Article first published online 26 January 2017.
Supplemental Digital Content is Available in the Text
Key Words: anti–tumor necrosis factor, open-label extension, clinical remission, linear growth velocity
Abstract
Background:
IMAgINE 1 assessed 52-week efficacy and safety of adalimumab in children with moderate to severe Crohn's disease. Long-term efficacy and safety of adalimumab for patients who entered the IMAgINE 2 extension are reported.
Methods:
Patients who completed IMAgINE 1 could enroll in IMAgINE 2. Endpoints assessed from weeks 0 to 240 of IMAgINE 2 were Pediatric Crohn's Disease Activity Index remission (Pediatric Crohn's Disease Activity Index ≤ 10) and response (Pediatric Crohn's Disease Activity Index decrease ≥15 from IMAgINE 1 baseline) using observed analysis and hybrid nonresponder imputation (hNRI). For hNRI, discontinued patients were imputed as failures unless they transitioned to commercial adalimumab (with study site closure) or adult care, where last observation was carried forward. Corticosteroid-free remission in patients receiving corticosteroids at IMAgINE 1 baseline, discontinuation of immunomodulators (IMMs) in patients receiving IMMs at IMAgINE 2 baseline, and linear growth improvement were reported as observed. Adverse events were assessed for patients receiving ≥1 adalimumab dose in IMAgINE 1 and 2 through January 2015.
Results:
Of 100 patients enrolled in IMAgINE 2, 41% and 48% achieved remission and response (hNRI) at IMAgINE 2 week 240. Remission rates were maintained by 45% (30/67, hNRI) of patients who entered IMAgINE 2 in remission. At IMAgINE 2 week 240, 63% (12/19) of patients receiving corticosteroids at IMAgINE 1 baseline achieved corticosteroid-free remission and 30% (6/20) of patients receiving IMMs at IMAgINE 2 baseline discontinued IMMs. Adalimumab treatment led to growth velocity normalization. No new safety signals were identified.
Conclusions:
Efficacy and safety profiles of prolonged adalimumab treatment in children with Crohn's disease were consistent with IMAgINE 1 and adult Crohn's disease adalimumab trials.
Crohn's disease (CD) is a progressive inflammatory disorder of the gastrointestinal tract. The incidence of CD is increasing worldwide, with 15% to 25% of inflammatory bowel disease cases manifesting in children and adolescents.1–3 Common complications of pediatric CD include growth retardation, undernutrition, and reduced health-related quality of life.4,5 Conventional therapies for treatment of children with CD include corticosteroids and immunomodulators (IMMs).6,7 However, a substantial number of cases are refractory to conventional therapy, and the use of these therapies has been associated with growth impairment,8,9 lymphoma, and nonmelanoma skin cancer.10–14
Adalimumab, a fully human monoclonal antibody, is approved for use in children aged 6 to 17 years with moderately to severely active CD (United States and Europe) and adolescents aged 13 to 17 years weighing ≥40 kg with severely active CD (Canada). The IMAgINE 1 trial was a 52-week, multicenter, randomized, double-blind phase 3 study of adalimumab for moderately to severely active pediatric CD. The results demonstrated that adalimumab was an effective treatment for inducing and maintaining clinical remission and response in this population, with a safety profile similar to that observed in adult CD trials.15
Children with moderately to severely active CD who completed the IMAgINE 1 trial through week 52 and who had responded any time were eligible to enter the open-label extension IMAgINE 2 study. We present the long-term efficacy and safety of adalimumab treatment for more than 5 years in pediatric patients with CD who enrolled in the IMAgINE 2 study.
MATERIALS AND METHODS
Patients and Study Design
The open-label extension study, IMAgINE 2 (ClinicalTrials.gov identifier, NCT00686374), has been conducted at 31 sites in Canada, Europe, and the United States since May 2008. The current analysis was based on data collected through January 31, 2015, when the study was ongoing. By design, conduct of IMAgINE 2 was to conclude at the time of adalimumab approval and reimbursement for pediatric CD in each individual country. Therefore, the duration of follow-up for an individual patient varied depending on the location of the study site and the timing of enrollment into IMAgINE 1. The study evaluated the long-term maintenance of clinical efficacy and safety of adalimumab treatment in pediatric patients with CD who completed IMAgINE 1. Eligible patients were those who successfully completed IMAgINE 1 through week 52 and achieved clinical response at any time point during the study. The details of the IMAgINE 1 study (ClinicalTrials.gov identifier, NCT00409682) have been published previously.15
The week 52 visit of IMAgINE 1 was the baseline visit for IMAgINE 2. Patients who entered the open-label extension from blinded (every other week [EOW] or every week [EW]) dosing in IMAgINE 1 (low dose: 20 mg adalimumab if ≥40 kg or 10 mg adalimumab if <40 kg; high dose [HD]: 40 mg adalimumab if ≥40 kg or 20 mg adalimumab if <40 kg) received open-label HD EOW adalimumab based on body weight at that visit (40 mg EOW adalimumab if ≥40 kg or 20 mg EOW adalimumab if <40 kg). Patients who entered the open-label extension from blinded EW dosing were changed to EOW dosing at that time point per protocol. Patients who entered IMAgINE 2 from open-label EW therapy in IMAgINE 1 (40 mg EW adalimumab if ≥40 kg or 20 mg EW adalimumab if <40 kg) continued to receive the same dose and frequency in IMAgINE 2.
