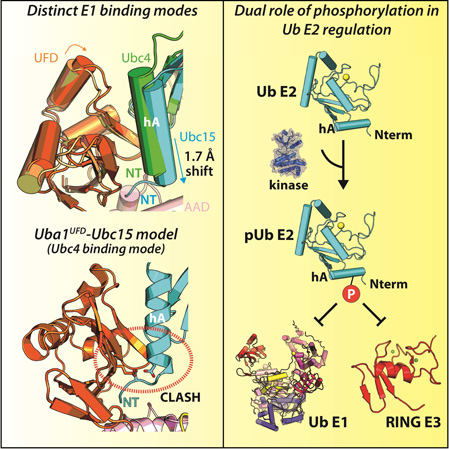
Summary
Ubiquitin (Ub) E1 initiates the Ub conjugation cascade by activating and transferring Ub to tens of different E2s. How Ub E1 cooperates with E2s that differ substantially in their predicted E1 interacting residues is unknown. Here, we report the structure of S. pombe Uba1 in complex with Ubc15, a Ub E2 with intrinsically low E1-E2 Ub thioester transfer activity. The structure reveals a distinct Ubc15 binding mode that substantially alters the network of interactions at the E1-E2 interface compared to the only other available Ub E1-E2 structure. Structure-function analysis reveals that the intrinsically low activity of Ubc15 largely results from the presence of an acidic residue at its N-terminal region. Notably, Ub E2 N-termini are serine/threonine-rich in many other Ub E2s, leading us to hypothesize that phosphorylation of these sites may serve as a novel negative regulatory mechanism of Ub E2 activity, which we demonstrate biochemically and in cell-based assays.
Keywords: E1, E2, ubiquitin, thioester transfer, kinase
Graphical abstract
Introduction
The reversible posttranslational modification of proteins by ubiquitin (Ub) is a regulatory mechanism that controls nearly all aspects of cellular function in eukaryotes (Swatek and Komander, 2016). Ub conjugation alters properties of the target protein such as its stability, subcellular localization, intermolecular interactions, and conformation/activity (Komander and Rape, 2012). Ub conjugation to target proteins requires the sequential interactions and activities of three enzymes, E1, E2, and E3 which function to activate, shuttle, and ligate Ub to target proteins, respectively (Streich and Lima, 2014).
Ub E1 is a modular enzyme comprising active and inactive adenylation domains (AAD and IAD, respectively) that interact with and adenylate the C-terminus of Ub, a Cys domain that is split into two globular half domains (first and second catalytic cysteine half domains, FCCH and SCCH, respectively) that harbors the catalytic cysteine residue involved in Ub thioester bond formation, and a ubiquitin fold domain (UFD) that is required for recruitment of E2 to E1 (Streich and Lima, 2014). All Ub E2s contain a conserved UBC domain that includes the active site and elements required for interactions with E1s and E3s but there are also structural variations across Ub E2s such as N- and C-terminal extensions, and loop insertions proximal to the active site cysteine that in some cases play additional functional roles (Wenzel et al., 2011). The UFD initially recruits E2 to E1 in a configuration in which the active sites are distant from each other, and then undergoes a conformational change that facilitates transfer of Ub from the E1 to the E2 catalytic cysteine in a process called thioester transfer (Huang et al., 2007; Olsen and Lima, 2013). After E1-E2 Ub thioester transfer, the resulting E2-Ub intermediate interacts with members of three different families of Ub E3 ligases that catalyze Ub conjugation to target proteins by distinct mechanisms (Buetow and Huang, 2016).
The Ub system is unique among Ub-like modifiers because it has 10–35 different E2s that must function with one or two E1s, depending on the organism, many of which perform clearly defined and distinct functions (Michelle et al., 2009; van Wijk and Timmers, 2010). The only Ub E1-E2 complex structure determined to date is an S. pombe Uba1-Ubc4/Ub adenylate (Ub(a)) ternary complex (Olsen and Lima, 2013). While this structure provided the first molecular insights into Ub E1 recognition of E2, this single structure was unable to explain the basis by which S. pombe Uba1 (hereafter, Uba1) is capable of promiscuously interacting with all of its Ub E2s, as the E2s exhibit only limited amino acid sequence identity and similarity at positions observed to interact with the UFD. This led to the hypothesis that structural plasticity at the E1-E2 interface may provide the molecular basis by which a single E1 interacts with many different E2s, but there is currently a lack of structural evidence supporting this hypothesis. Moreover, since S. pombe Ubc4 (hereafter, Ubc4) is a structurally minimalistic Ub E2 containing only the UBC domain, the role that additional structural elements play in thioester transfer from Uba1 to more complex Ub E2s is unknown.
Here, we present the 2.5 Å crystal structure of S. pombe Ubc15 (hereafter, Ubc15) in complex with Uba1 and Ub(a) which reveals that Ubc15 engages Uba1 via a distinct binding mode compared to Ubc4. Comparison of the structures reveals how structural elements unique to Ubc15, including the acidic loop insertion characteristic of CDC34-like E2s and a short N-terminal extension, play a role in determining its distinct E1 binding mode. Our structure-function analysis reveals that the presence of an N-terminal acidic residue accounts for the intrinsically low level of thioester transfer activity of Ubc15, likely due to electrostatic repulsion with an acidic patch on the UFD. The region encompassing Glu7 of Ubc15 is serine/threonine-rich in many other Ub E2s, and several of these residues have previously been shown to be phosphorylated in vivo by mass spectrometry (Table S1), however, the function of these phosphorylated residues is not understood. We provide extensive in vitro and in vivo data supporting the hypothesis that phosphorylation of residues at the N-termini of Ub E2s broadly inhibits their ability to function with Ub E1; furthermore, we propose that it may also serve as a dual regulatory mechanism of Ub E2 activity by also inhibiting its interactions with RING E3s.
Results & Discussion
S. pombe Uba1-Ubc15/Ub crystal structure reveals a novel Ub E1-E2 binding mode
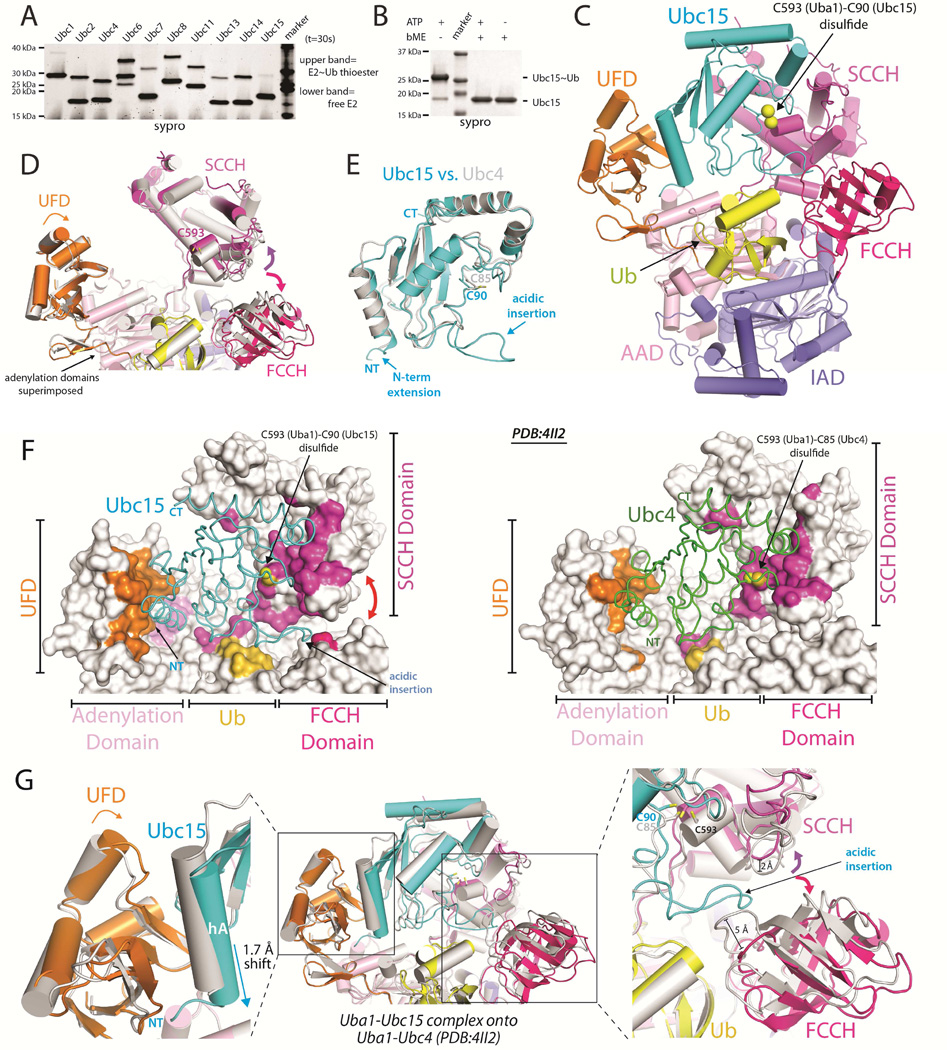
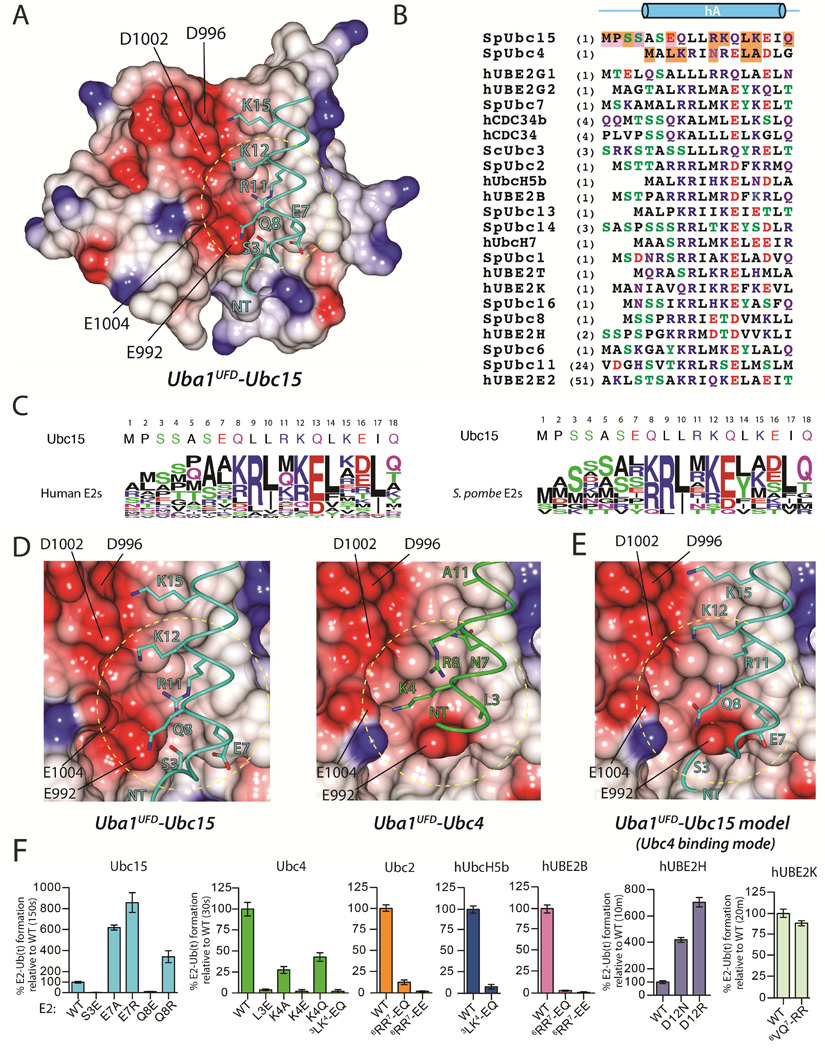
To guide our structural efforts aimed at understanding the molecular basis for promiscuity and specificity in E1-E2 interactions, we performed E1-E2 thioester transfer assays using Uba1 and a panel of 10 out of the 11 S. pombe Ub E2s in order to compare the efficiency with which Uba1 charges different E2s with Ub. While most E2s exhibited similar levels of E1-E2 thioester transfer activities, Ubc15 exhibited significantly lower activity relative to Ubc4 (Figures 1A and S1A). This comparatively low activity is not due to oxidation of the catalytic cysteine, as charging of Ubc15 with Ub is driven to near completion at higher E1 concentrations (Figure 1B). Compared to Ubc4, Ubc15 exhibits only 33% identity and 55% amino acid sequence similarity at positions predicted to interact with Uba1 (Figure S1B), and in light of its intrinsically low E1-E2 thioester transfer activity, we reasoned that a structure of Ubc15 in complex with Uba1 would provide significant insights into the molecular basis for promiscuity in Ub E1-E2 interactions.
Figure 1. Uba1-Ubc15/Ub structure reveals a distinct Ub E1 binding mode.
(A) E1-E2 Ub thioester transfer assays for the indicated Uba1-E2 pairs.
