Abstract
The central nervous system (CNS) is considered an “immunoprivileged” site with restricted access and a unique microenvironment that profoundly affects the capacity of T cells to exert their functions. The lymphocytic choriomeningitis virus model offers a unique system in which to evaluate the contrasting roles of specific T cells in causing lethal CNS disease or curing pervasive and life-long CNS infection. Specific T cell kinetics in the periphery is briefly discussed. The T cell–mediated mechanisms leading to fatal choriomeningitis are reviewed as are recent methodologic advances that will facilitate the study of antigen-specific T cells in disease pathogenesis. Understanding the specific constraints imposed by the CNS on local T cell activity has important consequences for the design of therapeutic strategies aimed at preventing or curing CNS infection.
With each new technological advance comes the opportunity to further refine our understanding of complex cellular organisms. Because of the daunting task biologists face in their attempt to understand the dynamics of cellular interactions in vivo, reductionism is commonly used to generate approachable model systems. Thus, faced with an impasse in vivo due to restrictions in existing methodologies, biologists often turn to the study of simplified cellular interactions in vitro. A case in point is the field of cellular immunology, which is often shaped by discoveries stemming from in vitro model systems. However, with the advent of more sophisticated tools, it is possible to break through methodologic barriers and elucidate the intricacies of immune responses in vivo.
Technology progression and refinement in the field of cellular immunology is engraved in the relationship between the lymphocytic choriomeningitis virus (LCMV) and its murine host, a model system that is one of the most widely known and well characterized in the field [1, 2]. LCMV infection can initiate a broad spectrum of disorders that reflect many of the virusinduced diseases observed in humans. Intraperitoneal infection with LCMV usually induces a massive expansion of virus-specific CD8 and CD4 T cells in peripheral lymphoid tissues, resulting in efficient viral clearance. Because of the prodigious expansion of virus-specific T cells and the relative ease with which these cells clear virus, this model system has been used to study nearly all defining elements of a successful adaptive immune response, including but not limited to activation, expansion, migration, contraction, and memory.
In contrast, intracerebral inoculation of LCMV initiates a lethal disease because the virus outraces the immune response to infect cells lining the brain. This disease process exemplifies the delicate balance between successful viral clearance and a life-threatening immunopathology. A field of immunologic research that has gained recent prominence studies specific T cell kinetics in secondary lymphatic tissues and their trafficking into extralymphatic tissues to control infection or cause immunopathology [3, 4]. Several recent reports provide important clues about the distribution and activation status of antigen-specific T cells in different tissues [5–7] and, by using the latest methodologic advances, the particular localization and function of these cells within the central nervous system (CNS) can now be investigated precisely in a variety of in vivo systems that rely on traceable populations of antigen-specific T cells [8].
Fatal Consequence of Immunity to LCMV in the CNS
LCMV per se does not kill the cells it infects. Cellular injury is immunopathologic and depends on the interactions of the antiviral immune response with infected cells of the host [2]. Following an intracerebral infection, LCMV replicates primarily in the choroid plexus, ependyma, and meninges [9–11]. This results in a choriomeningitis (the disease from which the name LCMV is derived) and death within 6–8 days. Because of the disruptive nature of the intracerebral injection, it is estimated that 190% of the inoculum escapes into the blood, while the remaining 10% is retained within the CNS [12]. A race then begins between the virus and the adaptive immune response. Virus released into the periphery primes the LCMV-specific immune response in secondary lymphoid tissues. Concurrent with this immune priming, LCMV replicates virtually unchecked in the CNS. When the virus and adaptive immune response finally converge, the result is destruction of infected cells, increased intracerebral pressure, brain edema, and death.
There is irrefutable evidence that the adaptive immune response is involved in this fatal meningitis. Early studies used immunosuppressive strategies to determine if the immune response was required for lethality of the disease process [9, 13]. In fact, immunosuppression with cyclophosphamide not only prevents development of CNS disease but also results in a chronic asymptomatic virus-carrier state [9]. It was also established by thymectomy [14] and depletion studies [15] that both CNS disease and viral clearance are dependent on thymusderived (T) lymphocytes.