Beginning at week 8 of IMAgINE 2, patients who experienced disease flares could move from open-label EOW treatment to weekly treatment, continuing with the same dose of adalimumab. Disease flare was defined as an increase in Pediatric Crohn's Disease Activity Index (PCDAI) of ≥15 points when compared with the PCDAI obtained at the previous study visit. Patients receiving weekly therapy could be discontinued from the study at the investigator's discretion if they developed a disease flare or PCDAI ≥15 points compared with baseline at any time during the study.
CD-related concomitant medications could not be adjusted during the first 8 weeks of the study except for safety reasons. After week 8 of IMAgINE 2, concomitant therapies could be reduced or discontinued at the investigator's discretion. Conversely, CD-related therapies could be initiated or reinitiated after week 8, with the exception of IMM, which could not be reinstated. Corticosteroid tapering continued without interruptions for patients who began their tapering in IMAgINE 1. At week 8, patients who were not experiencing disease flares could begin corticosteroid tapering and corticosteroid doses could be increased or reinitiated if a patient experienced a disease flare. Medications and therapies that were prohibited during the study included live vaccines, concurrent biological therapies, growth hormone, cyclosporine, and tacrolimus. Physical examination, PCDAI, and sample collection for laboratory tests were obtained at each visit.
Data Analysis
For this analysis, the long-term efficacy and safety of adalimumab were assessed up to January 31, 2015. Efficacy was assessed from weeks 0 to 240 of IMAgINE 2 (the latest visit that all patients could have reached by the data cutoff date) in all patients who entered IMAgINE 2 (N = 100). Adverse event (AE) rates were reported for any patient who entered IMAgINE 1 (N = 192) from the first dose through the cutoff date of January 31, 2015, or up to 70 days after the last dose of adalimumab.
Efficacy Assessments
Rates of clinical remission (PCDAI ≤ 10), clinical response (PCDAI decrease ≥15 points from IMAgINE 1 baseline), and mean PCDAI were assessed over time. Subgroup analyses of remission and response rates by previous infliximab use were performed. Discontinuation of corticosteroids and corticosteroid-free remission (PCDAI ≤ 10 and discontinued corticosteroid use) during IMAgINE 2 were assessed in patients who used corticosteroids at IMAgINE 1 baseline and entered IMAgINE 2 (n = 37). Discontinuation of IMM use during IMAgINE 2 was assessed in patients who received IMMs at IMAgINE 2 baseline (n = 44).
Linear growth velocity was assessed by sex-specific height velocity z score standardized by bone age, which was determined using the Greulich and Pyle method for reading x-rays.16 Observed height velocity z scores were calculated for each patient with reference to standard height velocity tables17 as previously described.15 Linear growth analysis was limited to patients with growth potential (female patients with bone age ≤13 yrs and male patients with bone age ≤14 yrs) based on reports about median bone age at puberty.18 Linear growth was assessed over time in patients with linear growth impairment (height velocity z scores ≤ −1.0) at IMAgINE 1 baseline.
Health-related quality of life was assessed at study visits in patients aged ≥10 years at IMAgINE 2 baseline using the IMPACT III questionnaire, which was developed to assess quality of life in pediatric patients with inflammatory bowel disease.19 IMPACT III contains 33 questions encompassing concerns in 6 domains: bowel, systemic function, emotional and social function, body image, and treatments or tests. Scores range from 35 to 175, and higher IMPACT III scores indicate a better quality of life.19
Safety Assessments
AEs, physical examination, vital signs, and laboratory data were evaluated throughout the study. Treatment-emergent AEs that occurred on or after the first adalimumab dose and up to 70 days after the last dose or until the cutoff date (January 31, 2015) were considered for this report. Safety data were collected at regular intervals and by spontaneous reporting throughout IMAgINE 1 and during the ongoing IMAgINE 2 trial. Patients who completed (or early terminated) the study were contacted 70 days after their last dose of adalimumab to obtain information on any ongoing or new AEs. AEs were summarized using the Medical Dictionary for Regulatory Activities, version 13.1. Rates of AEs of interest were assessed per 100 patient-years (PYs) of exposure.
Statistical Analyses
Not all patients had the same duration of follow-up because of different timing of enrollment into IMAgINE 1, country-specific adalimumab approval, and study site shut down. Consequently, rates of remission and response over time and maintenance of remission and response were reported as observed and analyzed using a hybrid nonresponder imputation (hNRI) method. Patients with missing data or who discontinued from the study for reasons other than study site closure or moving to adult care were imputed using NRI. Patients who discontinued from the study because of a study site closure and a documented change to commercial adalimumab (n = 18) or a move to adult treatment (n = 5) were imputed using last observation carried forward. For last-observation-carried-forward analysis, the patient's last nonmissing value after baseline was carried forward. Patients who escalated to weekly dosing were not imputed as nonresponders. Corticosteroid-free remission, discontinuation of corticosteroid use, discontinuation of IMM use, mean PCDAI, median height velocity z scores, and mean IMPACT III scores were reported as observed. For IMPACT III scores, P values were calculated based on a paired t-test.