(B) Uba1-Ubc15 thioester transfer assay under endpoint conditions, prepared in the presence and absence of reducing agent.
(C) Cartoon of the Uba1-Ubc15/Ub complex with Uba1 domains color-coded and labeled.
(D) Uba1 from the Uba1-Ubc15 structure is colored as in C and Uba1 from the Uba1-Ubc4 structure (PDB: 4II2) is colored gray. Uba1 adenylation domains superimposed (RMSD=0.207 Å). Domain rotations indicated with arrows.
(E) The UBC domains of Ubc15 (cyan) and Ubc4 (gray) were superimposed and the structures are shown as ribbons.
(F) Uba1-Ubc15 (left) and Uba1-Ubc4 (right) structures with Uba1 atoms contacting E2 colored by domain, as in C.
(G) Ubc15 and Ubc4 Uba1 binding modes. The adenlyation domains were superimposed and colored as in D and the E2s are shown as cartoons and colored as in E.
To facilitate our structural studies of the Uba1-Ubc15 complex, we employed the cross-linking strategy previously used to determine the Uba1-Ubc4 structure in which the E1 and E2 catalytic cysteines were cross-linked via disulfide bond (Olsen and Lima, 2013). The structure of Uba1-Ubc15/Ub (Figure 1C) was determined to 2.5 Å resolution (R/Rfree values of 0.219/0.251) (Table 1) with two complexes in the crystallographic asymmetric unit. The overall architecture of the Uba1-Ubc15 complex resembles that of the Uba1-Ubc4 complex, with Ubc15 sandwiched in the canyon between the UFD and SCCH domains of the E1, and Ub(a) engaging the adenlyation domains of Uba1 (Figure 1C) (Olsen and Lima, 2013). However, closer comparison of the complexes reveals that Ubc15 employs a distinct Uba1 binding mode compared to Ubc4 (Figures 1D, 1E, 1F and 1G). Superimposition of the Uba1 adenlyation domains of the Uba1-Ubc15 and Uba1-Ubc4 structures reveals subtle, but mechanistically important rigid body rotations of the UFD towards the SCCH domain, and of the SCCH and FCCH domains away from one another (Figures 1D, 1F, and 1G). While the structure of the UBC domains of Ubc15 and Ubc4 are very similar, the N-terminus of Ubc15 is longer than that of Ubc4 by four residues and Ubc15 harbors a 13-residue acidic loop insertion proximal to its active site (Figures 1E and S1B). These structural differences in E1 and E2, together with more detailed differences in the E1-E2 interactions discussed below, are defining features of the Ubc15 and Ubc4 modes of Uba1 binding.
Table 1.
Crystallographic Data and Refinement Statistics
| SpUba1-Ubc15/Ub | |
| PDB ID | 5KNL |
| Source | APS 22 ID |
| Wavelength (Å) | 1.00 |
| Resolution Limits (Å) | 29.7–2.50 (2.55–2.5) |
| Space Group | P1 |
| Unit Cell (Å) a, b, c | 77.1, 82.2, 135.4 |
| Unit Cell (°) α, β, γ | 102.1, 95.8, 90.9 |
| Number of observations | 378659 |
| Number of reflections | 108289 (5430) |
| Completeness (%) | 96.9 (93.2) |
| Mean I/σI | 10.2 (3.1) |
| R-merge on I a | 0.051 (0.29) |
| Refinement Statistics | |
| Resolution Limits (Å) | 28.3–2.50 (2.53–2.50) |
| # of reflections (work/free) | 97456/10828 |
| Completeness (%) | 96.9 (93.0) |
| Protein/ligand/water atoms | 19284/45/94 |
| Rcryst b | 0.216 (0.355) |
| Rfree (10% of data) | 0.251 (0.378) |
| Bonds (Å)/ Angles (°) | 0.003/0.699 |
| B-factors: protein/ligand/water (Å2) |
52.1/67.7/24.2 |
| Ramachandran plot statistics (%) |
|
| favored | 94.8 |
| allowed | 4.9 |
| outliers | 0.3 |
| MolProbity score | 1.11–100th percentile (N=6960, 2.50 Å ± 0.25Å) |
Parentheses indicate statistics for the high-resolution data bin for x-ray data.
With respect to how the E2s engage Uba1, comparison of the Uba1-Ubc15 and Uba1-Ubc4 structures reveals that relative to Ubc4, helix A (hA) of Ubc15 is translated by ~1.7 Å toward the ‘bottom’ of the UFD (Figures 1F and 1G). This results in a number of unique contacts between residues in hA of Ubc15 and the UFD as well as novel contacts between the extended N-terminus of Ubc15 and the AAD (Figures 1F and 1G; see below). By comparison, there were no contacts between Ubc4 and the AAD in the Uba1-Ubc4 structure (Figure 1F). Second, the acidic loop insertion of Ubc15 is wedged directly between the FCCH and SCCH domains of Uba1 (Figures 1F, 1G and S2A). While the FCCH and SCCH domains interact with each other in all previous Uba1 structures, breaking of the contacts between the FCCH and SCCH domains and rotation of these domains away from one another is required to accommodate the acidic loop insertion of Ubc15 which would otherwise sterically clash with Uba1 (Figures 1F, 1G, 2A and 2B). Rotation of the SCCH domain also results in a 3 Å translation of the active site cysteine, which together with the UFD rotation, allows the Uba1 and Ubc15 active site cysteines to be positioned proximal to each other during E1-E2 thioester transfer despite substantial differences in how Ubc15 interacts with the UFD (Figures 1D and 1G).
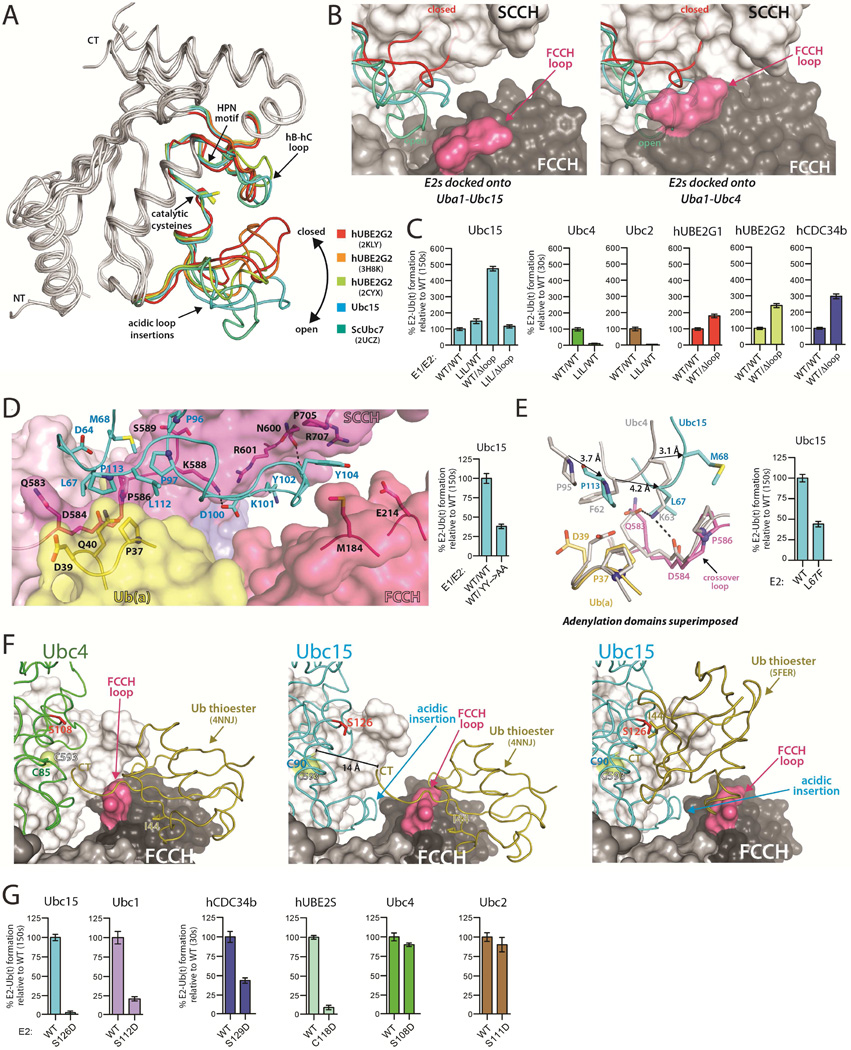
Figure 2. The Ubc15 acidic loop and architecture of the 'doubly loaded' Ub E1-E2 complex.
(A) The UBC domains of the indicated E2s were superimposed with the acidic loop insertions color-coded as indicated.
(B) The indicated E2s were superimposed onto the Uba1-Ubc15 structure (left) and Uba1-Ubc4 structure (right). E2s colored as in A.
(C) E1-E2 thioester transfer assays of the indicated proteins. Δloop indicates family three E2 constructs with the acidic loop deleted. LIL indicates a Uba1 mutant harboring an eleven amino acid insertion in the FCCH loop. Error bars in all figures represent ±1 standard deviation and were derived from three independent experiments.
(D) Uba1-Ubc15 structure colored as in Figure 1C with residues involved in key intermolecular contacts shown as sticks (left). E1-E2 thioester transfer assays of the indicated proteins (right).
(E) Uba1-Ubc15 and Uba1-Ubc4 structures shown as in Figure 1G with equivalent E2 residues that occupy different locations at the E1-E2 interface indicated by a black arrow. E1-E2 thioester transfer assays of the indicated proteins (right).
(F) The ubiquitin thioester (Ub(t)) was docked onto the FCCH domain of the Uba1-Ubc4 (left) and Uba1-Ubc15 (center) structures based on contacts observed in the ‘doubly loaded’ Uba1 structure (PDB: 4NNJ). Ub(t) was docked onto Ubc15 from the Uba1-Ubc15 structure based on the hUbcH5~Ub thioester intermediate mimic (PDB: 5FER), (right). The E2 residue on hC that was mutated to probe potential contacts between Ub(t) and E2 during thioester transfer is shown as red sticks.
(G) E1-E2 thioester transfer assays of the indicated proteins.
See also Figure S2.
The Ubc15 acidic loop insertion
Ubc15 is a member of the CDC34-like ‘family three’ of Ub E2s that is defined by the presence of a 12 to 13-residue loop insertion that lies in proximity to the E2 catalytic cysteine (Figures 2A and S2A) (Michelle et al., 2009). This loop has been shown to be critical for polyubiquitin chain extension by and biological activity of hCDC34 and hUBE2G2 (Gazdoiu et al., 2007; Liu et al., 2014; Petroski and Deshaies, 2005). Numerous structural studies have shown that the acidic loop insertion is highly flexible, adopting multiple conformations ranging from 'closed', where the loop folds back towards the E2 active site and partially occludes its solvent accessibility, to 'open' where the loop extends away from the active site, thereby increasing accessibility (Figure 2A). The Uba1-Ubc15 structure shows that the acidic loop of Ubc15 adopts a conformation in between the open-most and closed-most conformations (Figures 2A and S2A). As expected, superposition of family three E2 structures onto Ubc4 from the Uba1-Ubc4 structure shows that the acidic loop sterically clashes with the SCCH and FCCH domains regardless of its conformation (Figure 2B). This is consistent with rotation of the FCCH and SCCH domains away from each other in order to accommodate the acidic loop in the Uba1-Ubc15 structure. Superposition of family three E2 structures onto Ubc15 from the Uba1-Ubc15 structure further reveals that the E2 acidic loop also clashes with the SCCH domain when in the closed conformation whereas the acidic loop can be accommodated in more open conformations (Figure 2B). In the context of previous studies, our data suggests that opening of the acidic loop facilitates E1-E2 thioester transfer activity by: 1) increasing accessibility of the active site cysteine for attack of the E1~Ub thioester bond and 2) preventing steric clashes between the loop and E1 as the E1 and E2 active sites come into proximity during thioester transfer.
Previous qualitative studies showed that deletion of the acidic loop of CDC34 led to increased ‘charging’ by E1 (Lass et al., 2011). To test whether this is a general feature of family three E2s, we deleted the acidic loop of Ubc15, hCDC34b, and hUBE2G2 and found that the thioester transfer activities of the resulting E2s were increased by 2–5 fold (Figures 2C and S2C). We hypothesize that flexibility of the acidic loop and the need for it to fit into a relatively confined area on the surface of the E1 introduces a steric impediment that results in a general reduction of thioester transfer activity. Presumably the reduction in E1-E2 thioester transfer activity is outweighed by the positive role the acidic loop plays in RING E3-mediated ubiquitination of target proteins (Gazdoiu et al., 2007; Li et al., 2007; Petroski and Deshaies, 2005). Finally, an eleven amino acid insertion in the FCCH loop of Uba1 designed to partially occlude access to the 'front' side of the complex (Uba1LIL) substantially decreases thioester transfer to Ubc2 and Ubc4, whereas thioester transfer to Ubc15 is unaffected despite the presence of its acidic loop, presumably due to the rotation of the FCCH domain which redirects the FCCH loop insertion away from the ‘front’ of the complex (Figures 2C and S2B).