With the availability of more specific antibodies directed against T cell subsets came the opportunity to pinpoint the effector cell population required for disease. Both adoptive transfer [16, 17] and in vivo depletion studies [18, 19] convincingly showed that the CD8 T cell represented the key effector cell in the fatal meningitis. In contrast, B cells [20] and natural killer cells [21] were not required. The prerequisite for CD8 T cells was further supported by studies that induced a convulsive disease and death 1–4 days after intracerebral injection of cloned CD8 T cells into LCMV-infected mice [22–24]. When T cell clones specific for either the nucleoprotein or glycoprotein of LCMV were injected into infected mice that were immunosuppressed by irradiation or cyclophosphamide, results showed that in the absence of a host-derived immune response, a clonal population of CD8 T cells was capable of driving the entire disease process. When dose-response analyses were performed, it was established that as few as 102–103 cloned CD8 T cells were sufficient to induce mortality in 50% of infected syngeneic mice.
More recently, with the advent of technology to perform homologous recombination in embryonic stem cells, transgenic mouse strains have been established that have targeted deletions in genes responsible for the functionality of the CD8 T cell response. These strains have been used to analyze the requirements for the fatal meningitis induced by LCMV. For example, mutant mice with a targeted disruption of the CD8 gene showed increased survival after intracerebral LCMV infection. This is attributed to the inability of these mice to generate a population of class I major histocompatibility complex (MHC)-restricted cytotoxic T lymphocytes (CTL) [25]. Similar results were observed in β2-microglobulin (β2m)-deficient mice infected intracerebrally with LCMV [26, 27].
Of interest, despite the lack of a class I MHC-restricted immune response in both CD8- and b2m-deficient mice, a percentage of these animals eventually succumb to the disease unless their CD4 T cells are depleted by monoclonal antibody treatment [25, 26]. It was later found that mice deficient in CD8 T cells generate a population of CD4 class II MHC-restricted cytotoxic T cells capable of inducing fatal disease through a Fas-dependent mechanism [27]. Conversely, during the normal disease process, which is dependent entirely on CD8 T cells, perforin appears to be the critical effector molecule [28]. Finally, mice with a partial CD8 T cell deficiency develop an altered balance between the kinetics of virus growth and development of T cell responses [29]. A delayed CTL response in intracerebrally infected mice deficient for CD3d allows virus redistribution from the class I MHC-positive meninges to MHC class I–negative neurons. This results in protection from lethal disease despite the presence of persisting virus.
As expected on the basis of the requirement for CD8 T cells, disease susceptibility maps to the class I MHC genes [30]. To more precisely establish the MHC restriction constraints of adoptively transferred immune cells following intracerebral infection, a series of elegant studies were done with bone marrow radiation chimeras [31, 32]. In these studies, the most severe CNS inflammation was observed when the transferred immune cells shared an MHC haplotype with the radiation-resistant host and the bone marrow donor. Thus, virus-specific CD8 T cells are incapable of inducing disease without interacting with a radiation-resistant cell population in the CNS (presumed to be the endothelium) [33]. These studies again support the idea that maximal disease severity is dependent on the proliferation of CD8 T cells in peripheral lymphoid tissues prior to entering the infected CNS [34].
Because the development of neurologic deficits coincides with a massive extravasation of bloodborne cells into cerebrospinal fluid (CSF), flow cytometric analyses have been conducted to establish the kinetics of CNS infiltration as well as the phenotypic and functional characteristics of the mononuclear cells in CSF exudate. The CNS lacks the standard lymphatic drainage observed in most peripheral organs [35]; however, radio-labeled antigens administered to the ventricular CSF without disruption of the blood-brain barrier can eventually be found in the cervical lymph nodes (CLN) [36], demonstrating that brain-derived antigens can prime immune responses in peripheral lymphoid tissues.