Ethical Considerations
The IMAgINE 2 study protocol was approved by an independent ethics committee or an institutional review board at each site, and the study is being conducted in accordance with the International Conference on Harmonisation guidelines, appropriate regulations and guidelines governing clinical trial conduct, and the ethical principles originating in the Declaration of Helsinki. The parents/legal guardians of all participating patients provided verbal or written informed consent.
RESULTS
Patient Disposition, Demographics, and Baseline Characteristics
Of 192 patients who enrolled in IMAgINE 1, more than half (N = 100) entered IMAgINE 2. The disposition of patients who enrolled in IMAgINE 1 and those who later entered IMAgINE 2 is shown in Figure 1. During IMAgINE 2, 38 patients escalated to weekly dosing (Fig. 1). As of the data cutoff on January 31, 2015, there were 46 (46%) patients with efficacy endpoints at week 240 of IMAgINE 2 and 28 (28%) patients were ongoing in the study. A total of 72 patients have discontinued from IMAgINE 2 (Fig. 1). Lack of efficacy (n = 22) and AEs (n = 19) were the most frequent reasons for discontinuation. Other reasons for discontinuation included (but were not limited to) patients who transitioned to adult care (n = 5) or discontinued because adalimumab became commercially available in their country (n = 18).
FIGURE 1.
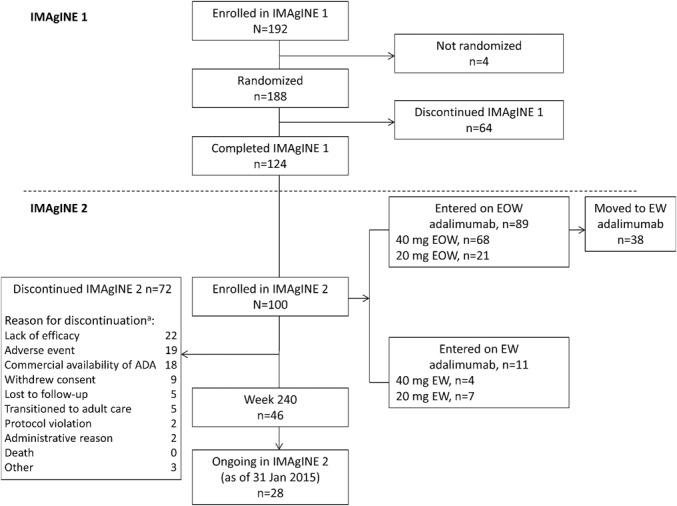
Flow diagram. Patient enrollment in the IMAgINE 1 and 2 studies as of January 31, 2015, data cutoff. ADA, adalimumab. aPatients may have had more than one reason for discontinuation.
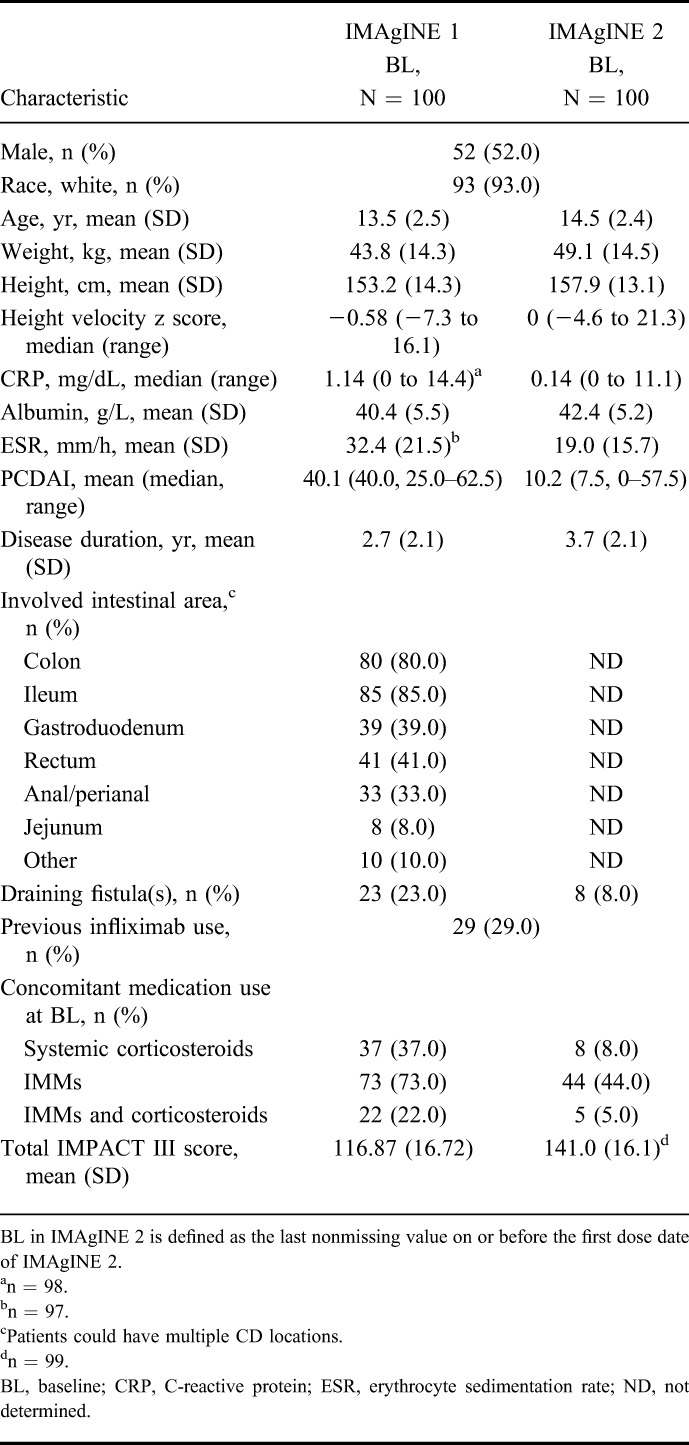
Patient characteristics at IMAgINE 2 baseline for the 100 patients who entered the open-label extension are shown in Table 1. Patient characteristics at IMAgINE 1 baseline for the patients who entered IMAgINE 2 are also shown in Table 1 for reference. On entry to IMAgINE 2, mean age was 14.5 years, mean disease duration was 3.7 years, and median PCDAI was 7.5. A lower proportion of patients were using concomitant IMMs at IMAgINE 2 baseline than at IMAgINE 1 baseline (44% versus 73%, respectively). A similar reduction in use of corticosteroids was noted at IMAgINE 2 baseline (8%) versus IMAgINE 1 baseline (37%).
