Abstract
Disruption of redox homeostasis is a key phenotype of many pathological conditions. Though multiple oxidizing compounds such as hydrogen peroxide are widely recognized as mediators and inducers of oxidative stress, increasingly, attention is focused on the role of lipid hydroperoxides as critical mediators of death and disease. As the main component of cellular membranes, lipids have an indispensible role in maintaining the structural integrity of cells. Excessive oxidation of lipids alters the physical properties of cellular membranes and can cause covalent modification of proteins and nucleic acids. This review discusses the synthesis, toxicity, degradation, and detection of lipid peroxides in biological systems. Additionally, the role of lipid peroxidation is highlighted in disease and death, and strategies to control the accumulation of lipid peroxides are discussed.
Keywords: Lipid oxidation, oxidation, peroxidation, antioxidant, ferroptosis, neurodegeneration
Introduction—overview of review structure
All biological systems exist in redox equilibrium, balancing oxidative and reducing reactions to achieve suitable conditions for life. Disruptions in redox homeostasis are caused by an accumulation of oxidizing molecules either by overproduction or loss of cellular reducing ability. In either case, the accumulated oxidizing agents are able to oxidize DNA, proteins, and lipids thereby altering their structure, activity, and physical properties. Given the potential severity of such widespread oxidative damage, perturbation of redox equilibrium can result in severe disruptions of biological homeostasis, potentially leading to disease or death.
Reactive oxygen species (ROS) are some of the most common oxidants in cells. ROS are formed by the partial reduction of molecular oxygen to superoxide (O2−•), hydrogen peroxide (H2O2), lipid peroxides (ROOH), or the corresponding hydroxyl (HO•) and peroxyl radicals (ROO•). A growing body of work implicates lipid peroxides as key mediators of many pathological states including inflammation, cancer, neurodegenerative disease, as well as ocular and kidney degeneration. Additionally, lipid peroxidation is the key downstream feature of ferroptosis, an emerging form of regulated non-apoptotic cell death.
Lipid peroxides exert their toxic effects through two general mechanisms. Since lipids are responsible for maintaining the integrity of cellular membranes, extensive peroxidation of lipids alters the assembly, composition, structure, and dynamics of lipid membranes. As highly reactive compounds, lipid peroxides are also able to propagate further generation of ROS, or degrade into reactive compounds capable of crosslinking DNA and proteins.
Finally, Lipid peroxides can be divided into two general classes: lipid endoperoxides and lipid hydroperoxides. Lipid endoperoxides are a key intermediate in the formation of prostaglandins and their importance in inflammation and disease has been reviewed extensively [1, 2]. More recently, lipid hydroperoxides have also been recognized as key mediators of cellular disease and death. This review will focus on lipid hydroperoxides, discussing their synthesis, toxicity, degradation, detection, their role in disease and death, and strategies to mitigate the deleterious effects of lipid peroxidation.
Biological Synthesis of Lipid Peroxides
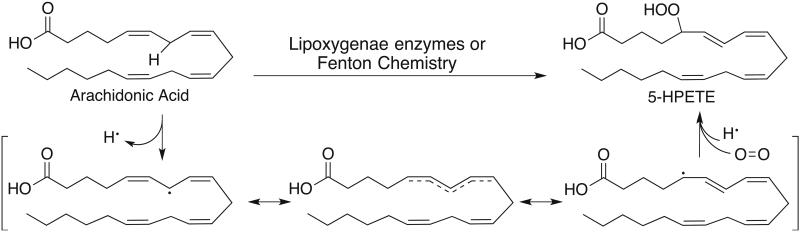
Lipid peroxides are produced in many cellular contexts and can serve as signaling molecules through post-translational modification of proteins [3]. The biosynthesis of lipid peroxides can be carried out by enzymes or via non-enzymatic processes. Yet, the substrate scope and general mechanism of lipid peroxidation is largely the same in both cases. Lipid peroxidation preferentially oxidizes polyunsaturated fatty acids (PUFAs) — long-chain fatty acids with more than one double bond, including linoleic, arachidonic, and docosahexaenoic acids. The PUFAs that are most susceptible to peroxidation contain a (1Z, 4Z) pentadiene moiety. In the first step of oxidation, a hydrogen atom is removed from the methylene carbon bridging the two double bonds. The weak C-H bond and the stability of the resulting bisallylic radical drive the selectivity in hydrogen atom removal. This resonance-stabilized pi system allows the lipid to isomerize into the more thermodynamically stable isomer, forming a (1Z, 3E) conjugated diene that reacts with molecular oxygen to form a lipid peroxide.
