Transplant glomerulopathy (TG), with no acute rejection detected, was present in the remaining 2 biopsies. We compared AT1R-Ab levels in patients with antibody and cell mediated rejection against those with no rejection and found a significant difference between the AMR and no rejection groups (P = 0.004) but no difference between the CMR and no rejection groups (P = 0.7) (Figure 1). The mean AT1R-Ab concentration was 18.8 ± 10.6 U/ml for patients diagnosed with AMR compared to 11.8 ± 7.4 U/ml for those with no rejection and 12.9 ± 6.7 U/ml for patients with CMR.
FIGURE 1.
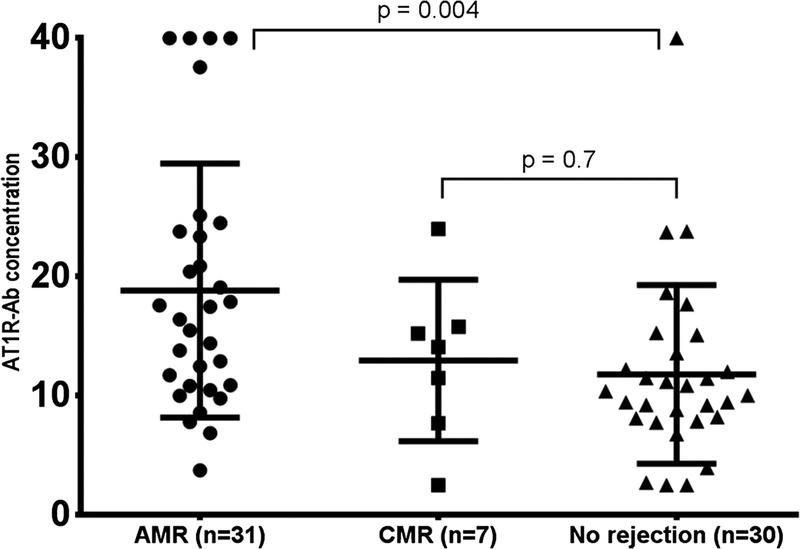
AT1R-Ab concentration in patients with AMR, CMR, and no rejection. AT1R-Ab concentrations were significantly higher in patients with AMR (mean AT1R-Ab concentration 18.8 ± 10.6 U/ml) compared to those with no rejection (mean AT1R-Ab concentration 11.8 ± 7.4 U/ml). No significant difference was observed for patients with CMR (mean AT1R-Ab concentration 12.9 ± 6.7 U/ml) versus no rejection. AT1R-Ab concentrations for two remaining biopsies were reported as transplant glomerulopathy and were 9.4 and 10.9 U/ml, respectively.
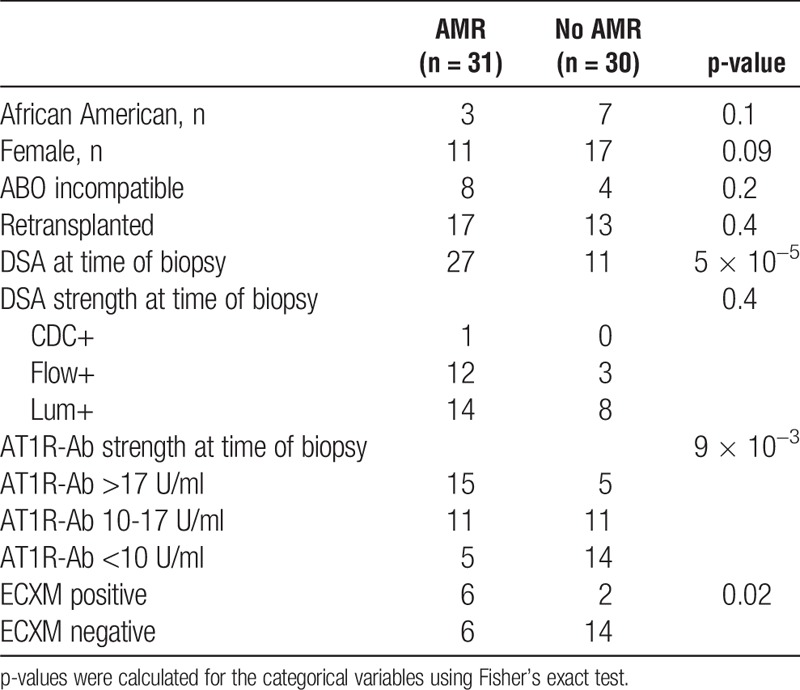
To further investigate the association between AT1R-Ab and antibody mediated rejection, we excluded the 7 patients with CMR only and reexamined risk factors for AMR. As expected, the number of patients with HLA-DSA was significantly higher in the AMR group compared to the group with no rejection (P = 0.00005). Similarly, the distribution of patients among the 3 AT1R-Ab levels was significantly different between AMR and no rejection (P = 0.009) (Table 2), with more AMR diagnosis in the higher AT1R-Ab levels (48% in the >17 U/ml group and 35% in the 10-17 U/ml group) compared to those at lowest AT1R-Ab levels (16% in the <10 U/ml group). Among patients with antibody mediated rejection, HLA-DSA was negative in 3/15 patients (20%) in the high AT1R-Ab group (>17 U/ml), 1/11 patients (9%) in the moderate AT1R-Ab group (10-17 U/ml), and none of the 5 patients (0%) in the low AT1R-Ab group (<10 U/ml).