TABLE 1.
Demographics and Characteristics at Baseline of IMAgINE 1 and 2 for Patients Who Entered IMAgINE 2
Long-term Efficacy Outcomes
At week 240 of IMAgINE 2, remission and response were achieved by 41% and 48% of patients who entered IMAgINE 2 (N = 100) per hNRI analysis, respectively (Fig. 2A, B). At IMAgINE 2 baseline, 67% of the 100 patients who entered the study were in remission and 95% had achieved response. Of the patients who entered IMAgINE 2 in remission, 45% (30/67, hNRI) maintained remission (Fig. 2C) and 50% (47/95, hNRI) of patients who entered IMAgINE 2 with response maintained their response at week 240, respectively (Fig. 2D). Remission and response rates remained stable over time regardless of previous infliximab use (see Fig. 1, Supplemental Digital Content 1, http://links.lww.com/IBD/B445).
FIGURE 2.
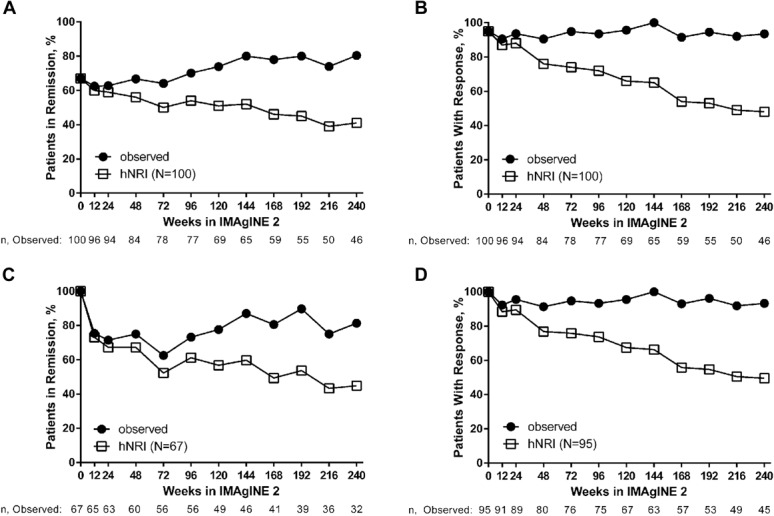
Long-term clinical remission and response rates with adalimumab treatment in patients who entered IMAgINE 2 (N = 100). A, Proportion of patients in remission over time. B, Proportion of patients with response over time. C, Proportion of patients in remission over time who entered IMAgINE 2 in remission (n = 67). D, Proportion of patients with response over time who entered IMAgINE 2 with response (n = 95).
Mean PCDAI for patients who entered IMAgINE 2 was reduced from 40.1 at IMAgINE 1 baseline to 10.2 at IMAgINE 2 baseline after 52 weeks of adalimumab treatment, and reductions were sustained through week 240 of IMAgINE 2 (mean PCDAI, 6.5, observed analysis; Fig. 2, Supplemental Digital Content 2, http://links.lww.com/IBD/B446).
Corticosteroid-free remission rates in IMAgINE 2 among patients who used corticosteroids at IMAgINE 1 baseline increased from 40.5% (15/37) at enrollment into IMAgINE 2 to 63.2% (12/19, observed analysis) at week 240 of IMAgINE 2. Discontinuation of corticosteroid use increased from week 12 (86.5% [32/37]) through week 240 of IMAgINE 2 (100% [19/19], observed analysis). Discontinuation of IMM use among patients who received IMMs at IMAgINE 2 baseline increased over time from week 12 of IMAgINE 2 (2.4% [1/41]) through week 240 (30% [6/20], observed analysis) of IMAgINE 2.
