Abstract
Background
Several sickle cell clinical trials have closed due to inability to enroll patients. To limit the early cessation of a proposed clinical trial due to low accrual rates, we sought to better understand barriers and facilitators to enrolling parents of children with sickle cell anemia (SCD) into clinical trials.
Procedure
Focus groups (n = 3) were conducted with parents/guardians (n = 14) who had not previously been recruited for a clinical trial and were not administering hydroxyurea to their children.
Results
Three main themes related to barriers to clinical trial enrollment were identified during analysis of focus groups: general barriers to health related research (general mistrust of research studies, emotional and practical concerns), barriers to trial design (randomization), and barriers to hydroxyurea (long term unknown risks, cancer, myelosuppressive effects). Facilitators identified were need for more education, including request for peer education, and improved explanation of clinical trials or study rationale.
Conclusion
Engagement of parents/guardians of children with SCD in identifying barriers and facilitators to clinical trial enrollment may be critical to the development of strategies to enhance SCD trial completion.
Keywords: clinical trials, psychosocial, sickle cell anemia, sickle cell disease
INTRODUCTION
Enrolling patients and successfully completing clinical trials is essential to improving health outcomes. Patients with sickle cell disease (SCD) have benefited from prior clinical trials, reducing the risk of infant death from pneumococcal sepsis, preventing cerebral vascular events, and decreasing clinical complications of the disease [1–4]. Unfortunately, several clinical trials failed to answer their important research question due to inability to enroll patients. Barriers related to recruiting African Americans into clinical trials are not unique to SCD, but as a predominantly African American disease, these barriers may create a disparity in advancing health care for these patients [5–7]. Since 2008, sixteen clinical trials for patients with SCD registered at clinicaltrials.gov were terminated for slow enrollment/inability to meet enrollment goals including five very important NHLBI sponsored/collaborative trials (Table I; www.clinicaltrials.gov). Failure to enroll patients in a clinical trial is costly and could place patients who were enrolled at risk for toxicity without the benefit of completing the study [8].
TABLE I.
NHLBI Sponsored/Collaborative Trials Closed for Slow Enrollment or Poor Accrual Since 2008
| Hydroxyurea and magnesium pidolate to treat people with hemoglobin sickle cell disease |
| Dexamethasone to treat acute chest syndrome in people with sickle cell disease |
| Ketorolac versus ibuprofen to treat painful episodes of sickle cell disease |
| The improve trial: improving pain management and outcomes with various strategies of patient-controlled analgesia (PCA) |
| A study of patients having pulmonary hypertension associated with sickle cell disease and completing an asset study (ASSET-3) |
| 11 Additional non-NHLBI sponsored trials listed on clinical trials.gov closed for slow accrual |
Developing strategies to enhance clinical trial enrollment is vital. In one recent SCD trial closed for slow enrollment, the investigators determined that based on their rate of enrollment, their study would have required 25 clinical sites and 5 years of patient accrual [9]. This costly strategy of increasing clinical sites and enrollment time may not be feasible in the current funding climate. Instead, research must focus on understanding of how to effectively engage the sickle cell community in clinical trials. Exploring the barriers and facilitators to clinical trial enrollment cannot be identified through quantitative research alone. In contrast, qualitative research allows investigators to understand these issues from the perspective of the target audience so that interventions can be developed that specifically target families’ concerns from a socio-cultural perspective [10].
The silent infarct transfusion (SIT) trial is determining the efficacy of blood transfusion therapy for preventing recurrent brain injury in children with a silent cerebral infarct (SCI). At the completion of the SIT trial, a trial (SIT2) is planned to determine the efficacy of hydroxyurea to prevent recurrent brain injury in patients with SCI. A pilot trial using hydroxyurea (SIT2 Feasibility Trial) is recruiting patients to collect background data on hydroxyurea for secondary SCI prevention. We postulate that understanding and addressing barriers prior to the start of a definitive clinical trial will maximize study accrual, often the rate limiting steps for clinical trial. Therefore, one aim of the SIT2 Feasibility Trial was to identify facilitators and barriers to enrollment in clinical trials of hydroxyurea. Our overall goal is to develop culturally relevant recruitment and retention strategies for all SCD clinical trials based on the input from African American parents/guardians of infants and children with SCD.