TABLE 2.
Risk factors for antibody mediated rejection
We evaluated whether development of antibody mediated rejection correlated with an increase in HLA-DSA from a pretransplant timepoint to time of biopsy. We compared changes in HLA-DSA levels in the 3 AT1R-Ab levels. In this cross-sectional cohort, AMR was more closely associated with high AT1R-Ab levels and was independent of changes in the level of HLA-DSA; 72% of the patients with no change in HLA-DSA and with AT1R-Ab >17 U/ml developed AMR; 71% with a decrease in HLA-DSA and AT1R-Ab > 17 U/ml had an AMR. Additionally, 83% of patients who had an increase in HLA-DSA and moderate levels of AT1R-Ab had AMR (Table 3).
TABLE 3.
Variation in HLA-DSA levels from pretransplant to time of biopsy in the 3 AT1R-Ab groups

Of 18 patients with no HLA-DSA before transplantation, 3 (16%) developed de novo HLA-DSA at time of biopsy; 2 of the 3 patients were in the moderate AT1R-Ab group and both developed rejection (1 AMR and 1 CMR). The third patient was negative for AT1R-Ab and had no rejection.
Biopsy Results
We compared the biopsy scores with respect to AT1R-Ab levels for the 70 patients in this cohort, including those who were positive and negative for HLA-DSA (Figure 2). Scores for glomerulitis (g), inflammation (i), and peritubular capillaritis (ptc) were positively correlated with increased AT1R-Ab concentrations (P = 0.007, 0.03, and 0.005, respectively). For 28 patients who tested negative for HLA-DSA, the number of biopsies with scores of 2 and 3 were combined to allow for statistical analysis as biopsies in those groups were limited to 4 and 2, respectively. In the absence of HLA-DSA, higher AT1R-Ab concentrations were again associated with biopsies showing increased microvascular inflammation (glomerulitis and peritubular capillaritis) compared to those with scores of 0 and 1 (P = 0.03 for both injuries). Two biopsies were scored ≥2 for inflammation, in the absence of HLA-DSA, and the AT1R-Ab concentrations were higher in those 2 biopsies compared to the biopsies scored as 0 and 1 (Figure 2b). Microcirculation inflammation (MCI) sum scores (g score + ptc score) were higher in group 1 (high level AT1R-Ab) compared to groups 2 (moderate level AT1R-Ab) and 3 (low level AT1R-Ab), in the absence of HLA-DSA. These results approached statistical significance (Figure 2c; P = 0.07). Of 9 patients with MCI scores >2 and no HLA-DSA, 4 were diagnosed with AMR (3 with high levels of AT1R-Ab; >17 U/ml), 2 were diagnosed with cell mediated rejection (1 with AT1R-Ab >17 U/ml), and 3 with no rejection (none with AT1R-Ab >17 U/ml).
FIGURE 2.