By day 3 after an intracerebral LCMV infection, a 3- to 4-fold increase in mononuclear cells is observed in the CLN [37]. This is thought to be due to the nonspecific recruitment (or retention) of lymphocytes [33]. Over the next 2 or 3 days ex vivo CTL activity is detectable, and more cells bear the interleukin (IL)-2 receptor (IL-2R). Finally, between postinfection days 5 and 6, lymphocytes migrate from peripheral lymphoid tissues to the CNS. Analysis of CSF exudate at this time shows that about 30% of the mononuclear cells in the CSF are CD8 and one-third of these cells express the IL-2R [38]. Of interest, virtually none of the cells are CD4, consistent with the fact that this T lymphocyte population is not involved in the disease process.
After defining the kinetics of T cell priming, activation, and migration after intracerebral LCMV infection, researchers in recent years set out to determine the specific molecules required for directing lymphocytes to sites of viral infection in the CNS [39–44]. Interactions between integrins (e.g., leukocyte function antigen [LFA]-1 and very late activation antigen-4) and their ligands (e.g., intercellular adhesion molecule [ICAM]-1 and vascular cell adhesion molecule [VCAM]-1) on activated endothelium play an important role in regulating lymphocyte migration and extravasation [45, 46]. Furthermore, the expression of chemokines also facilitates the migration of lymphocytes to areas of viral infection [47–49]. Of interest, after an intracerebral infection with LCMV, maximal expression of ICAM-1/VCAM-1 on endothelial cells [40] and chemokines within the CNS [41] depends on the presence of infiltrating CD8 T cells. It appears that LCMV infection alone induces a low-level expression of adhesion molecules and chemokines that allows the initial T cell trafficking into the CNS; however, infiltrating T lymphocytes vastly amplify the expression profile (possibly through the secretion of interferon-g), thereby permitting more efficient mononuclear cell infiltration.
Strategies to Visualize Antigen-Specific T Cell Trafficking and Engagement in the CNS
We recently developed methodologies to visualize in vivo interactions between antigen-specific CD8 T cells and LCMV-infected targets that will allow analysis of molecules required for cell-cell interactions during the pathogenesis of a disease that is entirely dependent on these interactions. These methodologies can also be used to visualize cellular immune interactions in vivo and to address immunologic questions that to date have only been approachable in vitro.
Since its inception in the early 1940s [50], immunocytochemistry has stood the test of time and allowed the visualization of antigens through the use of antigen-antibody interactions. Progress in immunocytochemical methods and microscopy has significantly advanced both life and biomedical sciences by allowing researchers to identify, phenotype, and quantify a multitude of molecules and cell populations in anatomically intact tissues of interest. In the field of cellular immunology, immunocytochemical techniques are now routinely used to detect various cell populations involved in immune function. For example, it is possible to identify the T lymphocyte populations involved in an inflammatory disease of the CNS (e.g., that induced by an intracerebral LCMV infection) through the use of cell type-specific antibodies [40]. While this type of analysis is more sophisticated than a histochemical stain that simply demonstrates the presence of mononuclear cells, it fails to identify the antigen-specific T cells capable of recognizing peptide-MHC complexes and causing immunopathology.
Several strategies have been used to trace specific lymphocytes that traffic to target tissues including the CNS [8], and most involve some in vitro manipulation to tag the cells of interest. For example, in a recent study, encephalitogenic CD4 T cells were transduced with a retrovirus genetically engineered to express green fluorescent protein (GFP) [51]. Upon adoptive transfer of these cells into recipients, the migration and phenotype of GFP-positive myelin-specific T cells were analyzed during the development of experimental autoimmune encephalomyelitis. Another recent study of note evaluated antigen-specific lymphocyte expansion and migration by adoptively transferring ovalbumin (OVA)-specific CD4+ Thy1.1+ T cells into a congenic Thy1.2+ recipient [5]. After mice were challenged with OVA, the whole body distribution of OVA-specific T cells was determined by use of an antibody directed against the congenic Thy1.1 marker. Both studies enhanced our understanding of T cell immunity in vivo and provided the foundation for novel strategies to track antigen-specific immune responses.