Clinical Outcomes in Patients Who Dose De-escalated at IMAgINE 2 Enrollment
Fifteen patients were receiving blinded HD weekly adalimumab at the end of IMAgINE 1 and, per protocol, de-escalated the frequency of adalimumab to HD EOW when entering IMAgINE 2. At week 240, remission and response rates for patients who dose de-escalated were 20% (3/15, hNRI) and 33% (5/15, hNRI), respectively.
Linear Growth Outcomes
Of the patients who entered IMAgINE 2, 58 patients had linear growth impairment at IMAgINE 1 baseline (median height velocity z score, −2.81 [range, −7.4 to −1.1]). Patients with linear growth retardation at IMAgINE 1 baseline sustained improvements in growth velocity during IMAgINE 2. Median height velocity z scores improved from the IMAgINE 1 baseline to 1.7 (range, −4.7 to 21.3; n = 33) at IMAgINE 2 baseline and to 0.85 (range, −7.6 to 9.6; n = 48) at week 48 of IMAgINE 2; as expected, after initial increase, the growth rate approached the median height velocity of the unaffected reference population over time and normalization of growth velocity was sustained through week 192 of IMAgINE 2 (median height velocity z score, 0 [range, −2.6 to 14.2]; n = 16; observed analysis).
Health-related Quality of Life
At IMAgINE 1 baseline, mean ± SD IMPACT III score was 116.9 ± 16.7 (n = 96) for patients who entered IMAgINE 2, indicating substantial impairment in quality of life before adalimumab treatment. Among these patients, mean ± SD IMPACT III score improved to 146.3 ± 12.3 (n = 58, P < 0.001) at week 52 of IMAgINE 1 (i.e., IMAgINE 2 baseline) and maintenance adalimumab treatment sustained improvements in IMPACT III scores through week 240 of IMAgINE 2 (mean ± SD IMPACT III score, 144.1 ± 18.7; n = 45; P < 0.001; observed analysis; Fig. 3).
FIGURE 3.
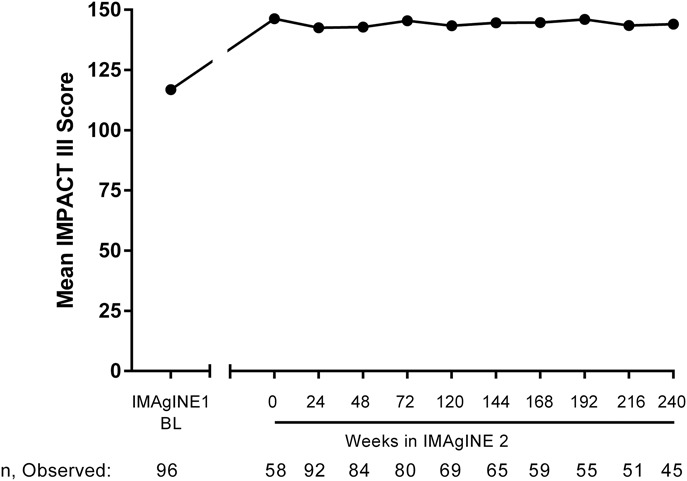
Mean IMPACT III score over time in patients who entered IMAgINE 2. Results at IMAgINE 1 baseline for patients who entered IMAgINE 2 are shown for reference, as observed analysis. BL, baseline.
Safety
As of January 31, 2015, a total of 192 pediatric patients received ≥1 dose of adalimumab in IMAgINE 1 and IMAgINE 2, representing 498.1 PY of exposure. The mean duration of exposure was 4.5 years, and 25% of patients were followed for a total of 286 weeks (i.e., 5.5 yrs including exposure in IMAgINE 1 and IMAgINE 2; Fig. 3, Supplemental Digital Content 3, http://links.lww.com/IBD/B447). Exposure-adjusted rates of treatment-emergent AEs of interest in all patients treated with adalimumab during the entire treatment period were similar to or lower than those observed during IMAgINE 1 (Table 2). Serious AEs (n = 92, 48%) and AEs leading to discontinuation (n = 61, 32%) of study drug were primarily due to worsening or flare of CD. The most frequently reported AEs were CD (n = 105, 55%), headache (n = 51, 27%), upper respiratory tract infection (n = 43, 22%), nasopharyngitis (n = 40, 21%), and diarrhea (n = 37, 19%). Most infectious AEs were nonserious and not related to treatment (Supplemental Table 1, Supplemental Digital Content 4, http://links.lww.com/IBD/B448). All opportunistic infections were also considered nonserious (oral candidiasis, n = 7; Aeromona infection, n = 1; fungal esophagitis, n = 1; and esophageal candidiasis, n = 1) with the exception of disseminated histoplasmosis (n = 1). The most common hematologic AE reported was anemia (22 events), and one event of noninfectious hepatitis was reported during the open-label extension phase. No deaths, demyelinating disease, malignancies, or active tuberculosis have been reported throughout the treatment period.