METHODS
Three focus groups were conducted with parents or guardians of children with no prior experience with clinical trials or hydroxyurea therapy (n = 14 parents). All participants were African Americans recruited during well child sickle cell clinic visits; three were male and 11 were female. The participants included two sets of parents (mother and father) and one mother/grandmother. Recorded demographic information included an average age of participants of 42 (31–56 years) and two thirds of participants attended school beyond high school. The overall goal of the focus groups was to better understand the parent perspective on enrollment in clinical trials, using a mock recruitment presentation of a feasibility trial of hydroxyurea for prevention of secondary silent cerebral infarcts (SIT2 Feasibility Trial) as a model to generate discussion. Purposeful sampling for the focus groups was conducted as the goal was to obtain parents/guardians of children not on hydroxyurea nor previously approached for clinical trial participation.
During well child sickle cell clinic visits, parents eligible for the focus groups received a list of the seven main questions that would be addressed during the focus group and a mock recruitment pamphlet explaining the SIT2 Feasibility Trial. A study coordinator for the SIT2 Feasibility Trial followed up by telephone providing time and date of the focus groups. Informed consent for focus group participation was signed on the night of the focus group and a demographic questionnaire completed. A PI of the SIT2 Feasibility Trial reviewed the trial pamphlet with parents prior to the focus group and answered all questions about the trial and hydroxyurea, similar to the recruitment process for eligible patients. In order to avoid bias or influence of the PI, once all questions were answered, the PI left the room and the moderator conducted the focus groups Focus groups lasted 60–90 min and were video recorded for accurate transcription. The focus group was lead by a trained African American female moderator.
Focus group transcripts were analyzed using a six stage iterative process [11–13]. (1) Coding: two researchers independently read and identified all codes (Lebensburger and Scarinci) in the transcript of focus groups/interviews. (2) Category development: researchers developed categories to place codes. (3) Constant comparison: each new segment of data (2nd/3rd focus group transcripts) was independently coded and compared to existing codes and categories, allowing for new relationships to be discovered. (4) Data saturation and categorization: time at which no new codes or categories were generated during data analysis (Supplemental Table I). Specifically, no new categories or themes were identified during the 3rd focus group so that identified categories were (1) exhaustive, (2) purposeful, (3) mutually exclusive, and (4) conceptually congruent. (5) Development of themes: researchers placed categories into relevant themes within the theoretical framework. (6) Establishment of validity and reliability: the independent coding from the two researchers were compared and contrasted for validity and reliability [13].
RESULTS
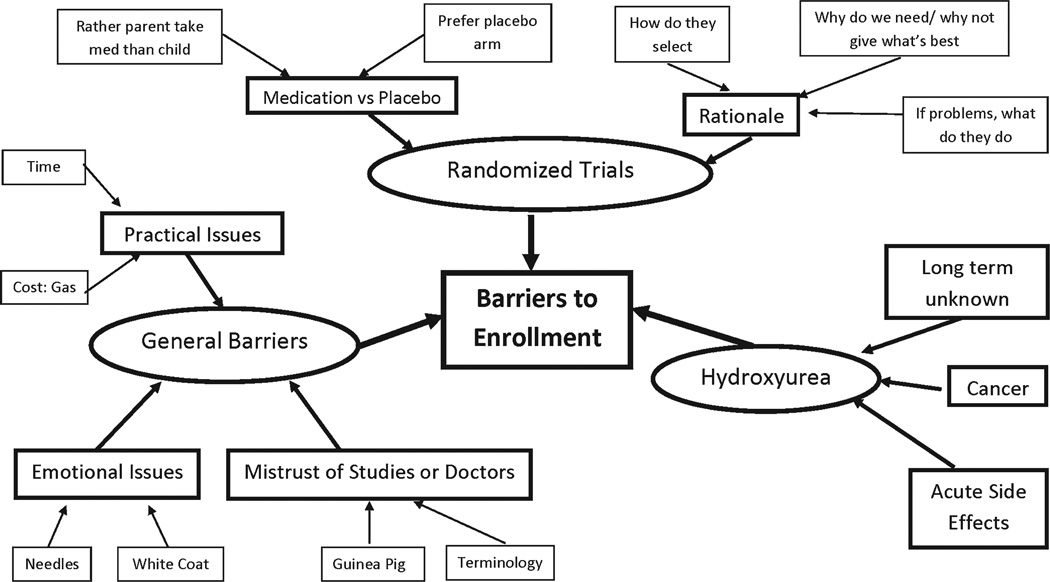
Fourteen African American parents or guardians of children with sickle cell anemia attended three focus group sessions. Three main themes emerged that related to barriers for these parents/ guardians to participate in clinical trials (Fig. 1): participation in health related research in general, trial design (randomization), and hydroxyurea.