Although there are not extensive contacts between the Ubc15 acidic loop insertion and Uba1, there are a few worth noting. First, Asp100 and Tyr102 of Ubc15 engage in hydrogen bonds to Lys588 and Asn600 of Uba1 (Figure 2D). Second, Tyr104 of Ubc15 stacks between the side chains of Met184 in the FCCH domain and Pro705 of the SCCH domain (Figure 2D), and it is interesting to note that these two Uba1 residues stack directly against each other in all previous structures of Uba1 both in the presence and absence of E2 (Lee and Schindelin, 2008; Olsen and Lima, 2013; Schafer et al., 2014). There is also a salt bridge between Glu214 of the FCCH domain and Arg707 of the SCCH domain in all previous Uba1 structures that does not take place in the Uba1-Ubc15 complex due to rotation of the FCCH and SCCH domains. Although the FCCH and SCCH domains do not make direct contacts in the Uba1-Ubc15 structure, indirect contacts mediated through Tyr104 of Ubc15 may serve to stabilize the conformation of the FCCH and SCCH domains relative to each other. Indeed, we observed a modest loss of function for a Y102A/Y104A Ubc15 mutant (Figure 2D) which suggests that the contacts observed between the acidic loop and Uba1 contribute to thioester transfer when the loop is present, perhaps by helping to lock the loop into the more open conformation that is compatible with Uba1 binding. Finally, Tyr102 and Tyr104 of the Ubc15 acidic loop insertion are not fully conserved among family three E2s, including hCDC34a and hCDC34b. If the hCDC34 acidic loop insertion adopts the same conformation as that of Ubc15 in the Uba1-Ubc15 structure, Pro104 and Gln105 of hCDC34b would be positioned in proximity to the FCCH and SCCH domains where they might participate in intermolecular interactions. However, P104A and Q105A substitutions do not decrease the thioester transfer activity of hCDC34b (Figure S3B), suggesting that there may be plasticity in the interactions that take place between Uba1 and the acidic loop insertion of family three E2 members.
Ubc15 interface with the Uba1 crossover loop and Ub(a)
A tripartite interface between Ubc4, the Uba1 crossover loop, and Ub(a) was previously observed in the Uba1-Ubc4 structure (Olsen and Lima, 2013). Differences in the topology of the E1-E2 interface in the Uba1-Ubc15 structure resulting from the distinct Ubc15 binding mode leads to substantial differences in the nature of these interactions. In the Uba1-Ubc4 structure Phe62 of Ubc4 of makes van der Waals contacts to Asp39 of Ub(a) and Gln583 of Uba1, and Lys63 of Ubc4 engages in hydrogen bonds to Gln583 and Asp584 of Uba1 (Olsen and Lima, 2013). In terms of primary sequence, Phe62 and Lys63 of Ubc4 correspond to Leu67 and Met68 of Ubc15, however, as a result of their distinct E1 binding modes these E2 residues are offset by 4.2 and 3.1Å, respectively, relative to Uba1 (Figure 2E). This results in Leu67 making structurally equivalent contacts as Lys63 of Ubc4 (rather than Phe62) (Figure 2E). In the Uba1-Ubc15 structure the side chain of Ubc15 Leu67 is wedged between the side chains of Gln583 and Pro586 of the Uba1 crossover loop (Figure 2E), and analysis of the structure shows that there would be little room for a phenylalanine (as in Ubc4). Indeed, an L67F substitution in Ubc15 results in a 2-fold reduction in thioester transfer activity (Figure 2E). Other contacts at this region of the Uba1-Ubc15 structure take place between Met68 (sequence equivalent of Lys63) and Pro113 of Ubc15, which engage in unique van der Waals contacts with Pro586 of the Uba1 crossover loop, and Pro37 of Ub(a), respectively (Figure 2E). Importantly, this provides an example of the structural basis by which plasticity at the E1-E2 interface arises. Relatively subtle amino acid differences, together with changes in the topology of the E1-E2 complex, result in contacts taking place between residues that differ in their physicochemical properties and their position in the primary sequence.
Architecture of the 'doubly loaded' Ub E1-E2 complex
The cross-linking strategy used to trap the Uba1-Ubc15 complex is incompatible with the presence of the Ub that is linked by thioester bond to the E1 catalytic cysteine during thioester transfer (Ub(t)). Nevertheless, our previous results (Olsen and Lima, 2013) together with the recently determined structure of ‘doubly loaded’ Uba1 (PDB: 4NNJ) (Schafer et al., 2014) are consistent with Ub(t) residing on the same side of the E1 where Ub(a) binds during thioester transfer (termed the ‘front’ of the complex). In the doubly loaded Uba1 structure, Ub(t) sits on top of the FCCH domain and modeling onto the Uba1-Ubc4 structure suggests that contacts between Ub(t) and the FCCH domain are compatible with thioester transfer to Ubc4 (Figures 2F and S2B) (Olsen and Lima, 2013; Schafer et al., 2014). In contrast, modeling Ub(t) onto the Uba1-Ubc15 complex shows that the observed contacts between Ub(t) and the FCCH domain are incompatible with thioester transfer to Ubc15 primarily due to the aforementioned rotation of the FCCH domain translating the C-terminus of Ub approximately 14 Å away from the Uba1 catalytic cysteine (Figures 2F and S2B). There are also clashes between Ub(t) and the aforementioned FCCH loop that adopts a different conformation than in the Uba1-Ubc4 structure (Figures 2F and S2B).
E2~Ub thioester intermediates adopt topologies ranging from ‘closed’ in which Ub(t) is proximal to and engages in contacts with helix C (hC) of E2, to ‘open’ and 'backbent' where Ub(t) projects away from the E2 (Page et al., 2012; Pruneda et al., 2011; Soss et al., 2013). Different E2~Ub thioesters (or thioester mimics) have been observed to differ in their tendency to adopt the 'closed', 'open' or 'backbent' conformation in the absence of E3 (Page et al., 2012; Pruneda et al., 2011). We previously noted that the surface of E2 that makes contacts to Ub(t) in the 'closed' conformation is positioned on the ‘front’ of the E1-E2 complex (Olsen and Lima, 2013). In line with our previous observations, neither a S108D mutation on Ubc4, nor a S111D mutation on Ubc2 that are predicted to disrupt this E2-Ub(t) interaction appreciably affect E1-E2 thioester transfer activity (Figures 2G and S2C) (Olsen and Lima, 2013). In contrast, thioester transfer activities of the corresponding Ubc15 (S126D), Ubc1 (S112D), hCDC34b (S129D), and hUBE2S (C118D) mutants are significantly reduced (Figures 2G and S2C), suggesting that contacts between Ub(t) and hC of E2 facilitates thioester transfer of Ub to this subset of E2s. Our structural and biochemical data, together with previously published data indicates that the involvement of Ub(t) contacts to hC of E2 correlates with E2s that have a greater tendency to adopt the 'closed' E2-Ub conformation (ScUbc1, hUBE2S, hCDC34) (Hamilton et al., 2001; Pruneda et al., 2011; Saha et al., 2011; Wickliffe et al., 2011) and/or the presence of the acidic loop. Ubc4~Ub has neither a strong tendency to adopt the closed conformation in the absence of E3 (Pruneda et al., 2011) nor does it harbor an acidic loop insertion (Figures 1E and S1B), which is consistent with the S108D mutation failing to affect the efficiency of thioester transfer (Figures 2G and S2C).
Considering that Ub(t) engages the FCCH domain of E1 in the absence of E2, it is intriguing to speculate that formation of contacts between Ub(t) and hC of the E2 during thioester transfer may lower the affinity of the product of thioester transfer (E2~Ub) for the enzyme (Uba1), thereby serving as a mechanism to drive the reaction forward. Though the details of the contacts between the E2 and the ubiquitin-like modifier differ, this idea is conceptually consistent with a model for E1-E2 thioester transfer in the Nedd8 system posited by Schulman and colleagues (Huang et al., 2007).
The Ubc15-FCCH/SCCH interface
Ubc15 makes extensive interactions with the SCCH domain that are distributed into two distinct but contiguous clusters (Figures 3A and S3A). The first cluster of interactions surrounds the E1 (Cys593) and E2 (Cys90) active sites cysteines where there are four Ubc15 residues (His84, His95, Ser135, and Ala137) that are positioned to potentially participate in catalysis of E1-E2 thioester transfer (Figure 3B). Ser135 is located on the highly flexible hB-hC loop of Ubc15 and its side chain hydroxyl is less than 5 Å away from the sulfur atoms of the catalytic cysteines of both Uba1 and Ubc15. We found that a S135A substitution in Ubc15 severely diminishes thioester transfer activity (Figures 3B and S3B) indicating a potential role for this residue in optimally positioning the Ub thioester for nucleophilic attack by the Ub E2 catalytic cysteine and/or positioning of the E2 catalytic cysteine itself. This result was surprising because mutation of the corresponding residue of Ubc4 (Asp117) to alanine did not have a significant effect on E1-E2 thioester transfer activity (Olsen and Lima, 2013), and interestingly, a Ubc15 mutant in which Ser135 was mutated to an Asp, as in Ubc4, exhibited wild type levels of thioester transfer activity (Figure S3B). Residues at this position of Ub E2s are referred to as ‘gateway residues’ as they are positioned at the opening of the cleft that leads to the Ub E2 catalytic cysteine. Most Ub E2s harbor a Ser or Asp at this position and mutation of these residues has been demonstrated to impact lysine selectivity and/or reactivity during RING E3 catalyzed ubiquitination (Plechanovova et al., 2012; Sadowski et al., 2010). Together, these results suggest that the gateway residues of Ub E2s may play distinct roles in thioester transfer that could reflect differences in E2 active site architecture that have evolved for optimal ubiquitination of specific lysine residues in different molecular contexts.
Figure 3. The Ubc15 mode of Uba1 binding.
(A) Uba1-Ubc15 structure colored as in Figure 1C with residues involved in key intermolecular contacts shown as sticks.
(B) Close-up view of the environment surrounding the Uba1 and Ubc15 active site cysteines (left). E1-E2 thioester transfer assays of the indicated proteins (right).
(C) Selected equivalent E2 residues that occupy different locations at the E2/Cys domain interface indicated by a black arrow. SCCH domains superimposed (left). E1-E2 thioester transfer assays of the indicated proteins (right).
(D) Surface representation of the Ubc15/UFD (left) and Ubc4/UFD (PDB: 4II2; right) interfaces in open book representation with residues involved in intermolecular contacts colored as in Figure 1. Outlines of the Ubc15/UFD and Ubc4/UFD interfaces are mapped onto the opposing structures as a black outline. Ribbon representations of the Ubc15/UFD and Ubc4/UFD interfaces with residues involved in key intermolecular contacts shown as sticks (bottom).
(E) Structure-based sequence alignment highlighting regions at the E2/UFD interface. Residues contacting the UFD and AAD are shaded orange and pink, respectively. Residues involved in unique contacts are labeled as ‘specific contacts’ above the alignment, those involved in equivalent contacts are labeled as ‘conserved’. Residues that our structure-function studies show are important in determining the Ubc15 and Ubc4 modes of binding are termed ‘specificity factors’. Coloring: acidic residues (red), basic residues (blue), Ser/Thr/Tyr (green), Gln/Asn (purple) and all others black.
(F) E1-E2 thioester transfer assays of the indicated proteins.
See also Figure S3.
Ubc15 is among a small subset of Ub E2s that lack a bulky hydrophobic residue at positions corresponding to either His84 or Ala137 (Figure S3C), which are in proximity to both Uba1 and Ubc15 catalytic cysteines. For the larger subset of Ub E2s harboring a hydrophobic residues at one of these positions, structure-function analysis has shown that the bulky hydrophobic side chain is critical for thioester transfer, perhaps by providing a platform for catalysis (Olsen and Lima, 2013). Because Ubc15 has an intrinsically low thioester transfer activity and is one of the Ub E2s that lacks a bulky hydrophobic residue at either of these positions, we introduced an A137L substitution into Ubc15 and found that it had a 4-fold increase in thioester transfer activity (Figures 3B and S3B). Finally, although the side chain of Ubc15 His95 projects towards the E1 and E2 catalytic cysteines and is positioned such that it could potentially serve as a catalytic base during thioester transfer (Figure 3B), alanine substitution did not have an effect on activity (not shown).
The second cluster of interactions between Ubc15 and the SCCH domain is centered around a hydrophobic pocket formed by Phe598, Leu685, Phe689, and Phe701 of Uba1, into which the hydrophobic side chains of Ubc15 Pro136 and Ile139 insert (Figure 3A). This interaction is conserved in the Ubc4 structure where Pro118 and Pro121 engage in homologous interactions (Figures S3A and S3C) (Olsen and Lima, 2013). As expected, mutation of the Uba1 hydrophobic patch (FFF→AAA) substantially reduces the thioester transfer activity of Ubc15 (Figures S3B and S3D), underscoring the importance of this conserved set of interactions for thioester transfer. One notable difference at the Ubc15-SCCH domain interface is a pair of hydrogen bonds between the side chains of Ubc15 Asp133 and Uba1 Thr695 and Ser696 (Figure 3C). Despite being offset by one position in the primary sequence alignment, Asp116 of Ubc4 engages in a structurally equivalent set of interactions at the Ubc4/SCCH domain interface and it was previously shown that D116A mutation of Ubc4 substantially reduced its thioester transfer activity (Olsen and Lima, 2013). Accordingly, a D133A substitution in Ubc15 also results in a substantial reduction in thioester transfer activity (Figure 3C), underscoring the importance of this unique interaction. Local structural differences in the loops harboring Ubc15 Asp133 and Ubc4 Asp116 together with the rotation of SCCH domain account for this observation, providing another example for how plasticity in E1-E2 interactions can arise.