Fenton-type chemistry
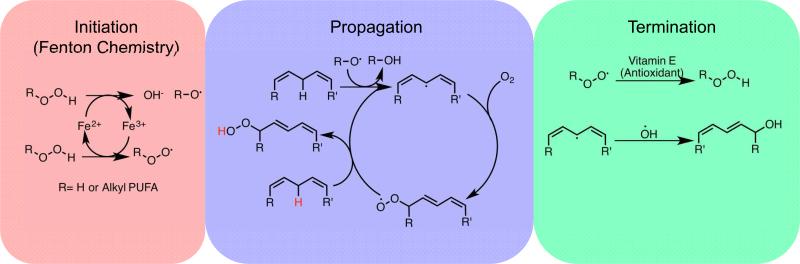
The non-enzymatic peroxidation of lipids is a process mediated by carbon- and oxygen-centered radicals. Like all radical reactions, this process can be broken down into three discreet phases: initiation, propagation, and termination. The initiation step is any process that generates radical compounds from non-radical molecules. In cells, this is accomplished using iron and, less frequently, copper. Cellular iron is under tight regulation in cells. Most iron is ligated by heme, bound in FeS clusters, or stored in the iron storage protein ferritin. Nevertheless there exists a small amount of iron that is loosely ligated (“labile”) and able to participate in redox reactions [4]. These redox reactions catalyzed by the labile iron pool are collectively referred to as “Fenton chemistry”, a series of reactions where labile iron reacts with endogenously produced hydrogen peroxide or superoxide to form oxygen centered radicals [5]. In the first step of Fenton chemistry, ferrous iron disproportionates hydrogen peroxide, oxidizing to ferric iron generating a hydroxide anion and a highly reactive hydroxyl radical. If another equivalent of hydrogen peroxide is available, the ferric iron can be reduced back to its ferrous state and generate a peroxyl radical. Having completed the initiation step, lipid peroxidation can proceed to the propagation step.
Both the hydroxyl and peroxyl radical produced in the Fenton reaction are able to abstract a hydrogen from the bisallylic methylene of a membrane PUFA, creating a resonance-stabilized, carbon-centered radical that can react with molecular oxygen in solution to form a lipid-peroxyl radical ROO• which can abstract another hydrogen from a different bisallylic methylene, generating a lipid peroxide (ROOH) and another carbon centered radical that can react with oxygen. Radical compounds giving rise to new radicals is the hallmark of the propagation step. In this way, non-enzymatic lipid peroxidation can be considered a chain reaction. If the concentration of radicals is high enough that two radicals can react with each other they will form a new bond between themselves, eliminating the radical. Alternately, molecules that are able to donate electrons to radical compounds without themselves becoming a radical can terminate radical propagation. These molecules, called antioxidants, are a primary mechanism of defense against uncontrolled lipid peroxidation and other oxidative damage and will be discussed in a later section of this review.
5-lipoxygenase
In addition to being formed through non-specific propagation of radicals, oxidized lipids can also be synthesized in a controlled manner by cyclooxygenases (COXs), cytochrome p450s (CYPs), and lipoxygenases (LOXs). COXs synthesize lipid endoperoxides and are partially responsible for the peroxidation of linoleic acid [6], CYPs synthesize epoxyeicosatrienoic acids (EETs), and LOX enzymes are the biggest contributor to the synthesis of lipid hydroperoxides. The lipoxygenases are named and classified based on the positional specificity of oxidation of arachidonic acid. The 5-lipoxygenase is an enzyme encoded by the ALOX5 gene that is responsible for oxidizing arachidonic acid at carbon 5, forming 5-hydrperoxyeicosatetraenoic acid (5-HPETE). 5-lipoxygenase can further convert 5-HPETE into Leukotriene A4, initiating leukotriene biosynthesis [7]. The expression and activity of 5-lipoxygenase is tissue specific and highly regulated [8-10]. 5-lipoxygenase activity is stimulated specifically by elevated Ca2+ levels while other divalent metal cations such as Cu2+ and Co2+ inhibit 5-HPETE production [11]. 5-lipoxygenase is also regulated by multiple protein kinases. Phosphorylation of 5-lipoxygenase by p38 MAPK and ERK1/2 increases enzyme activity in cells while phosphorylation by Protein Kinase A suppresses enzyme activity [10]. Once 5-lipoxygenase is activated it migrates to the nuclear membrane where it associates with two additional proteins: the 5-lipoxygeanse activating protein (FLAP) and cytosolic phospholipase A2 (cPLA2). cPLA2 is responsible for cleaving arachidonic acid from membrane phospholipids, increasing substrate availability for 5-lipoxygenase. The exact function of FLAP is still unclear, but it is believed that FLAP facilitates the delivery of arachidonic acid to 5-lipoxygenase. Pharmacologic inhibition of FLAP function prevents oxidation of endogenous arachidonic acid by 5-lipoxygenase, demonstrating the necessary role of FLAP in lipid peroxide formation [10, 12].
12/15-lipoxygenases
The 12- and 15-lipoxygenases are a class of enzymes encoded by for genes in humans: ALOX12, ALOX12B, ALOX15, and ALOX15B [13]. These enzymes synthesize 12-hydroperoxyeicosatetraenoic acid (12-HPETE) and 15-hydroperoxyeicosatetraenoic acid (15-HPETE) from arachidonic acid. In contrast to 5-lipoxygenase, some members of this class exhibit incomplete regioselectivity in forming lipid peroxides. For instance, 15-lipoxygenase 1 (encoded by ALOX15) converts 10-20% of the arachidonic acid it oxidizes to 12-HPETE where as 15-lipoxygenase 2 (encoded by ALOX15B) specifically oxidizes carbon 15 [14]. The 12- and 15-lipoxygenases also posses a greater substrate scope than the 5-lipoxygenase. Linoleic acid, a ubiquitous PUFA, can be oxidized to 13-hydroperoxyoctadecadienoic acid (13-HPODE) and 9-hydroperoxyoctadecadienoic acid (9-HPODE) by 15- and 12-lipoxygenases [15, 16]. Docosahexaenoic acid, an ω-3 PUFA is also oxidized by 15-lipoxygenase as part of the synthesis of resolvins and protectins [13, 15]. In keeping with their broadened substrate scope the 12- and 15-lipoxygenases are known to be reactive towards intact phospholipids, and do not require their hydrolysis for peroxidation [17, 18].
