Objectives
The treatment of AH is ultimately aimed at reducing CV morbidity and mortality.1-11 Clinical studies of outcome have provided scientific evidence of the benefits of the use of diuretics (DIUs) (GR: I; LE: A),5,10-15 beta-blockers (BBs) (GR: I; LE: A),10-13,16 calcium-channel blockers (CCBs) (GR: I; LE: A),10,11,15,17-23 angiotensin-converting-enzyme inhibitors (ACEIs) (GR: I; LE: A)10,11,15,17,18,24-26 and angiotensin-receptor blockers (ARBs) (GR: I; LE: A).10,11,27-33 It is worth noting that most of those studies have used an association of drugs. Based on the information available, the protection observed does not depend on the type of drug used, but mainly on BP reduction.7,9-11,34 Recent meta-analyses have reported that the benefits obtained from BB are smaller10,11,35-37 as compared to those provided by the other drug groups, and, thus, BBs should be reserved for specific situations. Regarding alpha-blockers and direct vasodilators, there is no effective information on the outcomes of morbidity and mortality. Regarding direct renin inhibitors, only one study of outcome in diabetic patients has been early interrupted due to lack of benefits and possible harm.38 The higher the CV risk, the greater the benefits, which occur even for small BP elevations.3-6,8,9,39
General principles of the pharmacological treatment
When pharmacological treatment is indicated, the patient should be instructed about the importance of its continuity, the occasional need for dose adjustment and change or association of drugs, and the occasional appearance of adverse effects.
For one medicine to be indicated, it should preferably:
have shown the ability to reduce CV morbidity and mortality;
be effective orally;
be well tolerated;
be taken the fewest possible times per day;
be started at the smallest effective doses;
be able to be used in association;
be used for at least four weeks, before any change, except for special situations;
have quality control in its production.
Choice of the medication
All antihypertensive drugs available can be used if specific indications and contraindications are observed (Table 1). The initial preference is always for those with confirmed action in decreasing CV events, being the others reserved for special cases that require the association of multiple drugs to achieve BP targets.
Table 1.
Antihypertensive drugs available
| - DIUs (GR: I; LE: A) |
| - adrenergic inhibitors |
| - Central action – central alpha-2 agonists (GR: IIb; LE: C) |
| - BBs – beta adrenergic blockers (GR: I; LE: A) |
| - Alpha-blockers – alpha-1 adrenergic blockers (GR: IIb; LE: C) |
| - Direct vasodilators (GR: IIb; LE: C) |
| - CCBs (GR: I; LE: A) |
| - ACEIs (GR: I; LE: A) |
| - ARBs (GR: I; LE: A) |
| - Direct renin inhibitors (GR: IIb; LE: C) |
General characteristics of antihypertensive drugs
Diuretics
The mechanisms of antihypertensive action of DIUs are initially related to their natriuretic effects, with a decrease in the extracellular volume. After 4-6 weeks, the circulating volume normalizes and a reduction in peripheral vascular resistance (PVR) occurs. Diuretics reduce BP and CV morbidity and mortality.12,14,15 Their antihypertensive effect is not directly related to their doses, but the side effects are.
Thiazide or similar DIUs (chlorthalidone, hydrochlorothiazide and indapamide) at low doses should be preferred, because they are milder and have a longer time of action. Loop DIUs (furosemide and bumetanide) should be reserved for cases of renal failure (creatinine > 2.0 mg/dL or estimated GFR < 30 mL/min/1.73m2) and edema (HF or renal failure). Potassium-sparing DIUs (spironolactone and amiloride) are usually associated with a thiazide or loop DIU.
Adverse effects
Their major adverse effects are weakness, cramps, hypovolemia and erectile dysfunction. From the metabolic viewpoint, hypopotassemia is the most common, occasionally accompanied by hypomagnesemia, which can induce ventricular arrhythmias, mainly extrasystole. Diuretics can cause glucose intolerance by reducing insulin release, increasing the risk for type 2 DM. Uric acid increase is an almost universal effect of DIUs, of undocumented clinical consequences, except for triggering gout crises in predisposed individuals. The use of low doses decreases the risk for adverse effects, without hindering the antihypertensive efficacy, especially when associated with other drug classes. Spironolactone can cause hyperpotassemia, particularly in patients with impaired renal function.
Central action agents
Alpha-agonists of central action stimulate alpha-2 receptors involved in sympatho-inhibitory mechanisms.40 Not all alpha-agonists of central action are selective. Their well-defined effects are as follows: a decrease in sympathetic activity and reflex of baroreceptors, contributing to relative bradycardia and postural hypotension; mild decrease in PVR and cardiac output; a reduction in serum levels of renin; and fluid retention.