The Ubc15 binding mode to the UFD and contacts to the AAD
The UFD is required for recruitment of E2 to Ub E1. The surface of Ubc15 formed by hA and the β1- β2 loop engages a contiguous surface of the UFD comprising the beta sheet (β28, β29, and β30), H32, and H33 (Figure 3D). As mentioned previously, a 1.7 Å translation of Ubc15 hA ‘down’ the UFD results in an altered network of contacts between Ubc15 and the UFD as well as a set of contacts between the N-terminal extension of Ubc15 and the AAD, whereas there were are contacts between Ubc4 and the AAD (Figure 3D).
Asn7 of Ubc4 is almost completely buried at the interface with the Uba1 where it predominantly engages in van der Waals contacts to Met944 at the center of the UFD (Figure 3D). As a result of the hA translation, the corresponding residue of Ubc15, Arg11, (Figure 3E) engages in a unique set of interactions with residues at the bottom of the UFD, proximal to the AAD. Specifically, Arg11 of Ubc15 engages in a side chain-side chain mediated hydrogen bond to S946 and van der Waals contacts to Met944, Glu992, and Cys994 of the UFD. In the Uba1-Ubc4 structure, the side chain of the Met944 projects towards the bottom of the UFD in a space that partially overlaps with Arg11 of Ubc15 in the Uba1-Ubc15 structure (Figure 3D). In order for the larger side chain of Arg11 to be accommodated in the context of the hA translation, the side chain of Met944 projects away from the surface of the UFD in the Uba1-Ubc15 structure (Figure 3D). In addition to intermolecular contacts with the UFD, Arg11 of Ubc15 engages in an intramolecular hydrogen bond to the side chain of Ser3 (Figure 3D). This interaction positions Ser3 for an intermolecular hydrogen bond to Glu992 of the UFD and appears to stabilize conformation of the N-terminal extension of Ubc15, which engages in unique intermolecular contacts with the AAD. Contacts between Ubc15 and the AAD include Pro2 of Ubc15, which inserts into a hydrophobic patch on Uba1 formed by residues both on the AAD (Val550 and Phe551) and the UFD (Val990) (Figure 3D). In addition, the backbone carbonyl oxygen of Pro2 engages in a hydrogen bond to the side chain of Arg547 of the AAD, and Ubc15 Met1 packs against the side chain of Tyr544 (Figure 3D). These unique contacts between Ubc15 and the AAD would not be possible without the 1.7 Å translation of hA, which is a defining feature of the Ubc15 binding mode.
Consistent with the structure, an R11N substitution in Ubc15 substantially reduces its E1-E2 thioester transfer activity (Figures 3F and S3F), presumably because the Asn7 side chain is not long enough to engage in equivalent contacts to the UFD. The reciprocal mutation in Ubc4 (N7R) leads to a slight gain of function (Figures 3F and S3F). Importantly, we extended our analysis to the human system by introducing an R11N substitution in hUBE2G1, the closest human ortholog to Ubc15 (Figures 3E and S3E). Similar to the R11N substitution of Ubc15, the R11N mutation of hUBE2G1 results in a significant loss of E1-E2 thioester transfer activity (Figures 3F and S3F), which suggests that this human E2 engages hUba1 via the Ubc15 binding mode. Further, a P2E substitution of Ubc15 results in a substantial loss of E1-E2 thioester transfer activity (Figures 3F and S3F), presumably due to being unfavorably positioned in the hydrophobic patch of the AAD. This further validates the distinct Ubc15 binding mode as well as the unique contacts observed between Ubc15 and the AAD. While the N-terminus of hUBE2G1 is poorly conserved with respect to Ubc15, T2D and L4D substitutions in hUBE2G1 decrease thioester transfer activity (Figure S3F), indicating that these residues are in also in proximity to Uba1.
Glu7 of Ubc15 is largely responsible for its reduced thioester transfer activity
The first structure of S. cerevisiae Uba1 (Lee and Schindelin, 2008) revealed a significant patch of acidity on the surface of the UFD (Figure 4A). With regards to electrostatics, Ubc15 is one of only three Ub E2s in the S. pombe and human systems harboring an acidic residue, Glu7, at positions predicted to project towards the acidic patch of the UFD (Figures 4B and 4C). We hypothesized that electrostatic repulsion between the acidic patch of the UFD and Glu7 of Ubc15 could partially account for its intrinsically low thioester transfer activity, and indeed, mutation of Glu7 to alanine or arginine leads to a 6–9 fold gain of thioester transfer activity (Figure 4F). While the 1.7 Å translation of Ubc15 hA increases the distance between Glu7 and the acidic patch of the UFD and positions the Glu7 side chain ~3.7 Å from the side chain of Arg547 of the AAD (Figure 4D), these changes are apparently insufficient to mitigate the negative effects of having an acidic residue at this region of Ubc15. Further, if Ubc15 interacted with Uba1 via the Ubc4 binding mode, Glu7 of Ubc15 would be positioned immediately proximal to Glu992 of the UFD (Figure 4E). Whereas the aforementioned the A137L Ubc15 mutation results in a 2-fold decrease in Km and 2.5-fold increase in Vmax, the Ubc15 E7R mutation results in an approximately 80-fold decrease in Km and a slight increase in Vmax, with both values falling in the range of wild type Ubc4 (Figures S4A, S4B, and S4C). These results are consistent with values for the reciprocal mutations of Ubc4 (L3E and L119A) (Figures S4A, S4B, and S4C).
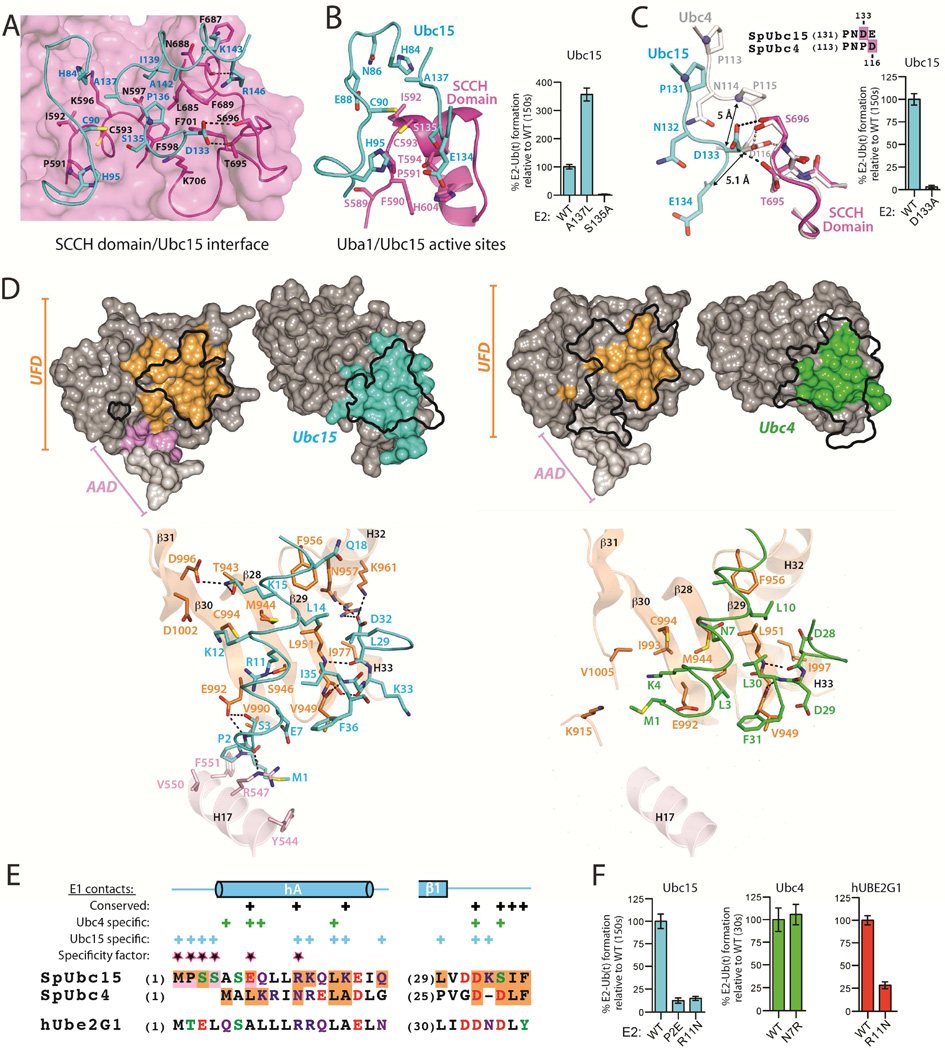
Figure 4. Acidity at the N-terminus of E2 diminishes Ub E1-E2 thioester transfer activity.
(A) The UFD from the Uba1-Ubc15 structure is shown as surface electrostatic representation. The N-terminus of Ubc15 shown as backbone worm with selected residues in sticks.
(B) Structure-based sequence alignment prepared as in Figure 3E, highlighting regions at the E2/UFD interface.
(C) Sequence logo of the N-termini of the thirty active human Ub E2s (left) and eleven S. pombe Ub E2s (right).
(D) Close-up view of electrostatics at the Ubc15/UFD (left) and Ubc4/UFD (right) interfaces. Structures are presented as in A.
(E) Model of Ubc15 superimposed onto Ubc4 from the Uba1-Ubc4 complex. Ubc4 is omitted for clarity.
(F) E1-E2 thioester transfer assays of the indicated proteins.
See also Figure S4.
Based on this data, we next posited that substitution of any E2 residue that projects towards the acidic patch of the UFD with an acidic residue is likely to diminish thioester transfer activity due to electrostatic repulsion. Indeed, S3E and Q8E substitutions in Ubc15 almost completely abrogate activity (Figures 4F and S4D). The result was similar for mutations of Ubc2, Ubc4, hUBE2B, and hUbcH5b (Figures 4F and S4D). Further, mutation of Asp12 of hUBE2H, which is one of the three E2s with a naturally occurring acidic residue at a position predicted to project towards the UFD acidic patch, to asparagine (as in Asn7 of Ubc4) or arginine (as in Arg11 of Ubc15) leads to a significant gain of function (Figures 4F and S4D) as does an E3A substitution in hUBE2G1 (Figures S3F).
Previously, a model for the E2/UFD interface involving direct interactions between relatively well-conserved basic residues at the N-terminus of E2s in a region corresponding to Glu7 of Ubc15 (Figures 4B and 4C), and the acidic patch of the UFD was proposed (Lee and Schindelin, 2008). In this study, a K4E substitution was found to almost completely abrogate the thioester transfer activity of ScUbc1. In the Uba1-Ubc4 structure, the analogous Lys4 residue of Ubc4 was found to be in the vicinity of the UFD acidic patch but did not engage in direct contacts to acidic residues on the UFD (Figure 4D) and a K4A substitution leads to a modest effect on thioester transfer activity compared to K4E (Figures 4F and S4D). Further, while a basic residue at the position corresponding to Lys4 is present in 10 of 11 S. pombe E2s and 21 of 30 active human Ub E2s, many highly active Ub E2s lack basic residues at and around this region (Michelle et al., 2009) (Figures 4B and 4C). Together, these results suggest that direct, short-range interactions between basic residues on the E2 at the position corresponding to Lys4 of Ubc4 and acidic residues on the UFD is not a major determinant of thioester transfer activity for all Ub E2s. To address this point, we tested whether introducing basic residues in an E2 naturally lacking them at the area corresponding to Lys4 of Ubc4 would lead to a gain of function. A 6VQ7→RR substitution in hUBE2K does not result in a significant difference in E1-E2 thioester transfer activity (Figures 4F and S4D). With that said, mutation of residues on the UFD acidic patch clearly had a significant effect on thioester transfer to ScUbc1 (Lee and Schindelin, 2008) and we cannot rule out direct contacts between basic E2 residues on hA and the UFD acidic patch for other Ub E2s such as ScUbc1. It is also worth noting that the positive dipole moment of hA of Ubc4 is situated proximal to the UFD acidic patch, Glu992 in particular (Figure 4D). It is tempting to speculate that one potential role of the UFD acidic patch is to engage in longer range electrostatic interactions with basic residues residing on hA of Ub E2s and/or the positive dipole moment of hA during formation of the encounter complex between E1 and E2 (Ubbink, 2009).