Degradation, Toxicity, and Detection of Lipid Peroxides
Though lipid peroxides are stable enough to persist and diffuse in lipid bilayers, they are still prone to degradation. These degradation products are often themselves reactive and can be broadly divided into two classes: hydroxy acids and reactive aldehydes. Lipid peroxide degradation products are the most useful tools for detecting and quantifying lipid peroxidation in biological samples.
Degradation of lipid peroxides
The hydroxy acids are the direct reduction products of the corresponding lipid peroxide. HPETEs and HPODEs can be reduced the corresponding HETEs and HODEs with a strong enough reducing agent. Chemical reducing agents like triphenylphosphine can reduce the peroxide bond [19], but the cell also possesses endogenous mechanisms to eliminate harmful peroxides on its own without creating additional radicals. The glutathione peroxidase (GPx) enzymes, particularly GPx4 (discussed more in a later section), are key regulators of lipid peroxides in cells, using GSH as a co-substrate to reduce lipid peroxides to the corresponding alcohol. Inactivation of this enzyme results in the accumulation of lipid peroxides and often death [19, 20].
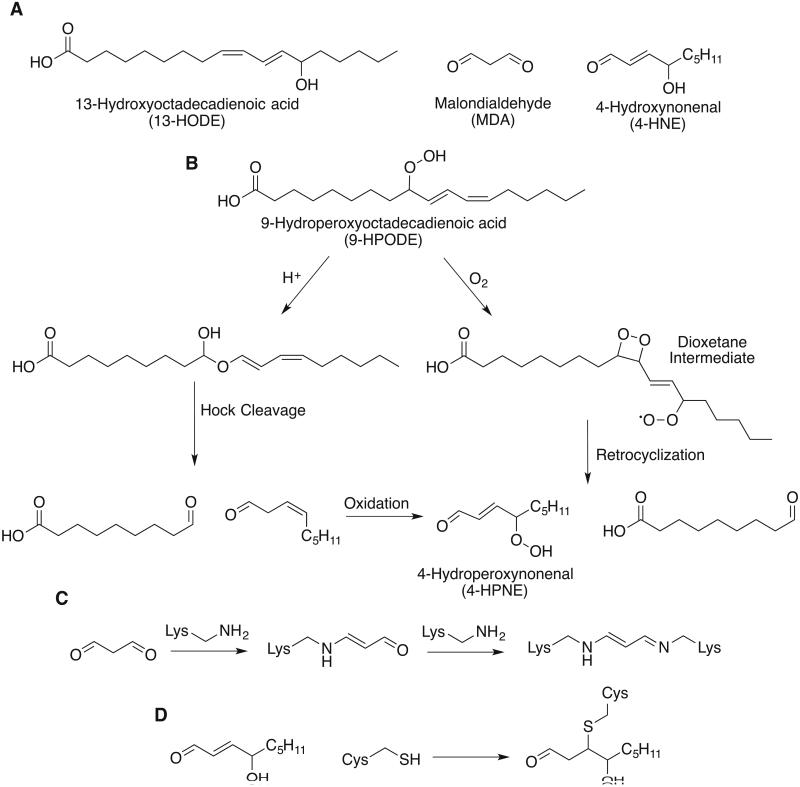
Aldehydes are the other major class of lipid peroxide degradation product, with 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) being the most well described and thoroughly researched members of this class [21, 22]. Multiple routes have been proposed for the biosynthesis of these molecules from lipid peroxides [22-28]. However, there is no clear consensus on how significantly each of these competing mechanisms may contribute to the degradation of lipid peroxides in vivo. What is common between these mechanisms is that ubiquitous downstream degradation products such as 4-HNE and MDA both require multiple oxidations of the parent substrate. 4-HNE, for instance, is believed to arise directly from a peroxide precursor 4-hydroperoxynonenal (4-HPNE) and that all nine carbons originate from the methyl end of a fatty acid. Two possible biosynthetic routes for 4-HNE are shown below. The peroxyl radical of 9-HPODE can attack the adjacent double bond, forming a dioxetane intermediate and reacting with another equivalent of oxygen. This strained intermediate can undergo retrocyclization to form 4-HPNE and an aliphatic aldehyde [23, 29]. Alternately, in sufficiently acidic environments, 9-HPODE can undergo Hock cleavage. 9-HPODE rearranges to form an intermediate hemiacetal that can cleave to furnish an aliphatic aldehyde and a β, γ-unsaturated aldehyde which can be further oxidized to 4-HPNE [27, 30].