Fig. 1.
Barriers to enrollment identified during focus groups. Circles represent the three themes. Large boxes represent categories. Smaller boxes represent subcategories.
Participation in Health Related Research
The most discussed theme identified barriers to study participation in general (Table II). The largest category within this first theme focused on general mistrust of research studies. An overall mistrust of trials/research was expressed by participants including, “Basically whenever you say research or clinical trials, I cringe. I don’t want my child to be poked and pricked or be the guinea pig.” Other parents stated “I just wouldn’t want (my child) to go in that situation” and “If it were me I would do it to help but it’s my child and I am supposed to protect him.” Most participants had a negative attitude towards the term ‘research’ with statements “If they use research, I’m like no” and “with research, I always picture an animal and (I) cringe.” While the term ‘clinical trial’ was described as “more subtle,” one participant stated that “the phrase clinical trial makes me think of the Tuskegee experiment.”
TABLE II.
Barriers Identified During Focus Groups and Possible Interventions
| Barrier theme | Category | Code and Possible intervention |
|---|---|---|
| General barriers to health related research |
General mistrust of studies |
Code: “With research, I always picture an animal, of course, and it puts a little cringe in your body when you hear that term” |
| Possible intervention: peer support groups including parents with previous experience enrolling their children in a SCD trial. “As much as we love our doctors, it’s the connection with other parents” | ||
| Emotional Issues |
Code: “If he sees someone in a white coat coming at him he asks them if they’re going to hurt him” |
|
| Possible intervention: discuss closer relationship with doctors and SCD staff. May overcome fears with frequent visits with same SCD staff members |
||
| Barriers to trial design including randomization |
Rationale | Code: “What I don’t understand is that if this medicine is (helpful) then why is a trial needed? |
| Possible intervention: discuss rationale for the study and why the research question is important | ||
| Design | Code: “Is there an out when you’re in a study? That would be my concern” | |
| Possible intervention: stress that patient will be treated as an individual and can be removed by parents or doctor request if complications occur on trial |
||
| Medication versus placebo |
Code “I think I would be interested in entering my child in a study or trial if she was in the group that didn’t have to take the medication. I don’t think I would be interested if it was the other way around” OR |
|
| “If it were my child I would want him to have the real thing that could help him” |
||
| Possible intervention: discuss benefits of randomized trial to improve under- standing of disease for all patients. Include prior history of trials that improved current care (altruism) |
||
| Barriers to hydroxyurea |
Unknown | Code: “That’s my biggest hesitant. He’s fine now but how is it going to affect him long term” |
| Intervention: discuss long term “unknown” effects which may underlie barriers |
||
| Cancer | Code: “As of now my child doesn’t have cancer but what about when my child is older and develops cancer cells and it is somehow related to HU” |
|
| Possible intervention: discuss prevalence of cancer among patients on/off hydroxyurea |
A second barrier category involved emotional issues that parents expressed when considering enrolling their child in a clinical trial. A few parents expressed a benefit of having increased clinic visits but several discussed the negative impact of clinic visits on their child. “She has enough with needles so I don’t want to put her through anymore than what she has to go through” or “the wait and anxiousness of having lab work done … he connects going to the hospital with getting stuck with needles and so you have to deal with the dramatic, emotional distress.”
Finally, participants discussed practical issues of enrolling in a clinical trial, focusing on the time required for clinic visits, cost of gas, and concerns for missing work. One parent described her concern for extra clinic visits as “who has time to be running back and forth”. Another parent was concerned about missing work, suggesting “I think a good thing would be to have after hour (study visits).”