Some representatives of that group are: methyldopa, clonidine, guanabenz and inhibitors of imidazoline receptors (moxonidine and rilmenidine).41
Clonidine can be useful in hypertensive situations associated with: restless legs syndrome,42 withdrawal of opioids,43 menopausal hot flushes,44 diarrhea associated with diabetic neuropathy,45 and sympathetic hyperactivity of patients with alcoholic cirrosis.46 These drugs have no unwanted metabolic effect, because they interfere with neither peripheral resistance to insulin nor lipid profile.
Adverse effects
Methyldopa can cause autoimmune reactions, such as fever, hemolytic anemia, galactorrhea and liver dysfunction, which, in most cases, disappear with use cessation. If an adverse reaction occurs, it can be replaced by another central alpha-agonist.41 Clonidine has a higher risk for the rebound effect with discontinuation, especially when associated with a BB, and can be dangerous in the preoperative period.40 The drugs in this class have adverse reactions due to their central action, such as drowsiness, sedation, dry mouth, fatigue, postural hypotension, and erectile dysfunction.40,41
Beta-blockers
Beta-blockers promote initial decrease in cardiac output and renin secretion, with readaptation of baroreceptors and decrease in catecholamines in nervous synapses.47,48 In addition to such actions, third-generation drugs (carvedilol, nebivolol) have a vasodilating effect via different mechanisms: carvedilol, via concomitant blockade of alpha-1 adrenergic receptor;47-50 and nebivolol, by increasing nitric oxide synthesis and release on the vascular endothelium.47,48,50 Propranolol is useful to patients with essential tremor, hyperkinetic syndromes, vascular headache and portal hypertension.47,48
Adverse effects
They consist of bronchospasm, bradycardia, atrioventricular conduction disorders, peripheral vasoconstriction, insomnia, nightmares, psychic depression, asthenia and sexual dysfunction. First- and second-generation BBs are formally contraindicated to patients with bronchial asthma, chronic obstructive pulmonary disease (COPD) and second- and third-degree atrioventricular blocks. They can cause glucose intolerance, induce new cases of DM, and lead to hypertriglyceridemia with LDL-cholesterol elevation and HDL-cholesterol reduction. The impact on glucose metabolism is potentiated when combined with DIUs. Third-generation BBs (carvedilol and nebivolol) have neutral impact or can even improve the glucose and lipid metabolism, possibly because of the vasodilating effect with decrease in insulin resistance and improvement of glucose uptake by peripheral tissues.47,50 Studies on nebivolol have shown less sexual dysfunction, possibly because of the effect on endothelial nitric oxide synthesis.47,50
Alpha-blockers
Alpha-blockers act as competitive antagonists of postsynaptic alpha-1 receptors, leading to a reduction in PVR without major changes in cardiac output.41 Some representatives of this drug class are doxazosin, prazosin and terazosin. The hypotensive effect is mild in monotherapy, the combined use being preferred. They have a favorable and discrete action on the lipid and glucose metabolisms, especially improving the symptoms related to benign prostate hypertrophy.41
Adverse effects
Alpha-blockers can cause symptomatic hypotension on the first dose. The phenomenon of tolerance is frequent, requiring increasing doses. Women can have urine incontinence. There is evidence that patients treated with doxazosin are at higher risk for CHF.41
Direct acting vasodilators
Representatives of this drug class are hydralazine and minoxidil. They act directly, relaxing arterial smooth muscle, leading to a PVR reduction.40
Adverse effects
The side effects of hydralazine are headache, flushing, reflex tachycardia and lupus-like reaction (dose-dependent).41 Hydralazine should be used carefully in patients with CAD, and avoided in those with dissecting aortic aneurysm and a recent cerebral hemorrhage episode. In addition, it can cause anorexia, nausea, vomiting and diarrhea. A common side effect of minoxidil is hirsutism, in approximately 80% of the patients. A less common side effect is the general expansion of the circulating volume and reflex tachycardia.
Calcium-channel blockers
Calcium channel blockers cause a reduction in PVR, because of the decreased calcium amount inside arteriolar smooth muscle cells, due to calcium channel blockade in their membranes.51 They are classified as dihydropyridine and nondihydropyridine CCBs.