TABLE 2.
Treatment-emergent AE Rates During IMAgINE 1 and 2
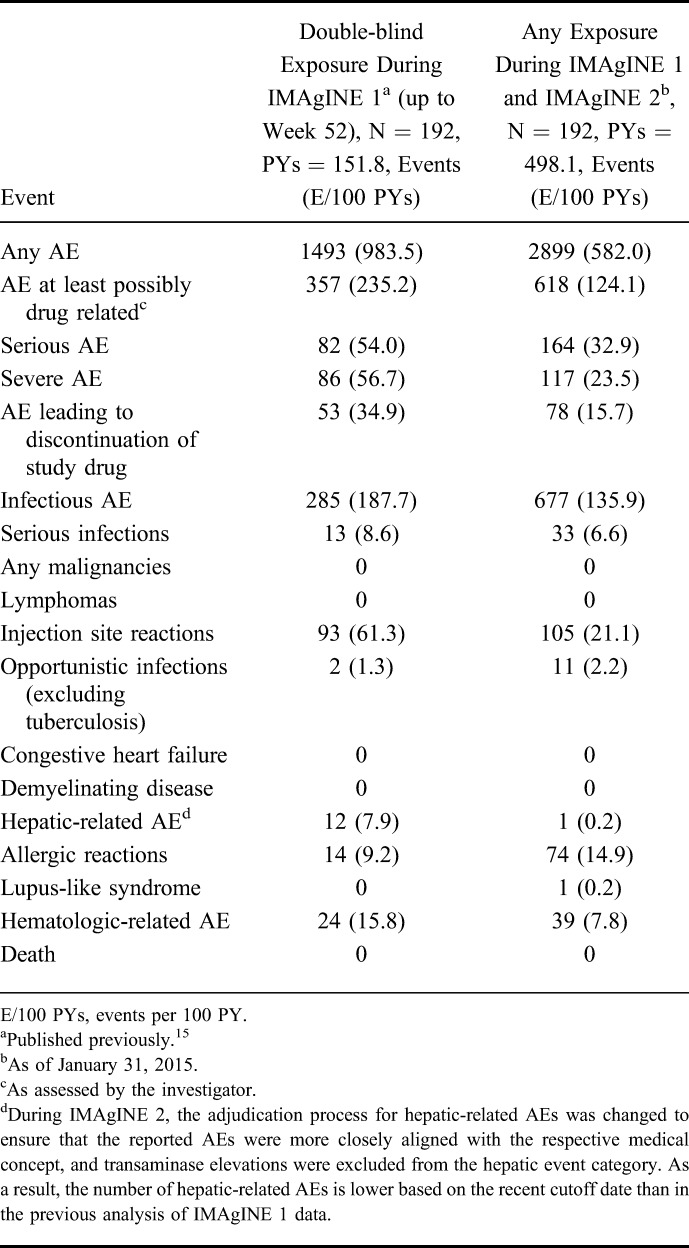
For patients whose adalimumab dosage was increased during IMAgINE 1 or IMAgINE 2 (n = 121), exposure-adjusted AE rates through the analysis cutoff date were similar or lower after dose escalation compared with AE rates before receiving the escalated dose (see Table 2, Supplemental Digital Content 5, http://links.lww.com/IBD/B449), indicating that in this limited sample size dosage adjustment did not affect AE rates. Patients receiving concomitant corticosteroids (n = 72) at IMAgINE 1 baseline experienced more serious AEs (43.3 events/100 PYs) than patients not receiving corticosteroids (n = 120; 26.6 events/100 PY; P = 0.002 by Poisson regression).
DISCUSSION
In the IMAgINE 1 study,15 adalimumab was shown to be effective for inducing and maintaining clinical remission and response in children with moderately to severely active CD who had failed conventional therapies or previous anti–tumor necrosis factor therapy. The long-term efficacy and safety profile of adalimumab in this patient population has not been previously reported beyond 52 weeks. In this analysis of patients who entered the ongoing open-label extension, IMAgINE 2, the long-term efficacy and safety of adalimumab in children with CD were assessed based on more than 5 years of exposure. Our findings showed that the clinical remission and response rates that were achieved with 1 year of adalimumab therapy in IMAgINE 1 were maintained in about half the patients at week 240 of IMAgINE 2. In addition, normalization of linear growth velocity and improvements in health-related quality of life that were achieved with 1 year of adalimumab therapy in IMAgINE 1 were maintained for up to another 4.5 years during IMAgINE 2.
We also demonstrated that >80% of patients were able to discontinue use of corticosteroids, and corticosteroid-free remission was achieved and sustained in >60% of patients receiving long-term adalimumab treatment up to week 240 of IMAgINE 2. These findings are of particular importance in children and adolescents because prolonged exposure to corticosteroids increases the risk of infection in children with inflammatory bowel disease,20 resulting in growth suppression21 and patients falling short of their target height as adults.22 Consequently, corticosteroid-free remission is a major therapeutic goal for children with CD based on current treatment guidelines.23 Furthermore, discontinuation of concomitant IMM, which was at the discretion of investigators, increased over time, possibly reflecting the increased awareness of the risk of lymphoma with IMMs.10,12,20
Results in patients who received weekly HD adalimumab at the end of IMAgINE 1 and de-escalated to EOW HD when entering IMAgINE 2 suggest that the dosing interval may be reduced to EOW in some patients who received weekly adalimumab; however, the overall number of patients was small, and results should be interpreted with caution.