Phosphorylation of the N-terminal region of E2s reduces E1-E2 thioester transfer
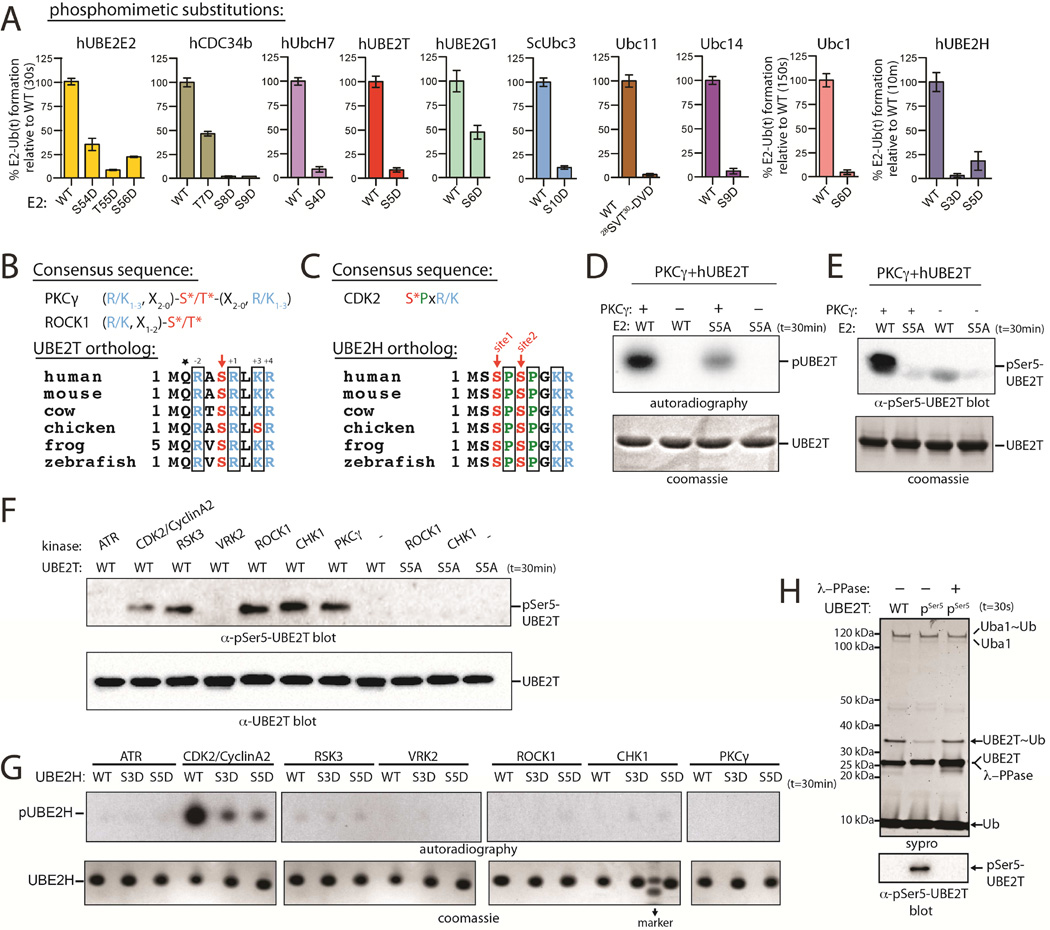
During our analysis of electrostatics at the E2/UFD interface, we noted a clustering of serine and threonine residues at the N-terminal region of Ub E2s (Figures 4B, 4C and S5A). Analysis of databases cataloging known protein phosphorylation sites revealed that ten sites from eight Ub E2s are phosphorylated at positions where we demonstrated that introduction of negative charge led to a significant decrease in thioester transfer activity in vitro (Table S1), but the potential regulatory effects of these phosphorylation events have not been investigated. In the context of the above results, we reasoned that if these residues were phosphorylated, it would serve as a mechanism to negatively regulate E2 function at the level of E1-E2 interaction. To test our hypothesis that phosphorylation of E2 N-terminal residues could negatively regulate E1-E2 Ub thioester transfer activity, we generated phosphomimetic substitutions in eight different S. pombe, S. cerevisiae, and human Ub E2s in the region encompassing Glu7 of Ubc15 and Lys4 of Ubc4. We found that for every Ub E2 we analyzed, phosphomimetic substitution at N-terminal residues predicted to interact with UFD led to a substantial decrease in E1-E2 thioester transfer activity (Figures 5A and S5B). In order to address the possibility that large structural perturbations resulting from the phosphomimetic substitutions account for the observed loss of activity, we compared the 1D proton NMR spectra for WT and S5D hUBE2T, which were nearly identical (Figure S5C).
Figure 5. Phosphomimetic substitution or phosphorylation of N-terminal Ub E2 residues diminishes Ub E1-E2 thioester transfer activity.
(A) E1-E2 thioester transfer assays of the indicated proteins.
(B) PKCγ and ROCK1 consensus sequences (top). ‘X’ is any residue. Sequence alignment of UBE2T orthologs (bottom). Conserved residues consistent with the PKC consensus are in black boxes.
(C) CDK2 consensus sequence (top). Sequence alignment of UBE2H orthologs (bottom). Conserved residues consistent with the CDK2 consensus are in black boxes.
(D) In vitro radiometric PKCγ kinase assays with the indicated Ub E2 substrate (top). Coomassie stained loading control (bottom).
(E) In vitro gel-based PKCγ kinase assay. Samples were probed by western blot with pSer5-specific hUBE2T antibody (top). Coomassie stained loading control (bottom).
(F) In vitro phosphorylation of hUBE2T with the indicated kinase (top).
(G) In vitro phosphorylation of hUBE2H using the in vitro radiometric assay as in D (top).
(H) E1-E2 thioester transfer assays of WT and pSer5 UBE2T in the presence and absence of lambda phosphatase (top). Coomassie stained loading control (bottom).
See also Figure S5.
To further investigate phosphorylation of the Ub E2 N-terminus we focused our attention on two E2s, hUBE2T and hUBE2H, due to the fact that the serine residues of interest (Ser5 and Ser3/Ser5, respectively) reside within the consensus sequences of basophilic and CDK/MAP kinases, respectively (Figures 5B and 5C). To test whether Ser5 of hUBE2T could be targeted by basophilic kinases, we screened a panel of thirty kinases for their ability to phosphorylate either wild type or an S5A substituted hUBE2T in a high-throughput in vitro radiometric assay. This assay led us to identify a subset of kinases that efficiently phosphorylated WT but not S5A hUBE2T, including PKCγ, CHK1, ROCK1, and RSK3 (Figures 5D and 5E). We generated a phosphoSer5 (pSer5) specific hUBE2T antibody and recapitulated the results of the radiometric assay (Figures 5E and 5F). We treated hUBE2H with the same panel of kinases and found that consistent with its predicted consensus site, CDK2 efficiently phosphorylated WT but not S3D or S5D hUBE2H (Figure 5G). Finally, we employed a tRNA-pSer amber codon suppressor system (Pirman et al., 2015) to recombinantly express site-specifically phosphorylated hUBE2T. As expected, pSer5 hUBE2T exhibits substantially reduced E1-E2 thioester transfer activity relative to unphosphorylated hUBE2T that is reversed with treatment with lambda phosphatase (Figure 5H), confirming that phosphorylation of Ser5 of hUBE2T negatively regulates E1-E2 thioester transfer activity in vitro and that this effect is reversible.
A dual mechanism for regulation of Ub E2 activity
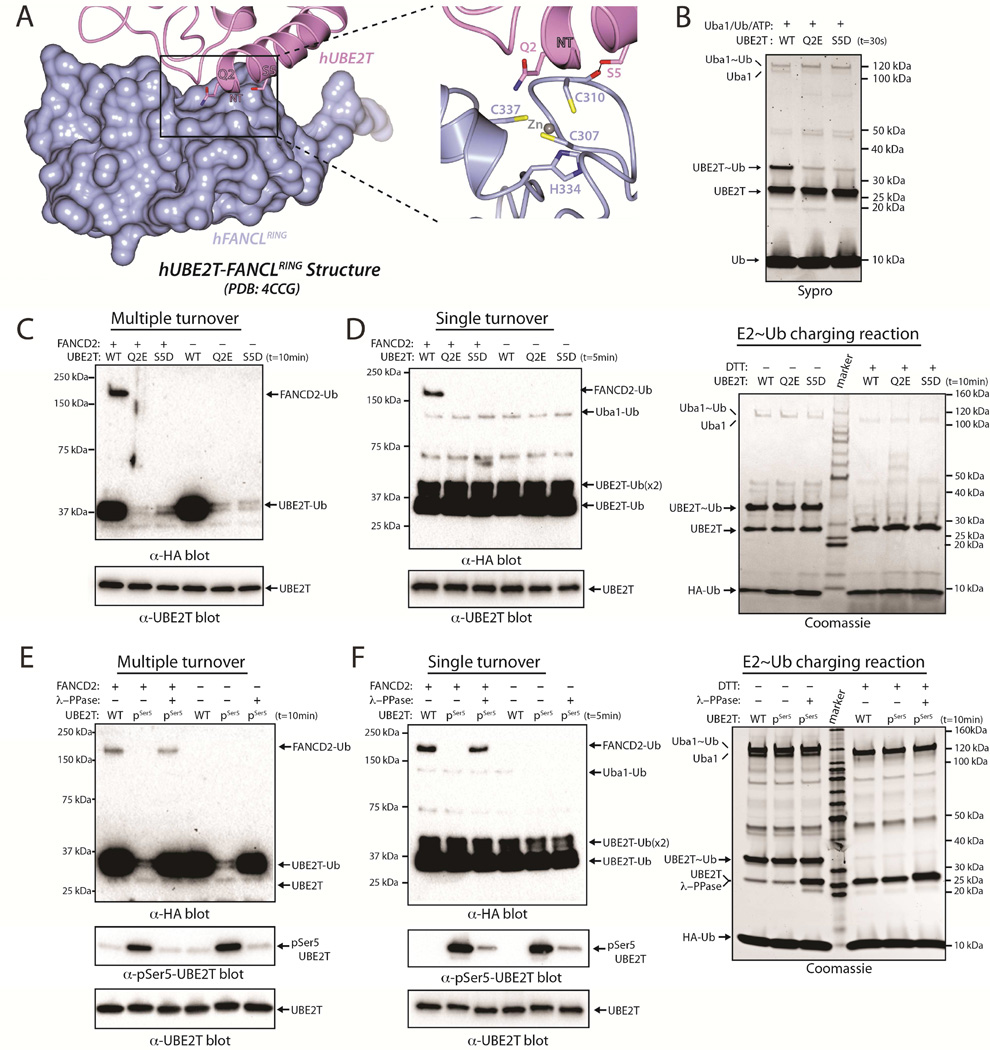
hUBE2T has a clearly defined biological function, having been identified as the Ub E2 in the Fanconi Anemia DNA repair pathway (Machida et al., 2006). hUBE2T functions with the RING E3 ligase FANCL to monoubiquitinate FANCD2, an event that is required for formation of foci at the sites of DNA interstrand cross-links (Alpi et al., 2007; Alpi et al., 2008; Machida et al., 2006; Wang and Smogorzewska, 2015). The crystal structure of hUBE2T in complex with the RING domain of FANCL was recently determined (Hodson et al., 2014), and consistent with other E2/RING complex structures, the surface of hUBE2T that interacts with the FANCL RING domain partially overlaps with the surface that interacts with the hUba1 UFD. This region of overlap includes the N-terminal region of hUBE2T and, interestingly, Ser5 of hUBE2T is proximal to the FANCL RING domain (Figure 6A). This observation, together with our data showing that hUBE2T Ser5 can be phosphorylated by basophilic kinases in vitro, led us to hypothesize that phosphorylation of hUBE2T Ser5 may also affect its ability to function with FANCL.
Figure 6. Acidity at the N-terminus of E2 negatively regulates FANCL-mediated UBE2T ubiquitination of FANCD2.
(A) Structure of hUBE2T (slate) in complex with the FANCL RING domain (rose) (PDB: 4CCG), with selected residues shown as sticks.
(B) E1-E2 thioester transfer assays of the indicated proteins.
(C) FANCL-mediated UBE2T monoubiquitination of FANCD2 performed under multiple turnover conditions (top). UBE2T loading control (bottom).
(D) FANCL-mediated UBE2T monoubiquitination of FANCD2 performed under single turnover conditions (left). E2~Ub charging control (right).
(E) Multiple turnover FANCD2 monoubiquitination assay as in C (top). E2s were treated or untreated with lambda phosphatase, as indicated. Anti-pSer5 UBE2T western blot (bottom).
(F) Single turnover FANCD2 monoubiquitination assay as in D (top). Anti-pSer5 UBE2T western blot (bottom).