Trial Design
The second major theme centered on the rationale and concerns regarding trial design with most concerns being specifically related to randomization. Several parents expressed displeasure in enrolling in a randomized trial as they believed that the physician would no longer treat their child as a patient and not make treatment decisions based on their child’s best interest. “I feel like they should scratch out randomized and sit down and think would this medicine help this child” or “If I am agreeing to a clinical trial, it’s because he needs it and I don’t want to risk that he doesn’t get the treatment.” Other participants wanted assurances that if their child had problems during the study, they would be removed and treated with the appropriate medications. Overall, no parent expressed an understanding for the equipoise of a clinical trial, instead believing that the doctor should know how to treat their child without having to participate in a trial.
Participants were split in the opinions of a placebo trial. Parents were interested in helping clinical trials but would not want to be randomized to the study drug (hydroxyurea). “I think I would be interested in entering my child in a study if she was in the group that didn’t have to take the medication. I don’t think I would be interested if it was the other way around. However, a few participants viewed being randomized to placebo would “be a waste of my time.”
Hydroxyurea
The final theme involved issues related to hydroxyurea therapy. The overwhelming barrier was the long term unknown risks, with a lesser concern of acute toxicity. Participants stated, “I’m concerned with the long term effects, that’s my biggest hesitancy” or “It sounds good now but how will it affect my child when he’s older… that would weigh on me.” Some participants expressed concerns about cancer and a few about the potential myelosuppressive effects of hydroxyurea. “As of now my child doesn’t have cancer but what about when my child is older and develops cancer cells and it is somehow related to HU” or “I did read something on the internet that said something about leukemia.”
Facilitators
Facilitators to clinical trial enrollment were also identified, and the clear breakdown in major themes identified with regard to barriers was not observed with regard to facilitators. In response to general fears about trial participation, parents expressed a desire to learn about how the clinical trial would improve their child’s life. Participants expressed a desire to discuss clinical trial enrollment with other families who were on hydroxyurea or enrolled in a clinical trial. “I’m really looking to connect with others who are walking the walk … it’s a matter of connecting with individuals who I can sit down with… As much as we love our doctors, it’s the connection with other parents (that’s important).”
While several parents viewed the time for extra clinic visits as a barrier, facilitators were also identified, particularly in regards to being monitored closely for side effects. Several participants expressed a desire to learn more about clinical trials and believed that more education was needed for parents. “I want more information. Why is this medicine chosen? Who did the research to say this medicine is going to help my child.” Another participant echoed this sentiment, “If you give me more information up front, I’m going to be more likely to participate.” Finally, participants expressed that researchers should make parents fully aware of their rights on trial and how they will be treated if their child has increased complications of their disease. “The doctor may know all along that we have the right to get out of this but unless you tell me, how am I going to know.” Overall, when specifically asked about which SCD team member should present the trial, participants desired that a doctor presents the trial rather than a research nurse or coordinator, but opinion was split if they wanted the doctor who was serving as PI or their primary doctor.
DISCUSSION
This study qualitatively explored barriers and facilitators to enrolling patients in a sickle cell clinical trial from the caregiver perspective during the pilot phase of a proposed clinical trial. While limited data exist about barriers to enrollment from the parent perspective, prior clinical trials have addressed barriers from the perspective of trial design and site recruitment capabilities. Acute intervention sickle cell trials have had difficulty enrolling patients leading to early study closure. One acute intervention trial that closed for slow enrollment required randomization within 24 h of the diagnosis of acute chest syndrome and required administration of oral therapy within 2 h of randomization [9]. Although the enrollment target was 112, only 12 participants were enrolled from only 5 of 10 sites over a 2 year period; consequently, this study was closed. The researchers postulated that slow enrollment was due to the challenge of acutely identifying and enrolling patients since acute chest syndrome was an unplanned event but no information was provided about parental barriers [9]. A second acute intervention trial evaluated the role of early transfusion to prevent acute chest syndrome in patients with vaso-occlusive pain and elevated serum markers [14]. Of the 420 eligible patients, only 10 participants were randomized. The researchers speculated that difficulty with staffing at sites and time constraints for enrollment played a role in poor accrual. No data on parental reasons for declining enrollment was provided. Finally an acute pain intervention study identified 1,116 potential participants with pain encounters at 31 centers, yet enrolled only 38 subjects [15]. The researchers surveyed their sites to determine barriers and identified a short enrollment period, protocol design, competing protocols, and limited research staff among barriers, but no mention was made of attitudes or beliefs of parents.