Toxicity of lipid peroxides
Peroxidation of membrane lipids is known to substantially alter the physical properties of lipid bilayers. In particular, the peroxidation alters lipid-lipid interactions, membrane permeability, ion gradients, membrane fluidity, and membrane permeability [31]. Molecular dynamics simulations initially suggested that peroxidized phospholipids reoriented themselves in a lipid bilayer so that the oxidized chain moved towards the water/lipid head-group interface, decreasing membrane thickness [32]. Later experimental evidence confirmed that the entire oxidized chain can protrude into the aqueous phase, allowing for macrophage recognition [33]. Increasing the concentration of oxidized lipids in liposomal membranes is also known to cause a corresponding decrease in membrane fluidity and slower lateral diffusion [34]. PUFA oxidation by the 5-lipoxygenase is known to require the hydrolysis of a fatty acid chain from a parent phospholipid, leaving behind lysophospholipids. In vitro data on lipid monolayers has shown that the resulting lysophospholipids are readily solubilized into the cytosol [35]. Both the desolvation of lysophospholipids as well as the conformational change of oxidized phsopholipids is believed to contribute to an increase in membrane permeability [32, 35].
Lipid peroxides exhibit additional toxicity from the degradation products they spontaneously form. Ferrous iron can react with a lipid peroxide to generate the corresponding alkoxy radical that can propagate new peroxidation reactions. The aldehyde degradation products of lipid peroxides are toxic to cells. Both 4-HNE and MDA are highly reactive molecules. MDA is a dialdehyde able to react with primary amines on proteins or DNA to form crosslinks. Additionally, MDA can form 1,4-dihydropyridine adducts with primary amines [22, 36]. 4-HNE also contains an aldehyde functional group and can form Schiff base adducts with primary amines and cyclization products similar to MDA [29]. 4-HNE is also a Michael acceptor, and can form covalent adducts with the side chains of nucleophilic amino acids. The covalent modifications carried out by these secondary messengers of lipid peroxidation alter the structure and function of proteins and nucleic acids and are responsible for the cytotoxicity of these molecules.
Detection of lipid peroxides and their degradation products
Many of the earliest methods to measure and quantify lipid peroxidation relied on the unique reactivity of aldehyde degradation products. The reaction of MDA and thiobarbituric acid yields a chromophore whose concentration can be quantified by absorbance [37]. Similarly, the reaction of the aldehyde moiety of 4-HNE with 2,4-dinitrophenylhydrazine has been used to measure the extent of protein carbonylation in biological samples[38]. Spectroscopic methods exist for direct detection of lipid peroxides. The absorbance of conjugated dienes that result from the isomerization of oxidized PUFAs and the absorbance of the triiodide anion from the reaction between iodide salts and peroxides are two well-validated methods for measuring lipid peroxides in vitro [37, 39]. While these methods are very useful for cell free in vitro systems, they are prone to interference and inaccuracy in cellular contexts, limiting the extent to which these assays can be regarded as quantitative [21]. To meet this challenge, LC-MS methods have been developed to quantitatively profile lipid peroxidation products in complex biological samples. LC-MS analysis of the HETE and HODE content of cells is used as a biomarker of lipid peroxidation [40]. Whereas many absorbance methods give a picture of lipid peroxidation generally, LC-MS also has the advantage of quantifying the oxidation of individual phospholipids, giving a more focused understanding of lipid peroxidation in pathological contexts [19].
Lipid peroxides in disease and death
Because of their reactivity and ability importance in generating secondary messengers, lipid peroxides have long been appreciated as critical for the progression and regulation of inflammation [9, 13]. The ability of lipid peroxides to generate toxic secondary messengers has also helped highlight their importance in multiple pathologies and cell death. One clinical area where lipid peroxidation is particularly important is degenerative disease of the brain and the central nervous system. The brain consumes a large volume of oxygen and generates a high quantity of ROS as a byproduct of ATP synthesis. Membrane phospholipids in the CNS are highly enriched in PUFAs, incorporating them rapidly from free fatty acids [41]. The combined result is an environment with all the necessary materials for peroxidation of lipids. Lipid peroxidation also plays a role in regulated cell death. The lipid degradation product 4-HNE has been shown to induce apoptosis in specific contexts [42]. Recent work has also identified lipid peroxidation as the primary driver of ferroptosis, a type of regulated necrotic cell death [43].
Alzheimer's disease
Alzheimer's disease is a progressive neurodegenerative disease characterized by the extracellular accumulation of amyloid-β (Aβ) and intracellular accumulation of neurofibrillary tangles composed of tau protein [44]. Insertion of Aβ into cellular membranes is known to generate hydrogen peroxide and, via Fenton chemistry, lipid peroxides and degradation products such as MDA [45]. Additionally, higher levels of 12- and 15-lipoxygenase and 12- and 15-HETE were found in post-mortem brain samples from Alzheimer's patients compared to controls [46]. Subsequent work in cell culture models of Alzheimer's disease showed that pharmacological inhibition of the 12- and 15-lipoxygenases reduced Aβ formation [47]. The growing body of evidence for the involvement of lipid peroxidation and general oxidative stress as a driving force of Alzheimer's disease has generated much interest in using antioxidants as therapeutics. Multiple clinical trials have tested the antioxidant vitamins E and C in patients with Alzheimer's disease but observed no change in the progression of disease [48, 49]. Another study found that while markers of lipid oxidation in the cerebrospinal fluid decreased with vitamin E and C treatment, no changes in Aβ production were detectable, and patients showed a faster onset of cognitive impairment compared to placebo treated patients [50]. Despite these disappointing results, interest in using antioxidants as therapeutics against Alzheimer's disease remains. Since the antioxidant capacity of a cell is dependent on a cocktail of multiple antioxidants, it is possible that supplementation with one or two antioxidants is not sufficient for changing the course of disease in humans. Additionally, since lipid peroxidation can be initiated at very early stages of the disease, antioxidant therapy may be more effective in patients with early stages of Alzheimer's disease, rather than late-stage patients [51].