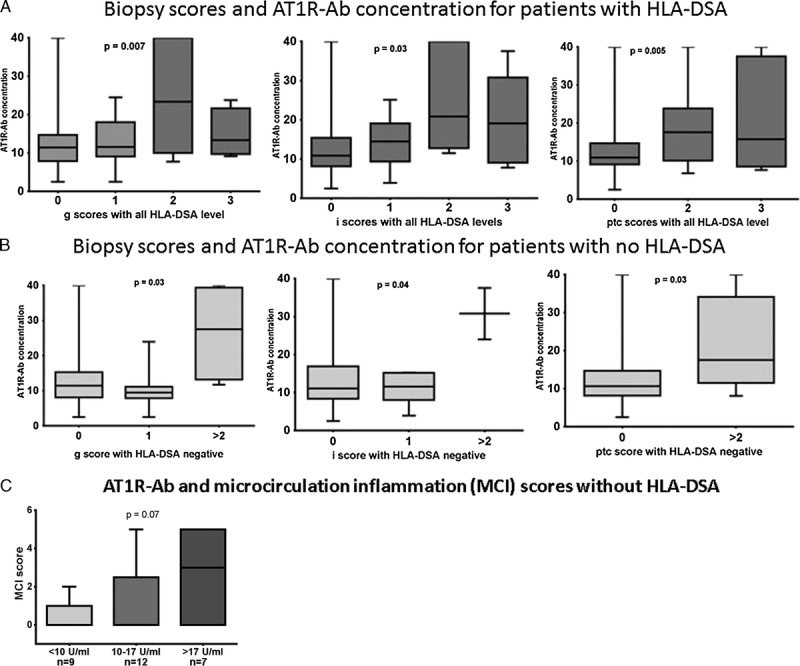
Scores for glomerulitis (g), inflammation (i), and peritubular capillaritis (ptc) for patients with and without HLA-DSA. AT1R-Ab concentrations were compared to Banff scores for glomerulitis (g), peritubular capillaritis (ptc), and inflammation (i) in the presence of HLA-DSA (score = 0; n = 29), score = 1; n = 26), score = 2; n = 11, score = 3; n = 4) and without HLA-DSA (score = 0; n = 15, score = 1; n = 7; score ≥2; n = 6). Higher AT1R-Ab concentrations correlated with increased scores for g, i, and ptc in presence of HLA-DSA (A) and without HLA-DSA (B). MCI consisted of the sum of g + ptc scores were compared in patients with no HLA-DSA. MCI scores were higher in patients with AT1R-Ab >17 U/ml but did not reach statistical significance (<10 U/ml, n = 9; 10-17 U/ml, n = 12; >17 U/ml, n = 7) (C).
Scores for tubulitis (t) and tubular inflammation (ti), which are elevated in cellular rejection, were not significantly correlated with increased AT1R-Ab concentration (data not shown). Similarly, intimal arteritis (v), interstitial fibrosis (ci), tubular atrophy (ct), intimal thickening (cv), C4d staining, and chronic glomerulitis (cg) did not correlate with changes in AT1R-Ab concentrations (data not shown).
Anti-Endothelial Cell Antibody Detection Posttransplantation
An endothelial cell flow cytometric crossmatch (ECXM) was available for 35 patients who had no HLA-DSA or low levels of HLA antibody that were insufficient to cause a positive flow cytometric crossmatch. Patients with a positive ECXM (n = 11) had stronger AT1R-Ab concentrations compared to a negative ECXM (n = 24) (P = 0.005; Figure 3a). Of the 11 patients with a positive ECXM, 6 were diagnosed with AMR, 2 with cell mediated rejection, 2 had no rejection, and 1 was diagnosed with transplant glomerulopathy (P = 0.02). The average time from transplant date to AMR was 20 months (SD 26.2) for patients with AECA and or/AT1R-Ab >17 U (n = 4) and 22 months (SD 49.7) for patients with HLA-DSA and non-HLA antibody (n = 27).
FIGURE 3.