Outpatient preventative clinical trials of hydroxyurea that do not require immediate enrollment decision have had better success than acute intervention trials. In the BABY HUG randomized controlled trial of hydroxyurea, 796 parents were approached to participate but 487 (61% declined) [16]. Study coordinators recorded reasons for rejection, but this involved selecting barriers from several predefined options that were derived from research staff insights [17]. Within this limitation, parents that refused enrollment cited too many clinic visits, fear of research, lack of perception of their child being ill and unwillingness to be randomized to placebo as reasons for not enrolling their child. In addition, only 234 (76%) of the 309 parents who initially stated they wanted to participate actually signed consent. In the SWiTCH trial, patients receiving chronic blood transfusions for stroke had the opportunity to participate in a randomized trial of hydroxyurea [18]. Both parent and physician barriers diminished enrollment as 15% of parents screened declined participation and 5% of investigators declined enrolling screened patients. The reasons that parents declined have not been published.
Finally, one trial (SIT Trial) enrolled patients with a newly diagnosed SCI. During the SIT Trial, education pamphlets were used to educate parents about the importance of knowing about silent infarcts. This education strategy may have influenced the excellent acceptance rate of parents (85%) for agreeing to a screening MRI for their children [19]. However, nearly 25% of parents did not consent to the trial, despite knowing that their child had a newly identified SCI. The reasons that parents rejected the SIT Trial are not published.
In our study, parents identified a number of specific barriers and facilitators to participation in clinical trials in the context of a concrete example of a hydroxyurea trial. Our results are similar to results among African American adults in oncology therapeutic trials. Mistrust of research and concerns about being a “guinea pig” are frequent themes among adult African American adult cancer patients [6,20]. Also, parents had expressed concerns regarding the randomization nature of most studies. Although parents expressed an overall negative initial opinion about clinical trial participation, several participants addressed the importance of discussing clinical trials with their peers, rather than a physician, may improve their initial distrust of studies. Of interest, some parents who initially commented they had no interest participating in a clinical trial at the beginning of the focus group asked for more information about SIT2 Feasibility trial at the end of the focus group, despite their child not being eligible for the trial (no proven silent infarct). Once mistrust is overcome, parents report needing more information and general knowledge about clinical trials. Parents need specific reassurances that their child would still be treated as an individual rather than a trial participant, including removing their child from a trial if needed. Finally, trials of hydroxyurea must address the underlying concern of many of these parents/guardians about unknown long-term side effects. While it is vital that informed consent focuses on acute, known complications of hydroxyurea, investigators must also discuss the unknown effects that were identified as a major barrier. To overcome these issues, some parents may want education about safety of hydroxyurea in infancy or prolonged survival in adults while others may desire an opportunity to discuss hydroxyurea with peers.
This study has some limitations that deserve mention. Focus groups were intended to include eight participants for each session but attendance was lower, reducing the diversity of the sample population and introducing the possibility of response bias. Despite this lower turn-out, data saturation was obtained as no new categories or themes emerged during the third focus group, the stopping point in data collection in qualitative research. Inherent to qualitative research, the themes and categories developed during this study reflect the opinion of parents/guardians who actively attended a night time focus group and may not reflect the general sickle cell population. Since demographic data was collected after informed consent, identifying differences in parent populations that attended or declined focus groups is not known. The qualitative categories and themes identified in these focus groups were similar to issues identified during the national quantitative BABY HUG recruitment which suggests these results could be generalizable [17].
Despite its limitations, this study makes two important contributions in the development of recruitment strategies of African American children with sickle cell anemia in clinical trials. First, it provides specific information on barriers and facilitators to participation in clinical trials from the parent perspective. Second, participants reacted to a concrete and real clinical trial (hydroxyurea) rather than a hypothetical situation or clinical trials in general. The themes identified in these focus groups should be considered by investigators during the design of clinical trials, rather than collected during a study. Without evidence that physician trust is increasing, future research should continue to focus on innovation in trial methodologies that incorporate patient opinions to enhance trial enrollment.