Ferroptosis
Ferroptosis is a non-apoptotic, iron dependent form of regulated cell death that is characterized by the accumulation of lipid peroxides. Ferroptosis can be suppressed by lipophilic antioxidants and iron chelators but is not affected by apoptosis or necroptosis inhibitors [19, 52]. Ferroptosis was discovered through high-throughput screening of lethal molecules, leading to the initial discovery of two ferroptosis inducing small molecules: erastin and RSL3. Both of these molecules exert their lethal effects by compromising the antioxidant capacity of cells. Erastin inhibits system Xc−, a transmembrane cystine/glutamate antiporter that supplies cysteine for the synthesis of the antioxidant tripeptide glutathione. RSL3 covalently inhibits glutathione peroxidase 4 (GPx4), which reduces lipid peroxides to the corresponding hydroxide, using glutathione as a cofactor. Monitoring oxidative stress in ferroptotic cells showed an increase in lipid peroxidation without an increase in other ROS such as superoxide [52]. Subsequent lipidomics showed a specific depletion of unoxidized PUFAs [53] and an increase in oxidized PUFAs [19]. Lipoxygenases contribute to the accumulation of lipid peroxides in ferroptosis. Genetic knockdown and pharmacological inhibition of lipoxygenases are both strongly protective against erastin treatment. Erastin treatment also induces the translocation of the 5-lipoxygenase to the nuclear membrane, which is necessary for its activation [53]. Mitochondria may have a central role in ferroptosis as well. Lipidomic profiling of GPX4 knockout mice shows an increase in oxidized cardiolipin, a mitochondria-specific lipid [19]. Correspondingly, treatment of cells undergoing ferroptosis with a mitochondria-targeted antioxidant capable of preventing the oxidation of cardiolipin potently restores cell viability [54].
Inhibition of lipid peroxidation
Because of their role in many disease and death, there has been intense effort to identify and develop compounds that ameliorate the toxic effects of lipid peroxides. Broadly these inhibitors can be divided into two classes: molecules that prevent peroxides from being formed and molecules that eliminate peroxides that have already been synthesized.
Inhibitors of peroxidation
The most common strategy for preventing the formation of lipid peroxides is to inhibit the enzymes responsible for synthesizing them. To this end, many inhibitors of the lipoxygenase enzymes have been developed. The 5-lipoxygenase has received a particularly large amount of attention due to its role in multiple inflammatory diseases, with many inhibitors advancing into clinical trials [55]. Of these, only Zileuton has been approved for clinical use. Zileuton is believed to ligate the active site iron through its N-hydroxy urea moiety [56]. Other classes of 5-lipoxygenase inhibitors similarly focus on the active site iron, targeting it with redox active inhibitors that prevent the iron from cycling between its ferrous and ferric states [57]. With the recognition of the importance of the 12- and 15-lipoxygenases in neurodegenerative diseases, efforts have increased to develop specific inhibitors of these lipoxygenases [58, 59].
An emerging strategy to prevent lipid peroxidation is supplementation with fatty acids labeled with heavier isotopes of hydrogen at sites prone to oxidation. The carbon-deuterium bond is stronger than the carbon-hydrogen bond, making it more difficult to remove deuterium atoms and thus slowing the rate of peroxide formation. This kinetic isotope effect is quite large for PUFAs that are deuterated at the bisallylic position [60]. The advantage of deuteration of PUFA substrates over direct enzyme inhibition is that D-PUFAs are expected to inhibit both enzymatic and non-enzymatic lipid peroxidation, making them more protective than inhibition of a single lipoxygenase enzyme. The protective effect of these D-PUFAs has been shown in multiple disease contexts, rescuing cells from oxidative stress and death [53, 61, 62].
Elimination of peroxides—Bioactive reducing agents
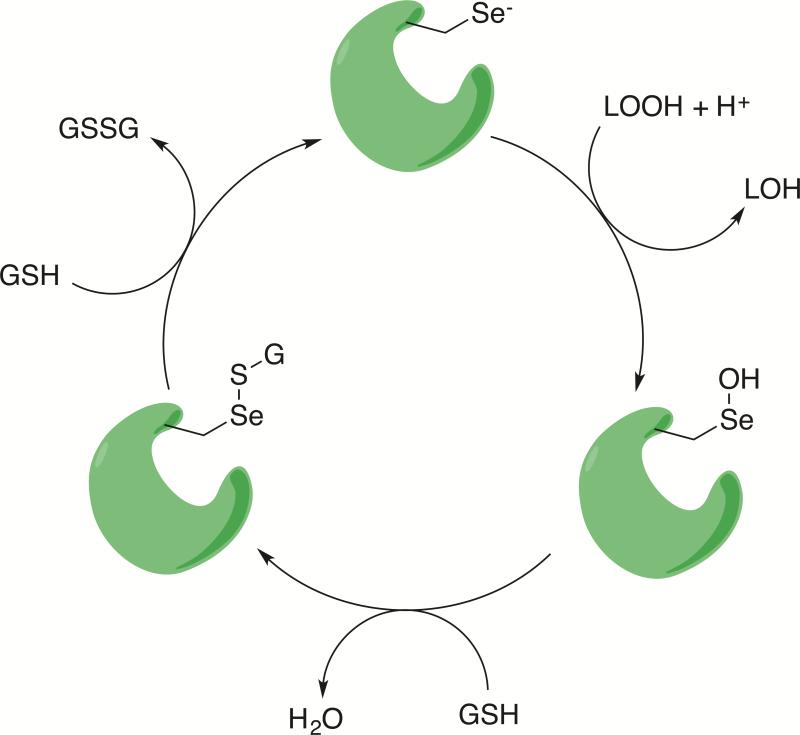
A complementary strategy to preventing lipid peroxide formation is the reduction of the peroxide or radical intermediate to a less cytotoxic compound. Biological systems have evolved multiple mechanisms for reducing lipid peroxides. In particular the glutathione peroxidase (GPx) class of enzymes are responsible for reducing lipid peroxides. There are eight GPx isoforms in humans, all with differing substrate specificities and tissue distributions. The GPx4 enzyme is the primary reductant of lipid peroxides. Inhibition or deletion of GPx4 leads to ferroptotic cell death and the accumulation of lipid peroxides [19, 20]. GPx4 reduces lipid peroxides using a selenocysteine to preform a nucleophilic attack on the terminal oxygen of the lipid peroxide, releasing a HETE or HODE [63, 64]. The intermediate selenenic acid is reduced by two equivalents of glutathione to regenerate the active enzyme and oxidized glutathione.