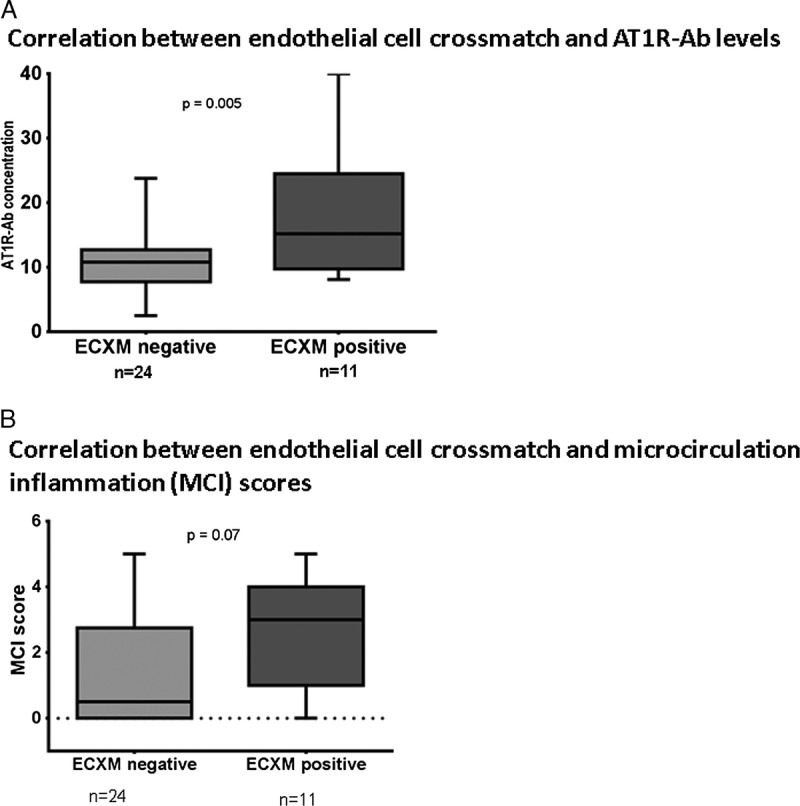
Endothelial cell crossmatch (ECXM) results compared to AT1R-Ab concentrations and MCI scores for 35 patients. A, a positive correlation between increased AT1R-Ab concentrations and ECXM positive (P = 0.005). B, MCI score consisted of the sum of g + ptc scores and were higher in ECXM positive group compared to ECXM negative but did not reach statistical significance.
We then examined the microcirculation inflammation (MCI) scores in patients who had a positive ECXM versus those with a negative ECXM. The mean MCI scores for patients with positive ECXMs (2.54 ± 0.5) were higher than for those with negative ECXMs (1.33 ± 0.3) but was not statistically significantly different (Figure 3b; P = 0.07).
DISCUSSION
The primary goal of this study was to correlate the characteristics of biopsy microcirculation inflammation with presence of non-HLA antibody identified in the ECXM and AT1R-Ab ELISA. Two histopathological features of endothelial injury, glomerulitis and peritubular capillaritis, emerged as significantly increased with increasing AT1R-Ab serum levels, independent of HLA-DSA. Glomerulitis and peritubular capillaritis are characterized by neutrophil and monocyte infiltration15 and likely reflect local immune activation. Angiotensin II type 1 receptors, the target for AT1R-Ab, are expressed at high levels on endothelial cells, and activation of this receptor in human microvascular endothelial cells in the presence of AT1R-Ab has been shown to cause endothelial cell dysfunction and neutrophil migration, and these effects were reduced in the presence of an angiotensin receptor blocker.16 Therefore, AT1R-Ab is thought to contribute to graft dysfunction by inducing activation of signaling pathways in a similar fashion as the endogenous ligand for this receptor, angiotensin II.16-18
A previous study from Jackson et al independently showed that anti-endothelial cell antibodies (AECA) identified in the endothelial cell crossmatch were associated with increased peritubular capillaritis in the absence of donor-specific HLA antibody,8 suggesting a similar pathophysiology with AECAs as with AT1R-Ab. Microcirculation inflammation (MCI) has been shown to predict worst graft outcome.19,20 In this cohort, MCI scores increased in patients with positive ECXM and AT1R-Ab >17 U/ml, although the numbers in this group were small and did not reach statistical significance. Thus, AT1R-Ab and AECA may be direct contributors to the initiation or maintenance of an inflammatory milieu through the activation of receptors on immune cells.16,17
Angiotensin II type 1 receptor is also expressed on monocytes, podocytes, renal vascular smooth muscle cells, and renal arteries.21 AT1R-Ab activation of this receptor has been shown to increase pro-inflammatory protein expression.16 An inflammatory environment is conducive to further upregulation of AT1R expression22 as well as upregulation of HLA expression.23 Inflammation scores were increased with increasing AT1R-Ab concentrations and HLA-DSA (Figure 2). We could not assess the contribution of AT1R-Ab alone on inflammation since there were only 2 biopsies with no HLA-DSA with an inflammation score ≥2. It is worth noting that both of these biopsies had AT1R-Ab levels >17 U/ml (23.9 and 37.5 U/ml) and positive ECXM, and both were diagnosed with acute rejection (Figure 2b). Further studies using larger cohorts of patients with no HLA-DSA will be required to determine the contribution of AT1R-Ab alone to the process of inflammation.