Because the aforementioned studies utilized either T cell clones [51] or T cell receptor (TCR)-transgenic (tg) cells [5] for tracing studies, it is important to develop strategies to visualize physiologic immune responses in an unmanipulated host. Recently Altman et al. [52] made the seminal observation that by multimerizing the peptide-MHC ligand for a virus-specific CD8 T cell population, soluble peptide-MHC tetramers could be generated. MHC class I tetramers are generated by tethering 4 peptide-loaded class I molecules to a streptavidin core. MHC tetramers can be used to label antigen-specific T cells because of the enhanced binding avidity this reagent has for the TCR.
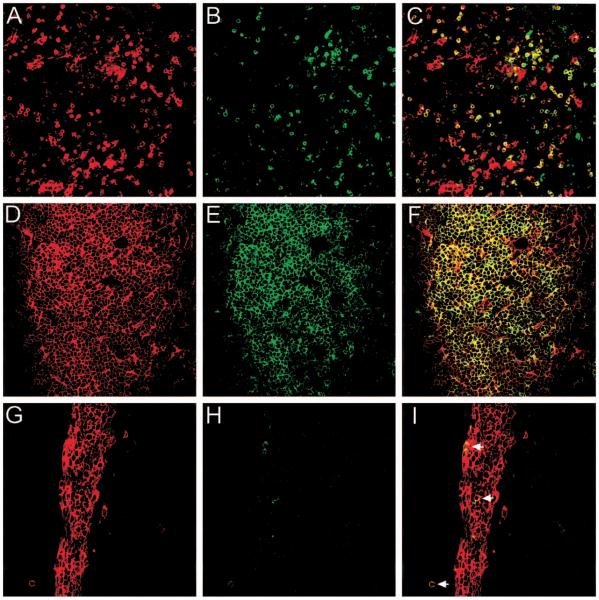
To date MHC tetramers have been used primarily to stain antigen-specific T cells in suspension, but two recent reports demonstrate an elegant strategy to visualize antigen-specific CD8 T cell responses in situ [53, 54]. With the use of class I MHC tetramers, both studies visualized antigen-specific CD8 T cells in anatomically intact unfixed tissue sections. The advantage of this technique is that the polyclonal antigen-specific immune response (regardless of TCR usage) can be analyzed in an unmanipulated host. Both studies relied heavily on the use of 200-mm unfixed vibratome sections [53, 54], which in our experience result in anatomic distortions (figure 1A–1C).
Figure 1.
Visualization of H-2Db-restricted GP33–41-specific CD8 T cells by use of in situ major histocompatibility class (MHC) I tetramer staining. MHC class I tetramers containing the GP33–41 peptide of lymphocytic choriomeningitis virus (LCMV) conjugated to the fluorophore allophycocyanin were used to visualize antigen-specific T cells (green) in tissue sections from GP33–41 T cell receptor transgenic mice (TCR-tg) (A–F) or B6 mice infected intracerebrally with LCMV Armstrong (G–I). Sections were colabeled with an anti-CD8 antibody (red) and analyzed by confocal microscopy. Tetramer-positive cells were easily visualized in 200-mm unfixed vibratome sections from the spleen of a naive TCR-tg mouse (A–C). However, when compared with 6-mm frozen sections (D–F), significant distortions in the anatomy were observed. A–F were captured in splenic white pulp. Each ring of staining represents a single cell; overlapping fluorescence between tetramer and CD8 cells appears in yellow. Separation between individual cells was observed with vibratome sections. At day 6 after an intracerebral infection with LCMV, GP33–41-specific CD8 T cells were visualized and quantified in CNS meninges, choroid plexus, and ependyma. G–I, Virus-specific cells (arrows) in dense meningeal infiltrate. Quantitative analyses revealed that during the terminal phase of disease, 4.5% of CNS CD8 T cells were GP33–41 specific. Comparable results were obtained by flow cytometry. This percentage reflects only 1 of 7 possible virus-specific CD8 T cell populations that expand after LCMV infection of C57BL/6 mice. Thus, the total percentage of virus-specific cells in the CNS is likely higher.