The vitamin E compounds are another group of compounds able to mitigate the toxicity arising from lipid peroxides. Vitamin E is not a strong enough reducing agent to reduce peroxide bonds, but it can serve as a radical scavenging agent. During the propagation of lipid peroxides, vitamin E can donate a single electron to the intermediate peroxyl radical, terminating the chain reaction propagation of peroxides. The radical vitamin E intermediate can be reduced by vitamin C [65]. Supplementation with vitamin E is protective against ferroptotic cell death [52]. The reduction of other ROS by vitamin E is quite limited, and vitamin E is believed to be a specific reductant of lipid peroxidation in vivo, and does not scavenge other radical intermediates such as the hydroxyl radical [65].
Discussion
Lipid peroxides are an important class of biomolecules generated by oxidative stress in cells. Though lipid peroxides have been observed in multiple disease states, it is often unclear to what extent they initiate disease or are the downstream products of other disease-promoting factors. Similarly, it remains to be determined what role general cellular processes like ferroptosis play in specific disease contexts such as Alzheimer's. Nonetheless, an increasing understanding how lipid peroxidation can be controlled is expected to have significant medical impact. For cancers that have evolved mechanisms to evade apoptotic signals, induction of lipid peroxidation and ferroptosis may provide a therapeutic alternative to current chemotherapeutic options. Similarly, despite the failures of vitamin E therapy in Alzheimer's disease, developing better techniques to prevent the oxidative stress accompanied by neurodegeneration has the potential to offer new therapeutic options and may be applicable to similar neurodegenerative diseases.
Supplementary Material
Figure 1.
General mechanism for peroxidation of PUFAs. A hydrogen atom at a bisallylic position is remove using either a radical or a redox active metal to generate a resonance stabilized carbon centered radical. The double bonds of the acid isomerize to form the more thermodynamically stable conjugated diene prior to reacting with molecular oxygen.
Figure 2.
The three steps of non-enzymatic lipid peroxidation. In the initiation step the first radicals are generated by redox active labile iron. In the propagation step radicals are able to react with new substrates, creating new radicals. The propagation step repeats until the termination step, where radicals are ‘quenched’ by antioxidants or reacting with another radical.
Figure 3.
Lipid peroxide degradation. (A) Structures of common lipid peroxide degradation products. (B) Two mechanistic hypotheses to explain the formation of 4-HNE via a peroxide intermediate 4-HPNE (C) Malondialdehyde can form Schiff bases with primary amines. In the first condensation, the resulting imine tautomerizes to the enamine before condensing with a second primary amine. (D) 4-hydroxynonenal is a Michael receptor that reacts with nucleophilic side chains such as cysteine.
Figure 4.
Catalytic reduction of lipid peroxides by GPX4. GPX4 (green) uses a highly nucleophilic selenocysteine to attack a lipid peroxide, generating a selenenic acid intermediate and a lipid hydroxide. The selenenic acid is further reduced by two successive equivalents of glutathione (GSH).
Lipid peroxides are an important class of reactive oxygen species.
Lipid peroxides can be synthesized in many contexts and are mediators of disease and death.
Lipid peroxidation is a hallmark of Alzheimer's disease and ferroptosis, an emerging form of regulated oxidative cell death.
Many mechanisms exist to ameliorate the deleterious effects of lipid peroxidation.