C4d scores were not significantly different among the 3 groups, and this is in agreement with most AT1R-Ab studies.24-26 It is now recognized that antibody mediated injury may occur in the absence of detectable complement activation27 through such mechanisms as antibody dependent cell mediated cytotoxicity or antibody mediated endothelial cell activation.12,28,29
Glomerulitis and peritubular capillaritis scores were highest when patients presented with both HLA-DSA and non-HLA antibody in their serum (Figure 2). Taniguchi also showed evidence of worst graft outcome in patients with HLA-DSA and non-HLA antibodies compared to either antibody alone.4 Therefore, our data support previously published reports showing that non-HLA antibodies and HLA-DSA may function in synergy.4,30
Different thresholds for positivity have been suggested in studies investigating AT1R-Ab levels in transplantation recipients.3,4,31 Our study confirms previous reports that AT1R-Ab and AECA are associated with antibody mediated rejection (Table 2). Moreover, we found a greater correlation with AMR and MCI scores when the AT1R-Ab was >17 U/ml. It is worth noting that among the 11 patients with moderate AT1R-Ab levels (10-17 U/ml), those with AMR had stronger HLA-DSA; 4/15 (26%) patients versus 8/11 (72%) patients in the high AT1R-Ab group and in the moderate AT1R-Ab group respectively had HLA-DSA sufficient to yield a positive flow cytometric crossmatch. This suggests that moderate levels of AT1R-Ab alone may not contribute to damage but in the presence of HLA-DSA may become pathogenic. This is further illustrated by the increased incidence of AMR in the 6 patients who had an increase in HLA-DSA at time of biopsy with moderate AT1R-Ab levels (Table 3). Conversely, an increase in HLA-DSA in the AT1R-Ab <10 U/ml did not result in AMR (Table 3). It is important to note that the HLA-DSA levels in both groups were at similar levels.
AMR was diagnosed in only 4 patients (5%) with AECA and or/AT1R-Ab >17 U, but no HLA-DSA. A similar frequency was determined by Tinckam et al when analyzing several reports linking AT1R-Ab with allograft dysfunction32 prompting the question of the relevance of testing for these antibodies. Taniguchi noted a few cases in which AT1R-Ab preceded development of HLA-DSA.4 The possibility that increased inflammation due to the presence of AT1R-Ab could result in production of de novo HLA-DSA may provide reason to consider screening for a selected group of patients. In this cohort, we found that of 18 patients with no HLA-DSA before transplantation, only 3 (16%) developed de novo HLA-DSA at time of biopsy; 2 of these patients had moderate levels of AT1R-Ab and experienced rejection. Further longitudinal studies with larger cohorts will determine whether presence of non-HLA antibodies can lead to development of HLA-DSA in patients who were not sensitized to HLA antibodies before transplantation.
In this study, we found no correlation between AT1R-Ab and cell mediated rejection. The median AT1R-Ab concentration for biopsies with cell mediated rejection was 14.66 U/ml (range 2.5 to 23.9 U/ml; mean: 12.9 ± 6.7 U/ml). These values are consistent with previous reports noting that high AT1R-Ab concentrations were not observed for patients diagnosed with cell mediated rejection alone.31 It is possible that our cohort was too small to definitively determine the contribution of AT1R-Ab in cell mediated rejection. Injuries related to CMR (tubulitis and tubular inflammation) were not increased with increased AT1R-Ab concentrations.