We recently improved the methodology to allow robust and specific staining of antigen-specific CD8 T cells on 6-mm frozen sections (figure 1D–1F). Because of the preserved anatomy observed with frozen sections, it is possible to analyze interactions between antigen-specific T cells and virus-infected targets in situ. In fact, we recently used in situ tetramer staining to visualize TCR by focusing at the interface between an antigen-specific CD8 T cell and an LCMV-infected target in the CNS (unpublished data), which likely represents in vivo “immunologic synapse” formation [55]. We also used this technique to visualize and quantify the frequency of virus-specific CD8 T cells present in the CNS during the development of the lethal immunopathologic meningitis induced by LCMV (figure 1G–1I). In situ tetramer staining should significantly advance our understanding of antigen-specific T cell–induced diseases by enabling researchers to distinguish these cells from bystanders that have an irrelevant specificity. Figure 1G illustrates a dense meningeal infiltrate that is CD8+. From this illustration it is impossible to determine which T cells are capable of recognizing virus-infected targets and of causing disease. However, with the addition of the tetramer stain shown in figure 1H, it becomes possible to identify these cells among a sea of potential bystanders (figure 1I).
In situ tetramer staining provides a very powerful approach for analysis of antigen-specific T cells involved in purging a host of a pathogen or for development of an autoimmune disease. However, one important caveat is that staining relies on detection of the antigen-specific TCR, which is in part internalized following engagement with a target displaying the appropriate peptide-MHC complex. Thus, it is likely that the sensitivity of the technique may decline as T cells traffic to tissues containing abundant target cells. A solution to this problem is a unique methodology we developed to visualize a virus-specific T cell population that does not depend on labeling the antigen-specific TCR (unpublished data). To this end we crossed C57BL/6-TgN(ACTbEGFP)10sb mice [56] (mice in which GFP is expressed under the actin promoter) with a TCR-tg mouse strain in which about 90% of the CD8 T cells recognize the glycoprotein (GP33–41) of LCMV [57]. GFP+/CD8+ GP33–41-specific T cells are isolated from F1 mice, and 105 of these cells are transferred intravenously into naive MHC-compatible recipients. After intracerebral infection of recipients with LCMV, the virus-specific GFP-positive cells expand massively within the spleen, traffic to sites of viral infection in the CNS (figure 2), and induce an immunopathologically mediated lethal meningitis.
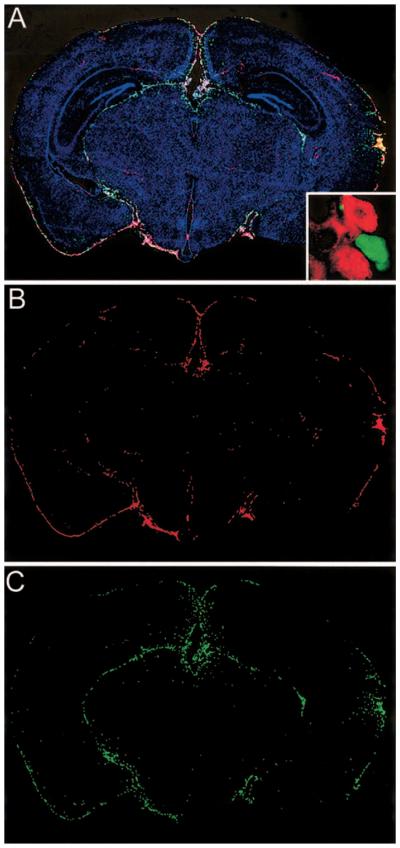
Figure 2.
Novel strategy to trace antigen-specific T cells in vivo. At day 3 after intravenous transfer of 105 green fluorescent protein (GFP)/ GP33–41-specific T cells, mice were challenged intracerebrally with lymphocytic choriomeningitis virus (LCMV) Armstrong. Coronal brain sections were reconstructed at postinfection day 5 to visualize the distribution of infiltrating GFP-positive effector cells in relation to LCMV-infected targets (A–C). Overlay of nuclei (blue), LCMV (red), and GFP-positive effectors (green) was made with 3-color immunofluorescence and image analysis software. Overlay (A) shows that GFP-positive effectors (C) localize primarily to sites of LCMV infection (B). Inset (A) shows resolution achieved with high-resolution 2D microscopy. Individual GP33–41-specific CD8 T cells (green) are seen interacting with LCMV-infected cells (red) in the CNS. These interactions are likely responsible for lethal disease.