To test this hypothesis, we performed both multiple and single turnover assays in order to uncouple the effects of pSer5 and S5D substitution on hUBE2T ‘charging’ by Uba1 (Figure 6B) from potential effects on FANCL-mediated FANCD2 monoubiquitination. The results of these assays show that compared to WT, FANCD2 monoubiquitination is severely diminished for hUBE2T S5D phosphomimetic (Figures 6C and 6D) and pSer5 hUBE2T (Figures 6E and 6F), and that the reduced activity of pSer5 hUBE2T is reversed upon lambda phosphatase treatment (Figures 6E and 6F). Together, this suggests that phosphorylation of Ser5 can serve as a dual regulatory mechanism of hUBE2T activity by inhibiting its charging by E1 and by inhibiting charged hUBE2T from functioning with FANCL to monoubiquitinate FANCD2. While phosphorylation of N-terminal residues appears to broadly inhibit E1-E2 interactions, it is likely that effects on E2-Ub/RING E3 interactions will depend on the particular E2/E3 pair, as not every E2/RING E3 interaction involves the corresponding N-terminal region of hA of Ub E2s.
During the course of our studies, a hUBE2T Q2E mutation was identified in individuals with FA (Hira et al., 2015). Since hUBE2T Gln2 is one turn of hA away from Ser5 and is also predicted to be at the hUba1-UBE2T interface, we hypothesized that the deleterious effects of the Q2E mutation could result from deficiencies in both E1-E2 and E2-E3 interactions. Indeed, Q2E hUBE2T exhibits severe deficiencies in both E1-E2 thioester transfer activity (Figures 6B and S5B) as well as FANCL-mediated FANCD2 monoubiquitination assessed using multiple and single turnover assays (Figures 6C and 6D).
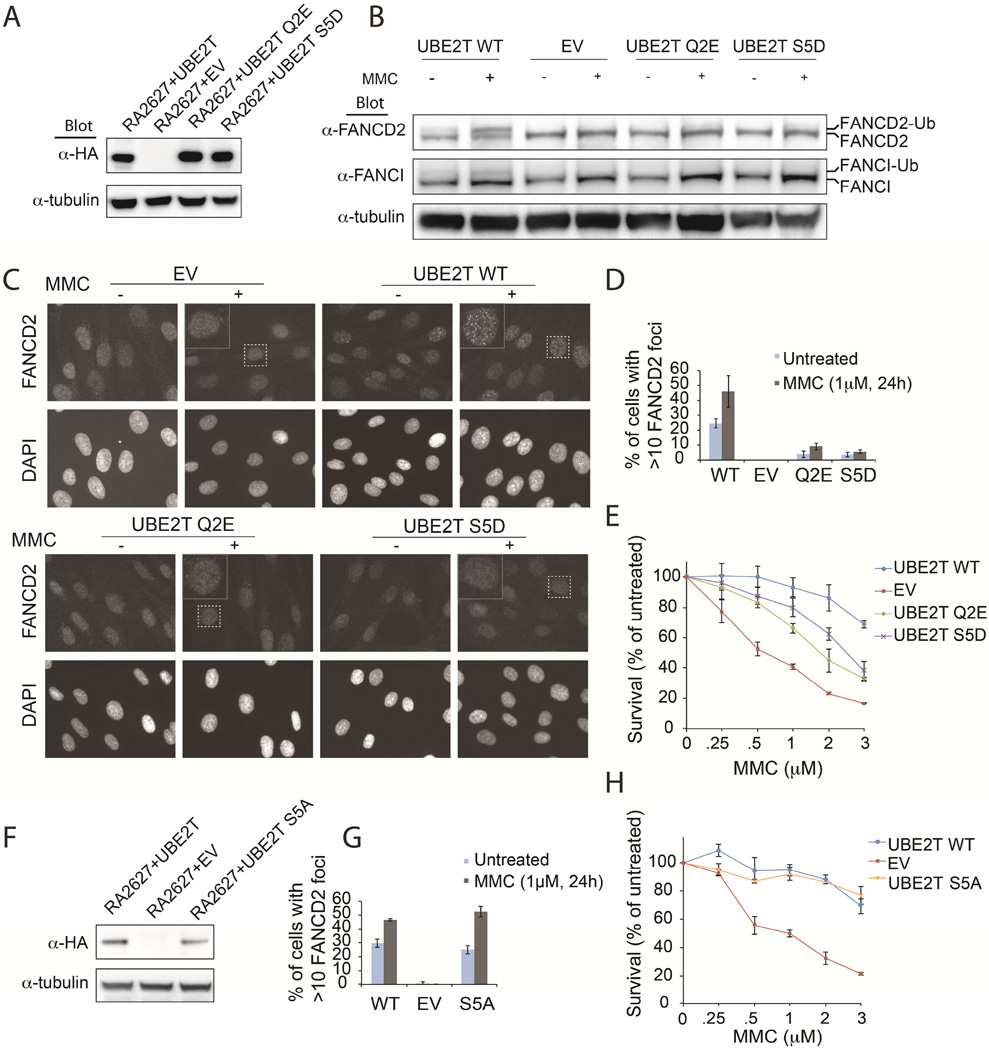
Finally, we wished to test whether the negative regulation of Ub E2 activity resulting from phosphorylation and phosphomimetic substitution in vitro can translate to a functional effect in cells. To that end, we performed complementation assays using the RA2627 cell line in which hUBE2T is not expressed (Rickman et al., 2015) and used FA pathway activation as a readout. Importantly, we found that complementation with the S5D phosphomimetic hUBE2T resulted in significantly reduced levels of FANCD2 and FANCI monoubiquitination upon treatment with the DNA interstrand cross-linking agent mitomycin C (MMC) compared to WT hUBE2T (Figures 7A, 7B, S6A, S6B, and S6D). Furthermore, RA2627 cells expressing S5D phosphomimetic hUBE2T displayed significantly reduced FANCD2 foci formation in response to MMC (Figures 7C, 7D, and S6A) and increased MMC sensitivity (Figures 7E, S6C, and S6D) compared to WT hUBE2T. Consistent with a negative regulatory effect of the phosphomimetic S5D hUBE2T mutant, and not nonspecific effects of mutating the Ser5 site, the expression of the S5A phosphomutant resulted in normal FANCD2 foci formation and ubiquitination following MMC treatment (Figures 7F, 7G, S6C, S6D, and S6E). Additionally, S5A hUBE2T expression in RA2627 cells fully rescued MMC resistance by cell survival assay (Figures 7H, S6C, and S6D). Although hUBE2T Ser5 has not yet been identified as being phosphorylated in cells, it is worth noting that in each of our cell-based assays the S5D mutant behaved similarly to the recently identified FA-causing Q2E mutant hUBE2T (Figures 7 and S6). Thus, our in vivo observations are fully consistent with our in vitro biochemical analyses, suggesting that phosphorylation at Ser/Thr residues at the N-terminal region of other Ub E2s can have a regulatory effect in cells.
Figure 7. hUBE2T-deficient RA2627 cells expressing hUBE2T S5D phosphomimetic are defective for FANCD2 and FANCI monoubiquitination.
(A) Anti-HA western blot of hUBE2T deficient transformed and immortalized RA2627 E6/E7/hTERT fibroblasts expressing the indicated protein. These cells were used in panels B-D.
(B) Western blot analysis of FANCD2 and FANCI monoubiquitination in hUBE2T complemented cells following 24hr treatment with 1µM MMC.
(C) FANCD2 foci formation in hUBE2T complemented cells following 24hr treatment with 1µM MMC.
(D) Quantification of FANCD2 foci formation visualized in C.
(E) MMC cell survival assay of hUBE2T complemented cells, see Figure S6C for expression levels.
(F) Western blot of HA-FLAG-tagged hUBE2T expression in immortalized RA2627 cells used in G-H.
(G) Quantification of FANCD2 foci formation of hUBE2T S5A mutant compared to EV and WT expressing RA2627 fibroblasts, see Figure S6E for visualization.
(H) MMC cell survival assay of hUBE2T complemented cells.
See also Figure S6.
Conclusions
The unique E1 binding mode revealed by the Uba1-Ubc15 structure provides the first evidence that structural plasticity at the Ub E1-E2 interface is the underlying mechanism by which a single E1 can function with many different E2s. While the Ub molecule undergoing thioester transfer from E1 to E2 catalytic cysteine is missing in our structure, our results suggest that there are differences in the architecture of the quaternary complex that are at least in part driven by the tendency of Ub to interact with a surface of the E2 formed predominantly by hC. The precise mechanism by which thioester transfer is catalyzed awaits structural characterization of a bona fide tetrahedral intermediate. Our observation that the intrinsically low thioester transfer activity of Ubc15 results largely from the presence of an acidic residue at a region that is Ser/Thr rich in a subset of other Ub E2s led to the discovery that phosphorylation of these residues can serve as a dual regulatory mechanism by inhibiting both E1 and RING E3 interactions. Importantly, the results of our in vivo complementation experiments demonstrate that the inhibitory effects on E2 activity we observe in vitro can have clear functional consequences in cells. Future efforts will focus on discovering the E1 binding mode of other Ub E2s in the hopes that a more complete understanding of how specificity and promiscuity are achieved in Ub E1-E2 interactions will facilitate development of therapeutic molecules designed to target particular complexes. Finally, given that eight different Ub E2s are known to be phosphorylated at positions we have shown have a regulatory effect on biochemical activity, an exciting future area of investigation will be elucidating the role that these modifications might play in cellular function.
CONTACT FOR REAGENT AND RESOURCE SHARING
Please direct any requests for further information or reagents to the Lead Contact, Shaun K. Olsen (olsensk@musc.edu), Department of Biochemistry & Molecular Biology, Medical University of South Carolina.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
E. coli strain BL21 (DE3) codon plus (Stratagene) was used to recombinantly express native protein.
E. coli strain EcAR7 was used to recombinantly express phosphorylated protein (Pirman et al., 2015).
Human fibroblast derived from an individual deficient for hUBE2T (Rickman et al., 2015) were immortalized by expression of the catalytic subunit of human telomerase (hTERT) and/or transformed by HPV16 E6E7 expression. Fibroblasts were cultured in Dulbecco Modified Eagle medium (DMEM) supplemented with 15% FBS, penicillin and streptomycin, nonessential amino acids, and glutamax.
METHOD DETAILS
Cloning
Human Uba1 and S. pombe Uba1 constructs were prepared as described (Olsen and Lima, 2013). Briefly, the DNA fragments encoding residues 13-1012 and 49-1058 of S. pombe UBA1 and human UBA1, respectively were cloned into vector pSMT3 to introduce an N-terminal Ulp1-cleavable His6-SMT3 tag. Saccharomyces cerevisiae UBA1 encoding residues 11-1024 was inserted into NcoI and XhoI sites of pET29NTEV vector.
Human UBE2G2 cDNA was the kind gift of Robert Gemmill; cDNA of other human and pombe Ub E2s were amplified from human cDNA library, and inserted into pET28 (hUBE2B, hUBE2H, hUBE2K, Ubc6 (residues: 1-206), Ubc7, Ubc15, Ubc16), pET29 (hUBE2S, Ubc1, Ubc2, Ubc4, Ubc8, Ubc11, Ubc13, Ubc14), and pMTTH (hUBE2D2, hUBE2E2, hUBE2G1, hUBE2G2, hUBE2T, hUBE2R2) vectors. All constructs are full-length except Ubc6 which was truncated to remove a C-terminal transmembrane domain. The DNA fragment encoding wild type S. pombe ubiquitin (SpUb; residues 1-76) and ubiquitin with its seven lysine residues mutated to arginine (Ub-K7R) were inserted into vector pET-28 to introduce an N-terminal hexahistidine tag. All point mutations were introduced using PCR-based mutagenesis.
Family three Ub E2 constructs with their acidic loop insertions deleted (referred to as ΔLOOP in text and figures) were created using PCR overlap extension. Residues deleted are as follows: Ubc15ΔLOOP (98-110); hUBE2G1ΔLOOP (98-110); hUBE2G2ΔLOOP (97-109); hCDC34bΔLOOP (101-113). A two-residue artifact (HG) was introduced between the N- and C-terminal E2 fragments during cloning.
The large insertion in the FCCH loop of Uba1 (referred to as Uba1LIL in the text and figures) was generated as described (Olsen and Lima, 2013). The insertion is between positions 198 and 199 in Uba1, and is composed of the following amino acid sequence: 'GRSRGRSRGTG'. X. laevis FANCD2 cDNA was the kind gift of Dr. David Long and was subcloned in pSMT3 vector. Codon optimized X. tropicalis FANCL cDNA was synthesized as a DNA fragment (Invitrogen) and subcloned into pSMT3 vector.