There were also more patients with high levels of AT1R-Ab (>17 U/ml) who had hypertension (29% in the high AT1R-Ab group versus 11% and 18% in the moderate and low AT1R-Ab groups) and were treated with an angiotensin receptor blocker (48% vs. 26% and 23% for groups 1, 2, and 3, respectively). These data are in line with reports linking AT1R-Abs with hypertension.17 Previous reports have shown that excessive activation of AT1R via tissue angiotensin II (Ang II) or AT1R-Ab negatively affects cardiovascular, renal, and endocrine systems to provoke hypertension and lead to kidney damage.33
The limitations of this study include the small sample size and the cross-sectional analysis that limits our analysis to a single time point posttransplant. Longitudinal samples collected after initiation of treatment would help determine whether current therapies are effective in preventing further damage associated with presence of non-HLA antibodies. Table 1 shows that 17 patients who were positive for AT1R-Ab (10 in the high level AT1R-Ab group and 7 in the moderate AT1R-Ab group) were treated with an angiotensin receptor blocker (ARB) at time of graft dysfunction primarily for treatment of hypertension. Increased posttransplant monitoring would be needed to assess whether treatment with an ARB results in some improvement of biopsy damage. The percentage of patients who were positive for AT1R-Ab and who were diagnosed with antibody mediated rejection is higher in this cohort compared to previous reports, and this is likely due to the selection of patients with suspected graft dysfunction or with biopsies showing subclinical rejection.
In conclusion, testing for AT1R-Ab and AECA may identify patients at higher risk for antibody mediated injury, particularly in the presence of HLA-DSA. Identifying histological features that lead to allograft loss and identifying targets for therapeutic intervention may improve the length of allograft survival.
MATERIALS AND METHODS
Study Population and Immunosuppression
Under an approved IRB protocol, demographics and clinical information were collected from the electronic patient information records (EPIC) of 77 kidney recipients who were transplanted between 1988 and 2014 and had posttransplant non-HLA antibody testing. Seventy of the 77 recipients were biopsied and 7 recipients did not have a biopsy (3 were unable to be biopsied because of the location of the allograft, 1 had health complications, and 3 experienced graft loss before a biopsy could be performed). Of the 70 patients with biopsy data, 47 were biopsied to investigate suspected dysfunction (for cause biopsies) and 23 were done on protocol. Data for AT1R-Ab, HLA antibody, AECA tests, and biopsy evaluation were collected for each patient between July 2009 and January 2015.
Pretransplant induction treatment was not available for 6 of the patients who were transplanted at other centers and were referred to Johns Hopkins for posttransplant management. For the remaining 64 patients, induction treatment consisted of either anti-IL2 receptor antibody (anti-CD25, daclizumab 2 mg/kg) or thymoglobulin (1.5 mg/kg per day for 5 days). HLA and ABO incompatible donor recipient pairs (n = 23) were additionally treated with single-volume plasmapheresis and 100 mg/kg IVIg (Cytogam; MedImmune, Gaithersburg, MD). The number of single-volume plasmapheresis treatments varied based on the level of HLA-DSA and ABO antibody before transplantation. Maintenance treatment consisted of mycophenolate mofetil (2 g/day) and tacrolimus (serum level of 8-10 ng/ml), and additional plasmapheresis treatments were given immediately posttransplantation as needed (n = 7). Twenty-eight patients were treated with angiotensin receptor blockers (ARB; n = 22) or angiotensin converting enzyme inhibitors (ACEi; n = 6); 6 patients received both ARB and ACEi.
Kidney Histology
Allograft biopsies were performed when serum creatinine was increased by ≥20% from baseline. Protocol biopsies are scheduled at this center at 1, 3, 6, and 12 months posttransplantation for HLA and ABO incompatible cases (n = 23). Biopsies were graded using the Banff' 2009-2013 criteria.12,34,35 The following Banff components were evaluated: tubulitis (t), intimal arteritis (v), interstitial inflammation (i), glomerulitis (g), peritubular capillaritis (ptc), interstitial fibrosis (ci), tubular atrophy (ct), allograft glomerulopathy (cg), vascular fibrous intimal thickening (cv), and C4d staining. Biopsy scores were compared to ECXM and AT1R-Ab levels.
HLA Antibody Detection and HLA-DSA Assignment
HLA-specific antibodies were evaluated before transplantation and at time of biopsy using solid-phase immunoassays (Lifecodes classes I and II phenotype panels; Immucor-Lifecodes, Stamford, CT and Single Antigen Beads; One Lambda, Canoga Park, CA) performed on a Luminex platform. Correlations between a median fluorescence intensity (MFI) value and crossmatch strength, cytotoxicity (CDC XM), or flow cytometric (FCXM) were established according to previously published report.36 Briefly, 3 levels of HLA-DSA strength were described; CDC XM level, FCXM level, and Luminex level were assigned for MFI values of 10000 or higher, 4000 to 9000, and 2000 MFI, respectively, for HLA-A, HLA-B, and HLA-DR, and 20000 or higher, 16000, and 4000 for HLA-C, HLA-DQ, and HLA-DP. MFI values below 1000 and lacking HLA specificity patterns were reported as negative.