By use of high-resolution 2D microscopy we visualized the plasma membrane distribution of molecules involved in attachment (LFA-1), signaling (Lck), and lytic activity (perforin) on GFP-positive effector cells that had engaged LCMV-infected targets in the CNS. These studies define the molecular requirements for antigen-specific CD8 T cell engagement in vivo and provide additional insights into the mechanisms involved in a fatal CNS immunopathology. Because of the relative ease with which this system can be established, it should greatly facilitate analysis of antigen-specific T cell activation, migration, engagement, memory, and other defining elements of adaptive immunity.
Conclusion
Although T cells are associated with pathogenesis in a multitude of experimental systems and in human disease, the precise mechanisms by which CD8 and CD4 T cells participate in control of CNS viral infections without causing “collateral damage” remain poorly defined. Recent advances in methodologies to visualize, quantify, and phenotype antigen-specific T cells in situ will open new avenues of study and allow in-depth analysis of multifaceted adaptive immune responses. These methodologies when used in combination with high-resolution microscopy will also permit detailed mapping of the molecular anatomy of antigen-specific T cell–target cell interactions. These contemporary analyses should contribute significantly to our understanding of adaptive immunity, autoimmune disease, vaccination, and immunocytotherapy. These tools will also aid in clarifying how T cells operate under the unique constraints exerted in the CNS microenvironment. An enhanced understanding of the precise factors that give rise to a successful immune response will ultimately permit more efficacious therapeutic interventions, which are required to avoid the functional alterations associated with the extended presence of virus in various organs.
References
- 1.Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MB. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 2.Borrow P, Oldstone MBA. Lymphocytic choriomeningitis virus. In: Nathanson N, editor. Viral pathogenesis. Lippincott-Raven; Philadelphia: 1997. pp. 593–67. [Google Scholar]
- 3.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 5.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 6.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 7.Marshall DR, Turner SJ, Belz GT, et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98:6313–8. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flugel A, Bradl M. New tools to trace populations of inflammatory cells in the CNS. Glia. 2001;36:125–36. doi: 10.1002/glia.1102. [DOI] [PubMed] [Google Scholar]
- 9.Gilden DH, Cole GA, Monjan AA, Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. I. Cyclophosphamide-mediated induction by the virus-carrier state in adult mice. J Exp Med. 1972;135:860–73. doi: 10.1084/jem.135.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole GA, Gilden DH, Monjan AA, Nathanson N. Lymphocytic choriomeningitis virus: pathogenesis of acute central nervous system disease. Fed Proc. 1971;30:1831–41. [PubMed] [Google Scholar]
- 11.Mims CA, Tosolini FA. Pathogenesis of lesions in lymphoid tissue of mice infected with lymphocytic choriomeningitis (LCM) virus. Br J Exp Pathol. 1969;50:584–92. [PMC free article] [PubMed] [Google Scholar]
- 12.Mims CA. Intracerebral injections and the growth of viruses in the mouse brain. Br J Exp Pathol. 1960;41:52–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Hotchin J, Weigand H. The effects of pretreatment with x-rays on the pathogenesis of lymphocytic choriomeningitis in mice. I. Host survival, virus multiplication and leukocytosis. J Immunol. 1961;87:675–81. [PubMed] [Google Scholar]
- 14.Rowe WP, Black PH, Levey RH. Protective effect of neonatal thymectomy on mouse LCM infection. Proc Soc Exp Biol Med. 1963;114:248–51. doi: 10.3181/00379727-114-28643. [DOI] [PubMed] [Google Scholar]