Acknowledgments
Funding
M.M.G is supported by the Training Program in Molecular Biophysics Grant T32GM008281. This research of BRS is supported by NIH R01CA097061 and 1R35CA209896.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 2.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breuer W, Shvartsman M, Cabantchik ZI. Intracellular labile iron. Int J Biochem Cell Biol. 2008;40:350–354. doi: 10.1016/j.biocel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Marisa Repetto JS, Boveris Alberto. Lipid peroxidation: chemical mechanism, biological implications and analytical determination. In: Catala DA, editor. Lipid Peroxiation. InTech; 2012. [Google Scholar]
- 6.Laneuville O, Breuer DK, Xu N, Huang ZH, Gage DA, Watson JT, Lagarde M, DeWitt DL, Smith WL. Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z, 13E/Z, 15Z)-octadecatrienoic acids from alpha-linolenic acid. J Biol Chem. 1995;270:19330–19336. doi: 10.1074/jbc.270.33.19330. [DOI] [PubMed] [Google Scholar]
- 7.Rouzer CA, Matsumoto T, Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc Natl Acad Sci U S A. 1986;83:857–861. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radmark O. Arachidonate 5-lipoxygenase. Prostaglandins Other Lipid Mediat. 2002;68-69:211–234. doi: 10.1016/s0090-6980(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 9.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta. 2015;1851:331–339. doi: 10.1016/j.bbalip.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Percival MD, Denis D, Riendeau D, Gresser MJ. Investigation of the mechanism of non-turnover-dependent inactivation of purified human 5-lipoxygenase. Inactivation by H2O2 and inhibition by metal ions. Eur J Biochem. 1992;210:109–117. doi: 10.1111/j.1432-1033.1992.tb17397.x. [DOI] [PubMed] [Google Scholar]
- 12.Peters-Golden M, Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids. 2003;69:99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 13.Ackermann JA, Hofheinz K, Zaiss MM, Kronke G. The double-edged role of 12/15-lipoxygenase during inflammation and immunity. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbalip.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soberman RJ, Harper TW, Betteridge D, Lewis RA, Austen KF. Characterization and separation of the arachidonic acid 5-lipoxygenase and linoleic acid omega-6 lipoxygenase (arachidonic acid 15-lipoxygenase) of human polymorphonuclear leukocytes. J Biol Chem. 1985;260:4508–4515. [PubMed] [Google Scholar]
- 17.Takahashi Y, Glasgow WC, Suzuki H, Taketani Y, Yamamoto S, Anton M, Kuhn H, Brash AR. Investigation of the oxygenation of phospholipids by the porcine leukocyte and human platelet arachidonate 12-lipoxygenases. Eur J Biochem. 1993;218:165–171. doi: 10.1111/j.1432-1033.1993.tb18362.x. [DOI] [PubMed] [Google Scholar]
- 18.Jung G, Yang DC, Nakao A. Oxygenation of phosphatidylcholine by human polymorphonuclear leukocyte 15-lipoxygenase. Biochem Biophys Res Commun. 1985;130:559–566. doi: 10.1016/0006-291x(85)90453-x. [DOI] [PubMed] [Google Scholar]
- 19.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 23.Spickett CM. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol. 2013;1:145–152. doi: 10.1016/j.redox.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J Biol Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 25.Schneider C, Boeglin WE, Yin H, Ste DF, Hachey DL, Porter NA, Brash AR. Synthesis of dihydroperoxides of linoleic and linolenic acids and studies on their transformation to 4-hydroperoxynonenal. Lipids. 2005;40:1155–1162. doi: 10.1007/s11745-005-1480-3. [DOI] [PubMed] [Google Scholar]
- 26.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu X, Salomon RG. Fragmentation of a linoleate-derived gamma-hydroperoxy-alpha,beta-unsaturated epoxide to gamma-hydroxy- and gamma-oxo-alkenals involves a unique pseudo-symmetrical diepoxycarbinyl radical. Free Radic Biol Med. 2012;52:601–606. doi: 10.1016/j.freeradbiomed.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaur K, Salomon RG, O'Neil J, Hoff HF. (Carboxyalkyl)pyrroles in human plasma and oxidized low-density lipoproteins. Chem Res Toxicol. 1997;10:1387–1396. doi: 10.1021/tx970112c. [DOI] [PubMed] [Google Scholar]
- 30.Yin HY, Xu LB, Porter NA. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 31.Mario Diaz AC. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Frontiers in Physiology. 2016;7 doi: 10.3389/fphys.2016.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys J. 2007;93:4225–4236. doi: 10.1529/biophysj.107.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XM, Salomon RG, Qin J, Hazen SL. Conformation of an endogenous ligand in a membrane bilayer for the macrophage scavenger receptor CD36. Biochemistry. 2007;46:5009–5017. doi: 10.1021/bi700163y. [DOI] [PubMed] [Google Scholar]
- 34.Borst JW, Visser NV, Kouptsova O, Visser AJ. Oxidation of unsaturated phospholipids in membrane bilayer mixtures is accompanied by membrane fluidity changes. Biochim Biophys Acta. 2000;1487:61–73. doi: 10.1016/s1388-1981(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 35.Heffern CT, Pocivavsek L, Birukova AA, Moldobaeva N, Bochkov VN, Lee KY, Birukov KG. Thermodynamic and kinetic investigations of the release of oxidized phospholipids from lipid membranes and its effect on vascular integrity. Chem Phys Lipids. 2013;175-176:9–19. doi: 10.1016/j.chemphyslip.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurbuz G, Heinonen M. LC-MS investigations on interactions between isolated beta-lactoglobulin peptides and lipid oxidation product malondialdehyde. Food Chem. 2015;175:300–305. doi: 10.1016/j.foodchem.2014.11.154. [DOI] [PubMed] [Google Scholar]