Protein expression and purification
All proteins in this study were expressed by inducing E. coli strain BL21 (DE3) codon plus (Stratagene) transformed with plasmid carrying target cDNA. All large-scale cultures were grown at 37°C in baffled flasks to an A600 of 1.0 at which point the flasks were placed into ice water to cold-shock the cells. After 30 minutes isopropyl-β-D-1-thiogalactoside (IPTG) was added to a final concentration of 0.2 mM and the cultures were shaken at 18°C for an additional 18 hours. Cells were harvested by centrifugation and re-suspended in buffer containing 20 mM Tris-HCl pH 8.0, 350 mM NaCl and 20 mM imidazole and snap frozen in liquid nitrogen for later use. Cell pellets were rapidly thawed by placing tubes under warm running water. β-mercaptoethanol (β-ME) was added to a final concentration of 3 mM, and the cells were lysed by sonication. All lysates were cleared by centrifugation and applied to columns containing Ni-NTA superflow resin (Qiagen) by gravity. After elution in buffer containing 20 mM Tris HCl pH 8.0, 350 mM NaCl, 250 mM Imidazole, proteins harboring cleavable SMT3 tags were processed by adding Ulp1 at a ratio of 1:1000 (w/w) and incubating overnight at 4°C; proteins with TEV-clea vable hexahistidine tags were processed by adding TEV protease at a ratio of 1:100 (w/w) and incubating overnight at 4°C. All proteins used for crystallization and biochemical experiments were subjected to gel filtration (Superdex 200 or Superdex 75 based on protein size; Amersham), and anion-exchange if sufficient purity was not achieved (MonoQ or MonoS, Amersham) except for X. tropicalis FANCL, which is nickel column purified and buffer exchanged to remove imidazole. Proteins were concentrated to 2–10 mg/ml and snap frozen in liquid nitrogen.
Upon removal of His-tags, when possible, the following artifactual residues were present in the Ub E2 proteins used in this study:
| N-terminus | C-terminus | |
| Ubc1 | LEHHHHHH | |
| Ubc2 | LEHHHHHH | |
| Ubc4 | LEHHHHHH | |
| Ubc6 | LEHHHHHH | |
| Ubc7 | MG | LEHHHHHH |
| Ubc8 | LEHHHHHH | |
| Ubc11 | LEHHHHHH | |
| Ubc13 | LEHHHHHH | |
| Ubc14 | MG | LEHHHHHH |
| Ubc15 | LEHHHHHH | |
| ScUbc3 | GA | |
| UBE2B | MG | LEHHHHHH |
| UBE2D2 | KLENLYFQ | |
| UBE2E2 | LEHHHHHH | |
| UBE2G1 | KLENLYFQ | |
| UBE2G2 | KLENLYFQ | |
| UBE2H | MG | LEHHHHHH |
| UBE2K | LEHHHHHH | |
| UBE2L3 | LEHHHHHH | |
| UBE2R2 | KLENLYFQ | |
| UBE2S | LEHHHHHH | |
| UBE2T | LEHHHHHH |
E1-E2 cross-linking
E1-E2 cross-linking was performed according to methods described in (Olsen and Lima, 2013). Briefly E2 was treated with fresh 'activating buffer' (20 mM Tris pH 8.0, 50 mM NaCl, 2.5 mM 2,2'-Dipyridyldisulfide, 2.5% DMSO) and stored at 25°C f or 15-30 minutes. The 'activated' E2 samples were filtered and desalted. E1 and ‘activated’ E2 were mixed and incubated for 15 minutes at 25°C. The Uba1-Ubc15 cross-linking mixture was further purified over a MonoQ HR10/10 anion exchange column. The complex was concentrated to 10–25 mg/ml and either subjected to crystallization trials or snap frozen for later use.
Crystallization and data collection
S. pombe Uba1-Ubc15 cross-link was purified as described above at a final concentration of 23.8 mg/ml (180 µM). Ub (360 µM), MgCl2 (5 mM), and ATP (1 mM) were added to the Uba1-Ubc15 cross-link prior to sparse-matrix screening in Intelli-Plate (Art Robbins Instruments) (400 nl sitting drop vapor diffusion format) at 18°C. Diffraction quality crys tals of the Uba1-Ubc15/Ub complex were grown by mixing 0.9 µl protein sample (supplemental with ATP and MgCl2) with 0.9 µl crystallization buffer by hanging drop vapor diffusion at 18°C. Crystallization buffer contains 0.3-0.4 M Li2SO4, 0.1 M Tris-HCl pH 8.0-8.5, 15%-18% w/v PEG8000. Uba1-Ubc15 crystals were flash-frozen in liquid nitrogen in cryoprotectant comprised of mother liquor supplemented with 5 mM MgCl2, 1 mM ATP, and 20% ethylene glycol. X-ray diffraction data for the Uba1-Ubc15/Ub complex was collected on a Rayonix (Mar) 300HS high speed CCD detector at Advanced Photon Source (Argonne, Illinois, USA), SER-CAT beamline 22-ID. All data were indexed, integrated, and scaled using HKL2000 (Otwinowski and Minor, 1997). The Uba1-Ubc15/Ub crystal belongs to orthorhombic space group P1 with unit cell dimensions a=77.1, b=82.2, c=135.4. There are two Uba1-Ubc15/Ub complexes per asymmetric unit and the crystal has a solvent content of 60%.
Structure determination and refinement
A complete data set for the S. pombe Uba1-Ubc15/Ub crystals was collected to a resolution of 2.5 Å. The program PHASER was used to find a molecular replacement solution using the coordinates for two copies of the S. pombe Uba1/Ub complex from the S. pombe Uba1-Ubc4/Ub structure (PDB: 4II2). Uba1UFD and Ubc4 were removed from the search model. Electron density for the Uba1UFD and Ubc15 were clear after a single round of refinement and these components were manually placed into the density after an additional refinement of ubiquitin and the adenylation and Cys domains of Uba1. The program Sculptor was used to create a model of Ubc15 using the structure of human UBE2G2 (PDB: 3H8K) as a starting point. The model was refined to R/Rfree values of 0.216/0.251 via iterative rounds of refinement and rebuilding using PHENIX (Adams et al., 2010), and COOT (Emsley and Cowtan, 2004). The final Uba1-Ubc15/Ub model contains amino acids 1-76 from both copies of Ub (chains B and E), amino acids 14-763 and 782-1012 from copy 'A' of Uba1, amino acids 13-763 and 787-1012 from copy 'D' of Uba1, amino acids 1-167 from copy ‘C’ of Ubc15 and amino acids 1-99 and 109-167 of copy ‘F’ of Ubc15. Four residues remaining from the C-terminal His-tag of Ubc15 are ordered in copy ‘C’ and three (LEH) are ordered in Chain ‘F’. There are nine ordered sulfates from the crystallization solution and 94 ordered water molecules. Although the acidic loop insertion of Ubc15 is sufficiently ordered to be fully modeled in only copy ‘C’ in the crystallographic asymmetric unit, rotation of the Uba1FCCH and Uba1SCCH domains away from each other is observed in both copies of Uba1. Also, ATP/Mg was required for crystallization but only an ordered sulfate ion was ordered in the active site of each Uba1 copy at the position corresponding to where the γ phosphate of ATP normally binds. The final model has good geometry, with 89.7%, 10.0%, 0.1%, and 0.1% of residues in the most favored, additional allowed, generously allowed, and disallowed regions of Ramachandran space. All molecular graphics representations of the structures were generated using PYMOL and CCP4mg.
E1-E2 thioester transfer assays
The E1-E2 thioester transfer assays were performed at 25°C as previously described (Olsen and Lima, 2013). For all experiments the Ub E1 ortholog corresponding to that of the E2 being tested (human, S. pombe, or S. cerevisiae) was used. For the comparison of S. pombe Ub E2 activities in Figure 1A, assays were performed with 2.5 nM E1, 500 nM E2, 5 µM Ub, 5 mM MgCl2, 200 µM ATP, 20 mM Hepes pH 7.5, 50 mM NaCl and were incubated for 30 s at room temperature. For the endpoint assay of Ubc15 activity in Figure 1B, reactions were performed with 1 µM E1, 500 nM E2, 5 µM Ub, 5 mM MgCl2, 200 µM ATP, 20 mM Hepes pH 7.5, 50 mM NaCl and were incubated for 5 min at room temperature. For all subsequent structure-function analysis throughout the paper, thioester transfer assays were performed with 2.5 nM E1, 500 nM E2, 5 µM Ub, 5 mM MgCl2, 200 µM ATP, 20 mM Hepes pH 7.5, 50 mM NaCl and the incubation times were adjusted based on activity levels, in order to remain in the linear range. Incubation times were as follows: 150 s for Ubc15 and Ubc1, 600 s UBE2H, 20 min for hUBE2K, 30 s for all other E2s. Reactions were terminated though the addition of non-reducing Urea SDS-PAGE buffer and subjected to SDS-PAGE, 150 V constant, at 4°C. The gels were stained with Sypro Ruby (BioRad) and visualized with a ChemiDoc MP (BioRad).
Kinetic analysis of Ubc15 and Ubc4 wild type and mutant proteins
Kinetic analyses were carried out similar to the E1-E2 thioester transfer assays described above, with a few modifications. Reactions contained 0.4 nM E1, 5 mM MgCl2, 1 mM ATP, 20 mM Na-HEPES pH 7.5, 50 mM NaCl, 50 µM ubiquitin and increasing E2 concentrations as indicated in Figure S4. Due to differing activities, the Ubc15 and Ubc4 proteins were incubated for different lengths of time: 40 s for Ubc15 E7R, 120 s Ubc4 WT, 14 min for Ubc15 WT, Ubc15 A137L, Ubc4 L3E, Ubc4 L119A. Reactions were terminated through the addition of non-reducing Urea SDS-PAGE buffer and subjected to SDS-PAGE, 150 V constant, at 4°C. The gels were stained with Sypro Ruby (Biorad) and visualized with a ChemiDoc MP (Biorad). Signal was quantified using ImageJ and analyzed using Prism7 (GraphPad) by fitting data points into Michaelis-Menten model.
FANCD2 mono-ubiquitination multi-turnover and single-turnover assays
Multiple turnover-
The ability of UBE2T and mutants function with Uba1 and FANCL to monoubiquitinate FANCD2 was assessed by a multiple turnover in vitro ubiquitination assay using a previously published protocol (Hodson et al., 2014) with some variations. Reaction volumes of 50 µl contained 2 nM Uba1, 100 nM E2, 300 nM FancL, 2 µM HA-Ub (Boston Biochem U-110), 500 nM FANCD2, 2 mM ATP, 1 mM DTT, 25 mM MgCl2, 20 mM HEPES pH 7.5. Reactions were left for 10 min at RT, and stopped with SDS sample buffer. Samples were subjected to SDS-page, and blotted with anti-HA antibody (Rockland 600-401-384), anti-UBE2T antibody (ThermoFisher: PA5-28464) or anti-pSer5-UBE2T antibody.
Single turnover-
UBE2T and mutants were first charged with ubiquitin by incubating, in a 20 µl reaction volume, 100 nM Uba1, 10 µM E2, 20 µM HA-Ub, 500 µM ATP, 25 mM MgCl2, 20 mM HEPES pH 7.5 for 10 min at room temperature to ensure equal loading of WT and mutant E2. E1-E2 thioester transfer was terminated by adding 1 unit Apyrase (New England BioLabs Inc.), and incubating on ice for 10 min. E2-thioester is analyzed on SDS-page under reducing and non-reducing conditions for quality control.
E2-Ub thioester from the previous step was immediately used for FANCD2 monoubiquitination. Reaction includes 500 nM E2~Ub, 600 nM FANCL, 500 nM FANCD2, 25 mM MgCl2, 20 mM HEPES pH 7.5, and is incubated for 5 min at RT. Samples were analyzed the same way as for the multiple turnover reactions.
Kinase assays
Several servers were used to determine kinases more likely to phosphorylate Ub E2s: NetPhos 2.0, GPS and NetPhorest. 30 kinases were chosen and tested with UBE2T and mutant proteins by a radiometric assay by Kinexus. Reactions contain 10–50 nM kinase, 8 µM Ub E2, 0.5 µCi [γ-33P] ATP and kinase buffer. Reactions were incubated at ambient temperature for 20–40 minutes, depending on the protein kinase tested. After the incubation period, the assay was terminated by spotting 10 µl of the reaction mixture onto a multiscreen phosphocellulose P81 plate. The P81 plate was washed 3 times for approximately 15 minutes each in a 1% phospohric acid solution. The radioactivity on the P81 plate was counted in the presence of scintillation fluid in a Trilus scintillation counter. The signal obtained WT E2 was compared to a serine to alanine mutant in order to identify kinases that specifically modified the site of interest.
ROCK1, PKCγ, CHK1, CDK2/CyclinA2, RSK3 were tested again in our laboratory to verify the above radiometric results. VRK2 and ATR/ATRIP were included as negative controls. Reactions contain 10 ng Kinase, 2 µg Ub E2, 6 µM ATP, 5 µCi [γ−33P] ATP in appropriate buffer recommended by manufacturer. UBE2T and UBE2H were each tested with a panel of kinases using recommended conditions by supplier (ROCK1 Promega V3411; CDK2/CyclinA2: Promega V2961; RSK3: SignalChem R16-10G; VRK2: Promega V4494; ATR/ATRIP: eurofins 14-953; PKCγ: SignalChem P66-10G).
Generation of site-specifically phosphorylated protein
pSer5 UBE2T was generated using Rinehart lab protocol (Pirman et al., 2015). cDNA of UBE2T Ser5 was mutated to Amber codon “TAG” by PCR, and was inserted into pGEX6p-2 vector. Resulting vector was co-transformed with SepOTSλ (Addgene plasmid # 68292) into EcAR7 strain (Addgene plasmid # 52055), and both Ampicillin and Kanamycin were used for selection. Multiple colonies were picked and mixed, and grown at 30°C in the presence of phosphoserine and induced with IPTG at 18°C for 24 h. Protein was purified the same way as native protein. The incorporation of phospho-Ser5 in UBE2T was assessed by western blot using phospho-Ser5 specific antibody in the presence and absence of Lambda phosphatase (NEB).