- 15.Cole GA, Nathanson N, Prendergast RA. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus–induced central nervous system disease. Nature. 1972;238:335–7. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- 16.Dixon JE, Allan JE, Doherty PC. The acute inflammatory process in murine lymphocytic choriomeningitis is dependent on Lyt-2+ immune T cells. Cell Immunol. 1987;107:8–14. doi: 10.1016/0008-8749(87)90260-7. [DOI] [PubMed] [Google Scholar]
- 17.Doherty PC, Allan JE, Ceredig R. Contributions of host and donor T cells to the inflammatory process in murine lymphocytic choriomeningitis. Cell Immunol. 1988;116:475–81. doi: 10.1016/0008-8749(88)90246-8. [DOI] [PubMed] [Google Scholar]
- 18.Moskophidis D, Cobbold SP, Waldmann H, Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus–infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987;61:1867–74. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leist TP, Cobbold SP, Waldmann H, Aguet M, Zinkernagel RM. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987;138:2278–81. [PubMed] [Google Scholar]
- 20.Johnson ED, Monjan AA, Morse HC., III Lack of B-cell participation in acute lymphocyte choriomeningitis disease of the central nervous system. Cell Immunol. 1978;36:143–50. doi: 10.1016/0008-8749(78)90257-5. [DOI] [PubMed] [Google Scholar]
- 21.Allan JE, Doherty PC. Natural killer cells contribute to inflammation but do not appear to be essential for the induction of clinical lymphocytic choriomeningitis. Scand J Immunol. 1986;24:153–62. doi: 10.1111/j.1365-3083.1986.tb02081.x. [DOI] [PubMed] [Google Scholar]
- 22.Baenziger J, Hengartner H, Zinkernagel RM, Cole GA. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur J Immunol. 1986;16:387–93. doi: 10.1002/eji.1830160413. [DOI] [PubMed] [Google Scholar]
- 23.Klavinskis LS, Tishon A, Oldstone MB. Efficiency and effectiveness of cloned virus-specific cytotoxic T lymphocytes in vivo. J Immunol. 1989;143:2013–6. [PubMed] [Google Scholar]
- 24.Joly E, Mucke L, Oldstone MB. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–5. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- 25.Fung-Leung WP, Kundig TM, Zinkernagel RM, Mak TW. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425–9. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn DG, Zajac AJ, Frelinger JA, Muller D. Transfer of lymphocytic choriomeningitis disease in beta 2-microglobulin-deficient mice by CD4+ T cells. Int Immunol. 1993;5:1193–8. doi: 10.1093/intimm/5.10.1193. [DOI] [PubMed] [Google Scholar]
- 27.Zajac AJ, Quinn DG, Cohen PL, Frelinger JA. Fas-dependent CD4+ cytotoxic T-cell–mediated pathogenesis during virus infection. Proc Nat Acad Sci USA. 1996;93:14730–5. doi: 10.1073/pnas.93.25.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagi D, Ledermann B, Burki K, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–7. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 29.Kappes DJ, Lawrence DM, Vaughn MM, Dave VP, Belman AR, Rall GF. Protection of CD3 delta knockout mice from lymphocytic choriomeningitis virus–induced immunopathology: implications for viral neuroinvasion. Virology. 2000;269:248–56. doi: 10.1006/viro.2000.0224. [DOI] [PubMed] [Google Scholar]
- 30.Zinkernagel RM, Pfau CJ, Hengartner H, Althage A. Susceptibility to murine lymphocytic choriomeningitis maps to class I MHC genes—a model for MHC/disease associations. Nature. 1985;316:814–7. doi: 10.1038/316814a0. [DOI] [PubMed] [Google Scholar]
- 31.Doherty PC, Allan JE. Role of the major histocompatibility complex in targeting effector T cells into a site of virus infection. Eur J Immunol. 1986;16:1237–42. doi: 10.1002/eji.1830161009. [DOI] [PubMed] [Google Scholar]
- 32.Doherty PC, Allan JE. Differential effect of hybrid resistance on the localization of virus-immune effector T cells to spleen and brain. Immunogenetics. 1986;24:409–15. doi: 10.1007/BF00377960. [DOI] [PubMed] [Google Scholar]
- 33.Doherty PC, Allan JE, Lynch F, Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990;11:55–9. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- 34.Allan JE, Dixon JE, Doherty PC. Nature of the inflammatory process in the central nervous system of mice infected with lymphocytic choriomeningitis virus. Curr Top Microbiol Immunol. 1987;134:131–43. doi: 10.1007/978-3-642-71726-0_6. [DOI] [PubMed] [Google Scholar]