Based on current guidelines, one of the treatment goals for pediatric patients with CD, in addition to symptom relief, is improvement of growth outcomes. Linear growth retardation is one of the most common complications of pediatric CD, and approximately one-third of children and adolescents with CD experience growth failure.24,25 Results of the IMAgINE 1 study showed that adalimumab treatment significantly improved linear growth rate in children with CD, indicated by an increase in height velocity z scores at weeks 26 and 52.15 In this analysis, we assessed the long-term effect of adalimumab on growth outcomes in a selected population of patients who exhibited linear growth impairment but still had physiologic growth potential as assessed by bone age. Our findings showed that long-term adalimumab treatment achieved and sustained normal linear growth rates in patients with growth retardation at IMAgINE 1 baseline, demonstrating that long-term adalimumab therapy may address significant disease complications beyond mere symptom control. Furthermore, sustained improvements in IMPACT III scores through week 240 of IMAgINE 2 also suggest that adalimumab therapy has favorable effects on health-related quality of life, another important treatment goal for pediatric patients with CD.23
The safety profile of long-term adalimumab therapy in pediatric patients with CD is consistent with AE rates reported in other adult and pediatric CD trials15,26 and with the overall safety profile of adalimumab in multiple indications spanning over 12 years of experience.27 We identified no new safety signals with long-term adalimumab exposure, including all patients who received adalimumab in IMAgINE 1 and IMAgINE 2. No malignancies were reported, and AE rates remained stable or decreased over time. Furthermore, dose escalation to weekly dosing did not affect AE rates.
This is the first prospective multicenter trial of the long-term efficacy and safety of adalimumab in children with CD; however, this study is not without limitations. Because of the long-term open-label clinical study design, there was a loss of patient data over time after discontinuation for several reasons, including lack of efficacy, AEs, the commercial availability of adalimumab, or the transition to adult models of care. In addition, the sample size has likely been too small to capture potential rare events. Furthermore, as the study case report forms did not specifically query if patients discontinued due to a move to commercial adalimumab or a move to adult care and, in many cases, lacked documentation of the reason for discontinuation, it is possible that the hNRI method underestimated efficacy at the later time points. Per study protocol, patient selection was restricted because eligible patients who enrolled in IMAgINE 2 were required to have successfully completed IMAgINE 1 and achieved a clinical response at any time point during IMAgINE 1. Also, dose adjustment based on therapeutic drug monitoring was not a part of this long-term study. Finally, the IMAgINE 1 and 2 trials did not assess mucosal healing in pediatric patients with CD receiving adalimumab. Achievement of mucosal healing and clinical remission (deep remission) is gaining increasing interest and has emerged as a treatment goal in adult and pediatric CD trials.28,29
In conclusion, these results demonstrate that long-term adalimumab treatment is beneficial for pediatric patients with moderately to severely active CD who have failed conventional therapies and previous anti–tumor necrosis factor therapy. Our findings are consistent with previously published adalimumab studies in multiple indications26,27,30,31 and show that efficacy and safety of long-term adalimumab treatment remained stable for more than 5 years in a pediatric CD population.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.ibdjournal.org).
AbbVie Inc. funded the IMAgINE 1 and 2 studies. Medical writing support was provided by Ade Adebusola, PhD, of AbbVie Inc. and Maria Hovenden of Complete Publication Solutions, LLC (North Wales, PA); this support was funded by AbbVie Inc. AbbVie reviewed and approved the publication.
W. A. Faubion has received consultancy fees from Connecticut Children's Medical Center—Safety Office on subcontracted award through NIH for clinical trial; serves as a board member (no personal compensation) for AbbVie and UCB; and serves as a consultant (no personal compensation) for AbbVie, Boehringer Ingelheim Pharma, Janssen Research & Development, Celgene Corporation, Genentech, and Shire Development, LLC. M. Dubinsky has received consultancy fees from AbbVie, Janssen, Takeda, Pfizer, Celgene, Boehringer Ingelheim, Prometheus Labs, and UCB and has received research support from Janssen. F. M. Ruemmele has received speaker fees from Schering-Plough, Nestlé, Mead Johnson, Ferring, MSD, Johnson & Johnson, Centocor, and AbbVie; serves as a board member for SAC:DEVELOP (Johnson & Johnson); and has been invited to MSD France, Nestlé Nutrition Institute, Nestlé Health Science, Danone, and Mead Johnson. J. Escher has received financial support for research from MSD; has received speakers fee(s) from MSD; has received consultancy fees from Janssen Biologics; and serves as a board member for scientific advisory committee of DEVELOP study (Janssen Biologics) and steering committee for CAPE registry (AbbVie). J. Rosh has received consultancy fees from AbbVie and Janssen; is a board member for GI Health Foundation; and has received financial support for research from AbbVie and Janssen. J. S. Hyams has received consultancy fees from Janssen Ortho Biotech, AbbVie, Celgene, Entera Health, Pfizer, Soligenix, Takeda, Lilly, Genentech, Boehringer Ingelheim, and AstraZeneca; has provided expert testimony on behalf of Janssen Ortho Biotech; has received speaker fees from Janssen Ortho Biotech; and has received payment for development of educational presentations from Janssen Ortho Biotech. S. Eichner, Y. Li, N. Reilly, R. B. Thakkar, A. M. Robinson, and A. Lazar are AbbVie employees and may own AbbVie stock or options.