- 37.Reilly CA, Aust SD. Measurement of lipid peroxidation. Curr Protoc Toxicol. 2001 doi: 10.1002/0471140856.tx0204s00. Chapter 2, Unit 2 4. [DOI] [PubMed] [Google Scholar]
- 38.Yan LJ, Forster MJ. Chemical probes for analysis of carbonylated proteins: a review. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1308–1315. doi: 10.1016/j.jchromb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braughler JM, Duncan LA, Chase RL. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem. 1986;261:10282–10289. [PubMed] [Google Scholar]
- 40.Niki E. Biomarkers of lipid peroxidation in clinical material. Biochim Biophys Acta. 2014;1840:809–817. doi: 10.1016/j.bbagen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Chen CT, Green JT, Orr SK, Bazinet RP. Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukot Essent Fatty Acids. 2008;79:85–91. doi: 10.1016/j.plefa.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Dalleau S, Baradat M, Gueraud F, Huc L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013;20:1615–1630. doi: 10.1038/cdd.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 46.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Succol F, Pratico D. A role for 12/15 lipoxygenase in the amyloid beta precursor protein metabolism. J Neurochem. 2007;103:380–387. doi: 10.1111/j.1471-4159.2007.04742.x. [DOI] [PubMed] [Google Scholar]
- 48.Arlt S, Muller-Thomsen T, Beisiegel U, Kontush A. Effect of one-year vitamin C- and E-supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer's disease. Neurochem Res. 2012;37:2706–2714. doi: 10.1007/s11064-012-0860-8. [DOI] [PubMed] [Google Scholar]
- 49.Tolonen M, Halme M, Sarna S. Vitamin E and selenium supplementation in geriatric patients : A double-blind preliminary clinical trial. Biol Trace Elem Res. 1985;7:161–168. doi: 10.1007/BF02916538. [DOI] [PubMed] [Google Scholar]
- 50.Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG, Aisen P, Alzheimer's Disease Cooperative S. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69:836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persson T, Popescu BO, Cedazo-Minguez A. Oxidative stress in Alzheimer's disease: why did antioxidant therapy fail? Oxid Med Cell Longev. 2014;2014:427318. doi: 10.1155/2014/427318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krainz T, Gaschler MM, Lim C, Sacher JR, Stockwell BR, Wipf P. A Mitochondrial-Targeted Nitroxide Is a Potent Inhibitor of Ferroptosis. ACS Central Science. 2016 doi: 10.1021/acscentsci.6b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinhilber D, Hofmann B. Recent advances in the search for novel 5-lipoxygenase inhibitors. Basic Clin Pharmacol Toxicol. 2014;114:70–77. doi: 10.1111/bcpt.12114. [DOI] [PubMed] [Google Scholar]
- 56.Bell RL, Young PR, Albert D, Lanni C, Summers JB, Brooks DW, Rubin P, Carter GW. The discovery and development of zileuton: an orally active 5-lipoxygenase inhibitor. Int J Immunopharmacol. 1992;14:505–510. doi: 10.1016/0192-0561(92)90182-k. [DOI] [PubMed] [Google Scholar]
- 57.Young RN. Inhibitors of 5-lipoxygenase: a therapeutic potential yet to be fully realized? European Journal of Medicinal Chemistry. 1999;34:671–685. [Google Scholar]
- 58.Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB, 2nd, Perry S, Joshi N, Bougie JM, Leister W, Taylor-Fishwick DA, Nadler JL, Holinstat M, Simeonov A, Maloney DJ, Holman TR. Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. J Med Chem. 2011;54:5485–5497. doi: 10.1021/jm2005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eleftheriadis N, Neochoritis CG, Leus NG, van der Wouden PE, Domling A, Dekker FJ. Rational Development of a Potent 15-Lipoxygenase-1 Inhibitor with in Vitro and ex Vivo Anti-inflammatory Properties. J Med Chem. 2015;58:7850–7862. doi: 10.1021/acs.jmedchem.5b01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill S, Lamberson CR, Xu L, To R, Tsui HS, Shmanai VV, Bekish AV, Awad AM, Marbois BN, Cantor CR, Porter NA, Clarke CF, Shchepinov MS. Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation. Free Radic Biol Med. 2012;53:893–906. doi: 10.1016/j.freeradbiomed.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andreyev AY, Tsui HS, Milne GL, Shmanai VV, Bekish AV, Fomich MA, Pham MN, Nong Y, Murphy AN, Clarke CF, Shchepinov MS. Isotope-reinforced polyunsaturated fatty acids protect mitochondria from oxidative stress. Free Radic Biol Med. 2015;82:63–72. doi: 10.1016/j.freeradbiomed.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 62.Cotticelli MG, Crabbe AM, Wilson RB, Shchepinov MS. Insights into the role of oxidative stress in the pathology of Friedreich ataxia using peroxidation resistant polyunsaturated fatty acids. Redox Biol. 2013;1:398–404. doi: 10.1016/j.redox.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maiorino M, Roveri A, Coassin M, Ursini F. Kinetic mechanism and substrate specificity of glutathione peroxidase activity of ebselen (PZ51) Biochem Pharmacol. 1988;37:2267–2271. doi: 10.1016/0006-2952(88)90591-6. [DOI] [PubMed] [Google Scholar]
- 64.Takebe G, Yarimizu J, Saito Y, Hayashi T, Nakamura H, Yodoi J, Nagasawa S, Takahashi K. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J Biol Chem. 2002;277:41254–41258. doi: 10.1074/jbc.M202773200. [DOI] [PubMed] [Google Scholar]
- 65.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.