- 35.Wekerle H, Linington C, Lassmann H, Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–7. [Google Scholar]
- 36.Cserr HF, DePasquale M, Harling-Berg CJ, Park JT, Knopf PM. Afferent and efferent arms of the humoral immune response to CSF-administered albumins in a rat model with normal blood-brain barrier permeability. J Neuroimmunol. 1992;41:195–202. doi: 10.1016/0165-5728(92)90070-2. [DOI] [PubMed] [Google Scholar]
- 37.Lynch F, Doherty PC, Ceredig R. Phenotypic and functional analysis of the cellular response in regional lymphoid tissue during an acute virus infection. J Immunol. 1989;142:3592–8. [PubMed] [Google Scholar]
- 38.Ceredig R, Allan JE, Tabi Z, Lynch F, Doherty PC. Phenotypic analysis of the inflammatory exudate in murine lymphocytic choriomeningitis. J Exp Med. 1987;165:1539–51. doi: 10.1084/jem.165.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nansen A, Marker O, Bartholdy C, Thomsen AR. CCR2+ and CCR5+ CD8+ T cells increase during viral infection and migrate to sites of infection. Eur J Immunol. 2000;30:1797–806. doi: 10.1002/1521-4141(200007)30:7<1797::AID-IMMU1797>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 40.Marker O, Scheynius A, Christensen JP, Thomsen AR. Virus-activated T cells regulate expression of adhesion molecules on endothelial cells in sites of infection. J Neuroimmunol. 1995;62:35–42. doi: 10.1016/0165-5728(95)00099-n. [DOI] [PubMed] [Google Scholar]
- 41.Andersson EC, Christensen JP, Scheynius A, Marker O, Thomsen AR. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand J Immunol. 1995;42:110–8. doi: 10.1111/j.1365-3083.1995.tb03633.x. [DOI] [PubMed] [Google Scholar]
- 42.Christensen JP, Andersson EC, Scheynius A, Marker O, Thomsen AR. Alpha 4 integrin directs virus-activated CD8+ T cells to sites of infection. J Immunol. 1995;154:5293–301. [PubMed] [Google Scholar]
- 43.Nielsen HV, Christensen JP, Andersson EC, Marker O, Thomsen AR. Expression of type 3 complement receptor on activated CD8+ T cells facilitates homing to inflammatory sites. J Immunol. 1994;153:2021–8. [PubMed] [Google Scholar]
- 44.Andersson EC, Christensen JP, Marker O, Thomsen AR. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–45. [PubMed] [Google Scholar]
- 45.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu Y, Newman W, Tanaka Y, Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992;13:106–12. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- 47.De Groot CJ, Woodroofe MN. The role of chemokines and chemokine receptors in CNS inflammation. Prog Brain Res. 2001;132:533–44. doi: 10.1016/s0079-6123(01)32101-5. [DOI] [PubMed] [Google Scholar]
- 48.Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57–110. doi: 10.1016/s0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- 49.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 50.Coons AH, Creech HL, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200–2. [Google Scholar]
- 51.Flugel A, Berkowicz T, Ritter T, et al. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–60. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 52.Altman JD, Moss PH, Goulder PR, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 53.Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: In situ tetramer staining of antigen-specific T cells in tissues. J Immunol. 2000;165:613–7. doi: 10.4049/jimmunol.165.2.613. [DOI] [PubMed] [Google Scholar]
- 54.Haanen JB, van OM, Tirion F, et al. In situ detection of virus- and tumor-specific T-cell immunity. Nat Med. 2000;6:1056–60. doi: 10.1038/79573. [DOI] [PubMed] [Google Scholar]
- 55.Krummel MF, Davis MM. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr Opin Immunol. 2002;14:66–74. doi: 10.1016/s0952-7915(01)00299-0. [DOI] [PubMed] [Google Scholar]
- 56.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–9. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 57.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–61. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]