Supplementary Material
Acknowledgments
Grant sponsor: Kaul Pediatric Research Institute grant from University of Alabama at Birmingham, Department of Pediatrics.
The authors would like to thank Latasha Carson, LGSW for her effort as focus group moderator, Mary Jones, RN and Kim Threadgill for coordinating focus groups, and Stacy Smith for transcription.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Falletta JM, Woods GM, Verter JI, et al. Discontinuing penicillin prophylaxis in children with sickle cell anemia. Prophylactic Penicillin Study II. J Pediatr. 1995;127:685–690. doi: 10.1016/s0022-3476(95)70154-0. [DOI] [PubMed] [Google Scholar]
- 2.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 3.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 4.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 5.Penberthy L, Brown R, Wilson-Genderson M, et al. Barriers to therapeutic clinical trials enrollment: Differences between African-American and White cancer patients identified at the time of eligibility assessment. Clin Trials. 2012;9:788–799. doi: 10.1177/1740774512458992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials: A pilot study. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 7.Braunstein JB, Sherber NS, Schulman SP, et al. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore) 2008;87:1–9. doi: 10.1097/MD.0b013e3181625d78. [DOI] [PubMed] [Google Scholar]
- 8.Kitterman DR, Cheng SK, Dilts DM, et al. The prevalence and economic impact of low-enrolling clinical studies at an academic medical center. Acad Med. 2011;86:1360–1366. doi: 10.1097/ACM.0b013e3182306440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn CT, Stuart MJ, Kesler K, et al. Tapered oral dexamethasone for the acute chest syndrome of sickle cell disease. Br J Haematol. 2011;155:263–267. doi: 10.1111/j.1365-2141.2011.08827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarinci IC, Bandura L, Hidalgo B, et al. Development of a theory-based (PEN-3 and health belief model), culturally relevant intervention on cervical cancer prevention among Latina immigrants using intervention mapping. Health Promot Pract. 2012;13:29–40. doi: 10.1177/1524839910366416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merriam SB. Qualitative research: A guide to design and implementation. Vol. 2. San Francisco: Jossey-Bass; 2009. pp. 169–207. [Google Scholar]
- 12.Saldana J. The coding manual for qualitative researchers. Thousand Oaks, CA: Sage Publications; 2012. [Google Scholar]
- 13.Creswell JW. Qualitative inquiry & research design: Choosing among five approaches. 2nd. xvii. Thousand Oaks: Sage Publications; 2007. pp. 202–209. [Google Scholar]
- 14.Styles L, Wager CG, Labotka RJ, et al. Refining the value of secretory phospholipase A2 as a predictor of acute chest syndrome in sickle cell disease: Results of a feasibility study (PROACTIVE) Br J Haematol. 2012;157:627–636. doi: 10.1111/j.1365-2141.2012.09105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters-Lawrence MH, Bell MC, Hsu LL, et al. Clinical trial implementation and recruitment: Lessons learned from the early closure of a randomized clinical trial. Contemp Clin Trials. 2012;33:291–297. doi: 10.1016/j.cct.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn L, Miller S, Faughnan L, et al. Recruitment of infants with sickle cell anemia to a Phase III trial: Data from the BABY HUG study. Contemp Clin Trials. 31:558–563. doi: 10.1016/j.cct.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware RE, Schultz WH, Yovetich N, et al. Stroke with transfusions changing to hydroxyurea (SWiTCH): A phase III randomized clinical trial for treatment of children with sickle cell anemia, stroke, and iron overload. Pediatr Blood Cancer. 2011;57:1011–1017. doi: 10.1002/pbc.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: Low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119:3684–3690. doi: 10.1182/blood-2011-05-349621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd GS, Edwards CL, Kelkar VA, et al. Recruiting intergenerational African American males for biomedical research Studies: A major research challenge. J Natl Med Assoc. 2011;103:480–487. doi: 10.1016/s0027-9684(15)30361-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.