AT1R-Ab and AECA Detection
Detection of antibodies against angiotensin II type 1 receptor (AT1R-Ab) was performed using quantitative ELISA (CellTrend GmbH, Luckenwalde, Germany). Duplicate samples of a 1:100 serum dilution were added to the 96-well polystyrene microtiter plate coated with human AT1R derived from transfected Chinese hamster ovary cell extracts37 and incubated at 4 °C for 2 hours. Because AT1R-Ab has been shown to bind specific amino acids within the second extracellular loop of the protein, maintenance of the conformational structure of the receptor is important and is achieved with the addition of 1 mM calcium chloride to each buffer. Following washing steps, a horseradish peroxidase-conjugated goat anti-human IgG detection antibody is added and incubated for 1 hour, followed by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. Presence of antibody bound to AT1R is detected by a colorimetric change. A standard curve is generated to allow the quantitation of AT1R-Ab, using a control sample at varying concentrations (2.5, 5, 10, 20, and >40 U/ml).
Anti-endothelial cell antibodies (AECAs) of the IgG isotype were detected using a flow cytometric crossmatch test (ECXM) performed using angiopoietin receptor positive (Tie-2+) peripheral blood ECPs (XM-ONE; Absorber AB, Stockholm, Sweden) acquired on a BD FACSAria using FACSDiva (version 6.1.1; BD Biosciences, Franklin Lakes, NJ).
HLA Typing for Patients and Donors
Recipient and donor HLA-A, B, C, DQ, DR, and DP typing were performed by solid-phase reverse sequence specific oligonucleotide assay (One Lambda LABType). HLA antigen typing ambiguities were resolved by DNA sequence based methods.
Statistical Calculation
Significant difference between the 3 groups was calculated using the analysis of variance (1-way ANOVA). Continuous variables were compared using Student’s t-test and categorical variables were compared using the chi-squared test and Fisher’s exact test. A P-value less than 0.05 was considered statistically significant.
Footnotes
The authors declare no funding or conflicts of interest.
M.C. Philogene participated in the design, data analysis, and writing of the manuscript. S. Bagnasco participated in the design, data analysis, and writing of the manuscript. E. Kraus participated in the writing of the manuscript. R.A. Montgomery participated in the writing of the manuscript. D.D. participated in the data analysis. M.S. Leffell participated in the data analysis and writing of the manuscript. A.A. Zachary participated in the data analysis and writing of the manuscript. A.M. Jackson participated in the design, data analysis, and writing of the manuscript.
REFERENCES
- 1.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. [DOI] [PubMed] [Google Scholar]
- 2.Kelsch R, Everding AS, Kuwertz-Broking E, et al. Accelerated kidney transplant rejection and hypertensive encephalopathy in a pediatric patient associated with antibodies against angiotensin type 1 receptor and HLA class II. Transplantation. 2011;92:e57–e59. [DOI] [PubMed] [Google Scholar]
- 3.Giral M, Foucher Y, Dufay A, et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013;13:2567–2576. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi M, Rebellato LM, Cai J, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013;13:2577–2589. [DOI] [PubMed] [Google Scholar]
- 5.Alachkar N, Gupta G, Montgomery RA. Angiotensin antibodies and focal segmental glomerulosclerosis. N Engl J Med. 2013;368:971–973. [DOI] [PubMed] [Google Scholar]
- 6.Pearl MH, Leuchter RK, Reed EF, et al. Accelerated rejection, thrombosis, and graft failure with angiotensin II type 1 receptor antibodies. Pediatr Nephrol. 2015;30:1371–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson AM, Kuperman MB, Montgomery RA. Multiple hyperacute rejections in the absence of detectable complement activation in a patient with endothelial cell reactive antibody. Am J Transplant. 2012;12:1643–1649. [DOI] [PubMed] [Google Scholar]
- 8.Jackson AM, Sigdel TK, Delville M, et al. Endothelial cell antibodies associated with novel targets and increased rejection. J Am Soc Nephrol. 2015;26:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukitsch I, Kehr J, Chaykovska L, et al. Renal ischemia and transplantation predispose to vascular constriction mediated by angiotensin II type 1 receptor-activating antibodies. Transplantation. 2012;94:8–13. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Zheng R, Yang L, et al. Angiotensin type 1 receptor autoantibody from preeclamptic patients induces human fetoplacental vasoconstriction. J Cell Physiol. 2013;228:142–148. [DOI] [PubMed] [Google Scholar]