REFERENCES
- 1.Malaty HM, Fan X, Opekun AR, et al. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. 2010;50:27–31. [DOI] [PubMed] [Google Scholar]
- 2.Walters TD, Griffiths AM. Mechanisms of growth impairment in pediatric Crohn's disease. Nat Rev Gastroenterol Hepatol. 2009;6:513–523. [DOI] [PubMed] [Google Scholar]
- 3.Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heuschkel R, Salvestrini C, Beattie RM, et al. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:839–849. [DOI] [PubMed] [Google Scholar]
- 5.Gasparetto M, Guariso G. Crohn's disease and growth deficiency in children and adolescents. World J Gastroenterol. 2014;20:13219–13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauer CG, Kugathasan S. Pediatric inflammatory bowel disease: highlighting pediatric differences in IBD. Med Clin North Am. 2010;94:35–52. [DOI] [PubMed] [Google Scholar]
- 7.Aloi M, Nuti F, Stronati L, et al. Advances in the medical management of paediatric IBD. Nat Rev Gastroenterol Hepatol. 2014;11:99–108. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz J, Grancher K, Rosa J, et al. Growth failure in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1993;16:373–380. [DOI] [PubMed] [Google Scholar]
- 9.Issenman RM. Bone mineral metabolism in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 1999;5:192–199. [DOI] [PubMed] [Google Scholar]
- 10.Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Askling J, Brandt L, Lapidus A, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005;54:617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashworth LA, Billett A, Mitchell P, et al. Lymphoma risk in children and young adults with inflammatory bowel disease: analysis of a large single-center cohort. Inflamm Bowel Dis. 2012;18:838–843. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–1628. [DOI] [PubMed] [Google Scholar]
- 15.Hyams JS, Griffiths A, Markowitz J, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012;143:365–374. [DOI] [PubMed] [Google Scholar]
- 16.Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed Stanford: Stanford University Press; 1959. [Google Scholar]
- 17.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Lustig RH, Kohn MA, et al. Menarche in pediatric patients with Crohn's disease. Dig Dis Sci. 2012;57:2975–2981. [DOI] [PubMed] [Google Scholar]
- 19.Otley A, Smith C, Nicholas D, et al. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002;35:557–563. [DOI] [PubMed] [Google Scholar]
- 20.Dulai PS, Thompson KD, Blunt HB, et al. Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol. 2014;12:1443–1451. [DOI] [PubMed] [Google Scholar]
- 21.Pfefferkorn M, Burke G, Griffiths A, et al. Growth abnormalities persist in newly diagnosed children with crohn disease despite current treatment paradigms. J Pediatr Gastroenterol Nutr. 2009;48:168–174. [DOI] [PubMed] [Google Scholar]
- 22.Alemzadeh N, Rekers-Mombarg LT, Mearin ML, et al. Adult height in patients with early onset of Crohn's disease. Gut. 2002;51:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8:1179–1207. [DOI] [PubMed] [Google Scholar]
- 24.Kanof ME, Lake AM, Bayless TM. Decreased height velocity in children and adolescents before the diagnosis of Crohn's disease. Gastroenterology. 1988;95:1523–1527. [DOI] [PubMed] [Google Scholar]
- 25.Shamir R, Phillip M, Levine A. Growth retardation in pediatric Crohn's disease: pathogenesis and interventions. Inflamm Bowel Dis. 2007;13:620–628. [DOI] [PubMed] [Google Scholar]
- 26.Colombel JF, Sandborn WJ, Panaccione R, et al. Adalimumab safety in global clinical trials of patients with Crohn's disease. Inflamm Bowel Dis. 2009;15:1308–1319. [DOI] [PubMed] [Google Scholar]
- 27.Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis. 2013;72:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. [DOI] [PubMed] [Google Scholar]
- 29.Kierkus J, Dadalski M, Szymanska E, et al. The impact of infliximab induction therapy on mucosal healing and clinical remission in Polish pediatric patients with moderate-to-severe Crohn's disease. Eur J Gastroenterol Hepatol. 2012;24:495–500. [DOI] [PubMed] [Google Scholar]
- 30.Panaccione R, Colombel JF, Sandborn WJ, et al. Adalimumab sustains clinical remission and overall clinical benefit after 2 years of therapy for Crohn's disease. Aliment Pharmacol Ther. 2010;31:1296–1309. [DOI] [PubMed] [Google Scholar]
- 31.Colombel JF, Sandborn WJ, Ghosh S, et al. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: data from ULTRA 1, 2, and 3. Am J Gastroenterol. 2014;109:1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