Generation of pSer5 UBE2T antibody
PhosphoSer5-specific UBE2T antibody was raised at YenZym Antibodies, LLC. In brief, synthetic labeled peptide: QRA- pS – RLKRELHMLAC conjugated to carrier protein is used to immune rabbits. Antibody is purified from serum with the same phosphorylated peptide conjugated to resin and absorbed against native peptide. Antibody is further tested with native/phospho peptides to determine specificity and titration.
Cell lines and viral transfection/transduction
RA2627, human fibroblast derived from an individual deficient for hUBE2T (Rickman et al., 2015) were immortalized by expression of the catalytic subunit of human telomerase (hTERT) and/or transformed by HPV16 E6E7 expression. Fibroblasts were cultured in Dulbecco Modified Eagle medium (DMEM) supplemented with 15% FBS, penicillin and streptomycin, nonessential amino acids, and glutamax. hUBE2T cDNAs were delivered by retroviral transduction after packaging in HEK293T cells (Transit-293 transfection reagent, Mirus). Fibroblast transduction was in the presence of 4mg/ml polybrene. Stably expressing cells were selected by puromycin.
MMC treatment
For western blot and immunofluorescence studies, cells were seeded overnight and treated with 1μM MMC the next day. Analysis was performed 24 hours later. For cell survival studies, RA2627 fibroblast expressing the indicated UBE2T constructs or empty vector (EV) control were seeded overnight in triplicate and treated the next day with MMC for one hour. Cells were washed and drug-free media replaced. Cells were grown for 4–5 days and passaged at appropriate ratios once nearly confluent. Cells were counted on days 7–8 when nearly confluent and survival calculated relative to untreated controls.
Plasmids
hUBE2T cDNA (Human ORFeome V8.1 Library, GE Healthcare) was recombined into pDONR223 using Gateway system BP reaction (Invitrogen) (Rickman et al., 2015). Mutagenesis was performed using QuickChange Multi Site-Directed Mutagenesis Kit (Agilent). pDONR223 hUBE2T cDNA was recombined into a pMSCV retroviral vector using Gateway system LR reaction to produce a C-terminally tagged HA-FLAG-UBE2T.
Western Blot and Antibodies
Whole-cell lysates were prepared in Laemmli sample buffer (Bio-Rad), sonicated, and boiled. Samples were separated on 4–12% or 3–8% gradient gels by SDS-PAGE. Antibodies are as follows: FANCD2 (Novus NB100-182), FANCI (#589, Smogorzewska lab), and HA (Covance MMS-101R).
Immunofluorescence
Cells were fixed on coverslips with 3.7% formaldehyde and permeabilized by 0.5% Triton in PBS, blocked in 5% FBS in PBS, and incubated with FANCD2 antibody (1:1000). Cells were washed in blocking buffer and incubated with anti-rabbit Alexa 488 secondary antibody. Coverslips were washed and embedded with Fluoromount-G containing DAPI (SouthernBiotech).
QUANTIFICATION AND STATISTICAL ANALYSIS
Quantification of E1-E2 thioester transfer was achieved by measuring the intensity of relevant protein bands from Sypro Ruby stained gels using ImageJ, and data was analyzed using Prism7 (GraphPad). Kinetics analysis was achieved by fitting data points into Michaelis-Menten model.
DATA AND SOFTWARE AVAILABILITY
Atomic coordinates and structure factors are deposited in the RCSB with accession code 5KNL.
Supplementary Material
Acknowledgments
The authors thank Miklos Bekes, Christopher Davies, and James Atkison for critically reading the manuscript and Stuart Parnham for assistance with NMR. X-ray diffraction data were collected at SER-CAT 22-ID and NE-CAT 24-ID-C beamlines at the Advanced Photon Source, Argonne National Laboratory. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DEAC02- 06CH11357 and W-31-109-Eng-38. Research reported in this publication was supported by NIH R01 GM115568 (S.K.O.), NIH R01 CA088932 (B.O.), and NIH R01 CA555536 (P.H.H.). Work in A.S. laboratory was supported by the Rockefeller University, NIH RO1 HL120922 and grant #8 UL1 TR000043 from the National Center for Advancing Translational Sciences, NIH Clinical and Translational Science Award program. K.A.R. was supported by an NIH MSTP grant (T32GM007739) and A.S. is an HHMI Faculty Scholar. The X-ray crystallography facility used for this work is supported by the Office of the Vice President for Research at the Medical University of South Carolina. The liquid handling robot used was purchased via an NIH Shared Instrumentation Award (S10 RR027139-01). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions Structural and biochemical experiments were performed by Z.L., L.Y., K.W., and S.K.O. Cell studies were performed by K.A.R. under the supervision of A.S. P.H.H., B.O., A.N.W., and S.P.S. assisted in data interpretation. The manuscript was prepared by A.S. and S.K.O.
References
- 1.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpi A, Langevin F, Mosedale G, Machida YJ, Dutta A, Patel KJ. UBE2T, the Fanconi anemia core complex, and FANCD2 are recruited independently to chromatin: a basis for the regulation of FANCD2 monoubiquitination. Mol Cell Biol. 2007;27:8421–8430. doi: 10.1128/MCB.00504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpi AF, Pace PE, Babu MM, Patel KJ. Mechanistic insight into site-restricted monoubiquitination of FANCD2 by Ube2t, FANCL, and FANCI. Mol Cell. 2008;32:767–777. doi: 10.1016/j.molcel.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Buetow L, Huang DT. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat Rev Mol Cell Biol. 2016;17:626–642. doi: 10.1038/nrm.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 6.Gazdoiu S, Yamoah K, Wu K, Pan ZQ. Human Cdc34 employs distinct sites to coordinate attachment of ubiquitin to a substrate and assembly of polyubiquitin chains. Mol Cell Biol. 2007;27:7041–7052. doi: 10.1128/MCB.00812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton KS, Ellison MJ, Barber KR, Williams RS, Huzil JT, McKenna S, Ptak C, Glover M, Shaw GS. Structure of a conjugating enzyme-ubiquitin thiolester intermediate reveals a novel role for the ubiquitin tail. Structure. 2001;9:897–904. doi: 10.1016/s0969-2126(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 8.Hira A, Yoshida K, Sato K, Okuno Y, Shiraishi Y, Chiba K, Tanaka H, Miyano S, Shimamoto A, Tahara H, et al. Mutations in the gene encoding the E2 conjugating enzyme UBE2T cause Fanconi anemia. Am J Hum Genet. 2015;96:1001–1007. doi: 10.1016/j.ajhg.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodson C, Purkiss A, Miles JA, Walden H. Structure of the human FANCL RINGUbe2T complex reveals determinants of cognate E3-E2 selection. Structure. 2014;22:337–344. doi: 10.1016/j.str.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40:D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DT, Hunt HW, Zhuang M, Ohi MD, Holton JM, Schulman BA. Basis for a ubiquitin-like protein thioester switch toggling E1-E2 affinity. Nature. 2007;445:394–398. doi: 10.1038/nature05490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- Lass A, Cocklin R, Scaglione KM, Skowyra M, Korolev S, Goebl M, Skowyra D. The loop-less tmCdc34 E2 mutant defective in polyubiquitination in vitro and in vivo supports yeast growth in a manner dependent on Ubp14 and Cka2. Cell Div. 2011;6:7. doi: 10.1186/1747-1028-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Schindelin H. Structural insights into E1-catalyzed ubiquitin activation and transfer to conjugating enzymes. Cell. 2008;134:268–278. doi: 10.1016/j.cell.2008.05.046. [DOI] [PubMed] [Google Scholar]
- Li W, Tu D, Brunger AT, Ye Y. A ubiquitin ligase transfers preformed polyubiquitin chains from a conjugating enzyme to a substrate. Nature. 2007;446:333–337. doi: 10.1038/nature05542. [DOI] [PubMed] [Google Scholar]
- Liu W, Shang Y, Zeng Y, Liu C, Li Y, Zhai L, Wang P, Lou J, Xu P, Ye Y, et al. Dimeric Ube2g2 simultaneously engages donor and acceptor ubiquitins to form Lys48-linked ubiquitin chains. EMBO J. 2014;33:46–61. doi: 10.1002/embj.201385315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida YJ, Machida Y, Chen Y, Gurtan AM, Kupfer GM, D'Andrea AD, Dutta A. UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Mol Cell. 2006;23:589–596. doi: 10.1016/j.molcel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Michelle C, Vourc'h P, Mignon L, Andres CR. What was the set of ubiquitin and ubiquitin-like conjugating enzymes in the eukaryote common ancestor? J Mol Evol. 2009;68:616–628. doi: 10.1007/s00239-009-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SK, Lima CD. Structure of a ubiquitin E1-E2 complex: insights to E1-E2 thioester transfer. Mol Cell. 2013;49:884–896. doi: 10.1016/j.molcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Page RC, Pruneda JN, Amick J, Klevit RE, Misra S. Structural insights into the conformation and oligomerization of E2~ubiquitin conjugates. Biochemistry. 2012;51:4175–4187. doi: 10.1021/bi300058m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Mechanism of lysine 48-linked ubiquitin-chain synthesis by the cullin-RING ubiquitin-ligase complex SCF-Cdc34. Cell. 2005;123:1107–1120. doi: 10.1016/j.cell.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Pirman NL, Barber KW, Aerni HR, Ma NJ, Haimovich AD, Rogulina S, Isaacs FJ, Rinehart J. A flexible codon in genomically recoded Escherichia coli permits programmable protein phosphorylation. Nat Commun. 2015;6:8130. doi: 10.1038/ncomms9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plechanovova A, Jaffray EG, Tatham MH, Naismith JH, Hay RT. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature. 2012;489:115–120. doi: 10.1038/nature11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneda JN, Stoll KE, Bolton LJ, Brzovic PS, Klevit RE. Ubiquitin in motion: structural studies of the ubiquitin-conjugating enzyme approximately ubiquitin conjugate. Biochemistry. 2011;50:1624–1633. doi: 10.1021/bi101913m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman KA, Lach FP, Abhyankar A, Donovan FX, Sanborn EM, Kennedy JA, Sougnez C, Gabriel SB, Elemento O, Chandrasekharappa SC, et al. Deficiency of UBE2T, the E2 Ubiquitin Ligase Necessary for FANCD2 and FANCI Ubiquitination, Causes FA-T Subtype of Fanconi Anemia. Cell Rep. 2015;12:35–41. doi: 10.1016/j.celrep.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M, Suryadinata R, Lai X, Heierhorst J, Sarcevic B. Molecular basis for lysine specificity in the yeast ubiquitin-conjugating enzyme Cdc34. Mol Cell Biol. 2010;30:2316–2329. doi: 10.1128/MCB.01094-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A, Lewis S, Kleiger G, Kuhlman B, Deshaies RJ. Essential Role for Ubiquitin- Ubiquitin-Conjugating Enzyme Interaction in Ubiquitin Discharge from Cdc34 to Substrate. Molecular Cell. 2011;42:75–83. doi: 10.1016/j.molcel.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer A, Kuhn M, Schindelin H. Structure of the ubiquitin-activating enzyme loaded with two ubiquitin molecules. Acta Crystallogr D Biol Crystallogr. 2014;70:1311–1320. doi: 10.1107/S1399004714002910. [DOI] [PubMed] [Google Scholar]
- Soss SE, Klevit RE, Chazin WJ. Activation of UbcH5c~Ub is the result of a shift in interdomain motions of the conjugate bound to U-box E3 ligase E4B. Biochemistry. 2013;52:2991–2999. doi: 10.1021/bi3015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streich FC, Jr, Lima CD. Structural and functional insights to ubiquitin-like protein conjugation. Annu Rev Biophys. 2014;43:357–379. doi: 10.1146/annurev-biophys-051013-022958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatek KN, Komander D. Ubiquitin modifications. Cell Res. 2016;26:399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubbink M. The courtship of proteins: understanding the encounter complex. FEBS Lett. 2009;583:1060–1066. doi: 10.1016/j.febslet.2009.02.046. [DOI] [PubMed] [Google Scholar]
- van Wijk SJ, Timmers HT. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. 2010;24:981–993. doi: 10.1096/fj.09-136259. [DOI] [PubMed] [Google Scholar]
- Wang AT, Smogorzewska A. SnapShot: Fanconi anemia and associated proteins. Cell. 2015;160:354–354. e351. doi: 10.1016/j.cell.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Wenzel DM, Stoll KE, Klevit RE. E2s: structurally economical and functionally replete. Biochem J. 2011;433:31–42. doi: 10.1042/BJ20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickliffe KE, Lorenz S, Wemmer DE, Kuriyan J, Rape M. The Mechanism of Linkage-Specific Ubiquitin Chain Elongation by a Single-Subunit E2. Cell. 2011;144:769–781. doi: 10.1016/j.cell.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.