- 11.Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. [DOI] [PubMed] [Google Scholar]
- 12.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. [DOI] [PubMed] [Google Scholar]
- 13.Colvin RB. Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. [DOI] [PubMed] [Google Scholar]
- 14.Colvin RB. Dimensions of antibody-mediated rejection. Am J Transplant. 2010;10:1509–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racusen LC, Bagnasco SM. Peritubular capillaritis in the renal allograft takes center stage. Kidney Int. 2015;88:218–220. [DOI] [PubMed] [Google Scholar]
- 16.Kill A, Tabeling C, Undeutsch R, et al. Autoantibodies to angiotensin and endothelin receptors in systemic sclerosis induce cellular and systemic events associated with disease pathogenesis. Arthritis Res Ther. 2014;16:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dragun D. Agonistic antibody-triggered stimulation of angiotensin II type 1 receptor and renal allograft vascular pathology. Nephrol Dial Transplant. 2007;22:1819–1822. [DOI] [PubMed] [Google Scholar]
- 18.Gunther J, Kill A, Becker MO, et al. Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Res Ther. 2014;16:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. [DOI] [PubMed] [Google Scholar]
- 20.Loupy A, Hill GS, Nochy D, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2010;10:952; author reply 953. [DOI] [PubMed] [Google Scholar]
- 21.Harrison-Bernard LM, Navar LG, Ho MM, et al. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997;273(1 Pt 2):F170–F177. [DOI] [PubMed] [Google Scholar]
- 22.Rincon J, Correia D, Arcaya JL, et al. Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015;124:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muczynski KA, Ekle DM, Coder DM, et al. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: Characterization, isolation, and regulation of MHC class II expression. J Am Soc Nephrol. 2003;14:1336–1348. [DOI] [PubMed] [Google Scholar]
- 24.Dragun D, Catar R, Philippe A. Non-HLA antibodies in solid organ transplantation: Recent concepts and clinical relevance. Curr Opin Organ Transplant. 2013;18:430–435. [DOI] [PubMed] [Google Scholar]
- 25.Dragun DHB. Detection of C4d-fixing HLA antibodies in serum—a glass half full and half empty. Transpl Int. 2013;26:119–120. [DOI] [PubMed] [Google Scholar]
- 26.Dragun D. The detection of antibodies to the angiotensin II-type 1 receptor in transplantation. Methods Mol Biol. 2013;1034:331–333. [DOI] [PubMed] [Google Scholar]
- 27.Sis B. Endothelial molecules decipher the mechanisms and functional pathways in antibody-mediated rejection. Hum Immunol. 2012;73:1218–1225. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Reed EF. Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: Evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812–1822. [DOI] [PubMed] [Google Scholar]
- 30.Reinsmoen NL, Lai CH, Mirocha J, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014;97:595–601. [DOI] [PubMed] [Google Scholar]
- 31.Reinsmoen NL, Lai CH, Heidecke H, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010;90:1473–1477. [DOI] [PubMed] [Google Scholar]
- 32.Tinckam K, Campbell P. Angiotensin II type 1 receptor antibodies: Great expectations? Am J Transplant. 2013;13:2515–2516. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal S, Makris A, Hennessy A. Linking the old and new—do angiotensin II type 1 receptor antibodies provide the missing link in the pathophysiology of preeclampsia? Hypertens Pregnancy. 2015;34:369–382. [DOI] [PubMed] [Google Scholar]
- 34.Sis B, Mengel M, Haas M, et al. Banff '09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. [DOI] [PubMed] [Google Scholar]
- 35.Mengel M, Sis B, Haas M, et al. Banff 2011 meeting report: New concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zachary AA, Sholander JT, Houp JA, et al. Using real data for a virtual crossmatch. Hum Immunol. 2009;70:574–579. [DOI] [PubMed] [Google Scholar]
- 37.Riemekasten G, Philippe A, Näther M, et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70:530–536. [DOI] [PubMed] [Google Scholar]