Dihydropyridine CCBs (amlodipine, nifedipine, felodipine, nitrendipine, manidipine, lercanidipine, levamlodipine, lacidipine, isradipine, nisoldipine, nimodipine) have mainly a vasodilating effect, with minimum interference in HR and systolic function, being, thus, more often used as antihypertensive agents. Nondihydropyridine CCBs, such as phenylalkylamines (verapamil) and benzothiazepines (diltiazem), have a lower vasodilating effect, can cause bradycardia and have an antiarrhythmic effect, which limit their use to specific cases. Nondihydropyridine CCBs can depress the systolic function, mainly in patients with systolic dysfunction prior to their use, and, thus, should be avoided in that condition. Long-acting CCBs should be preferred to prevent unwanted oscillations in HR and BP. They are effective antihypertensive drugs that reduce CV morbidity and mortality.52-55 A study of outcome has reassured the efficacy, tolerability and safety of this drug class for the AH treatment of patients with CAD,56 being an alternative when BBs cannot be used, or even in association, in cases of refractory angina.
Adverse effects
Ankle swelling is usually the most common side effect, resulting from the vasodilating action (more arterial than venous), which causes capillary transudation. Throbbing headache and dizziness are not uncommon. Facial blushing is more common with fast-acting dihydropyridine CCBs. Hyperchromia of the distal third of the legs (ochre dermatitis) and gingival hypertrophy might occur. Such effects can be dose-dependent. Verapamil and diltiazem can worsen HF, bradycardia and atrioventricular block. Constipation is observed with verapamil.55
Angiotensin-converting-enzyme inhibitors
Angiotensin-converting-enzyme inhibitors are effective antihypertensive drugs whose major action is inhibition of angiotensin-converting-enzyme, hindering transformation of angiotensin I into angiotensin II, a vasoconstrictor. They are effective to treat AH, reducing CV morbidity and mortality.57 They are useful in many other CV conditions, such as HF with reduced ejection fraction, post-infarction anti-remodeling, and might have antiatherosclerotic properties. They delay renal function decline in patients with diabetic nephropathy or nephropathy of other etiologies.58
Adverse effects
Usually well-tolerated by most hypertensive patients, dry cough is their major side effect, affecting 5-20% of patients. Angioneurotic edema59 and skin rash are rare. Serum urea and creatinine elevation, usually small and reversible, is a transient phenomenon observed in the initial use of ACEIs in patients with renal failure.60 In the long run, ACEIs are effective to halt the progression of CKD. They can cause hyperpotassemia in patients with renal failure, mainly those with DM. They can reduce GFR and increase the levels of urea, creatinine and potassium in patients with bilateral stenosis of the renal arteries or renal artery stenosis in a single functioning kidney. They are contraindicated during pegnancy,61 because of the risk of fetal complicactions.62 Thus, they should be carefully used and often monitored in adolescents and childbearing-age women.
Angiotensin II AT1 receptor blockers
The ARBs antagonize the action of angiotensin II via the specific blockade of AT1 receptors, responsible for angiotensin II own actions of vasoconstriction, proliferation and stimulation of aldosterone release. In the AH treatment, especially of populations at high CV risk or with comorbidities, ARBs reduce CV and renal (diabetic nephropathy) morbidity and mortality.27-29,63-66
Adverse effects
Adverse effects related to ARBs are not common, exanthema being rarely observed. Similarly to ACEIs, ARBs are contraindicated during pregnancy, and the same care should be taken for childbearing-age women.
Direct renin inhibitors
Aliskiren, the only representative of this drug class available for clinical use, causes direct renin inhibition with consequent decrease in angiotensin II production.67 Other actions might contribute to BP lowering and tissue protection, such as the reduction in renin plasma activity,67 the blockade of a renin/prorenin receptor,68 and the decrease in intracellular angiotensin II production.69 Studies of antihypertensive efficacy have confirmed its ability in monotherapy to lower BP in an intensity similar to that of other antihypertensive drugs.70 There is, however, no evidence of its benefits on morbidity and mortality.
Adverse effects
They are well tolerated. Skin rash, diarrhea [especially at high doses (> 300 mg/day)], CPK increase, and cough are the most frequent events, whose incidence is usually < 1%. Their use is contraindicated during pregnancy.
The beginning of pharmacological treatment
Pharmacological treatment is indicated for individuals with stage 1 AH and at low and intermediate CV risk, when nonpharmacological measures proved ineffective after an initial period of at least 90 days. In especial situations, in which access and/or return to medical care is difficult, the initial use of antihypertensive drugs, even for that group of patients, might be considered. For individuals with stage 1 AH and at high CV risk or with established CVD, the use of antihypertensive agents should be started immediately. Likewise, for patients with stage 2 and 3 AH, regardless of the CV risk, pharmacological treatment should be started immediately. For prehypertensive individuals, pharmacological treatment might be an option, considering the CV risk and/or presence of CVD. For 60- to 79-year-old patients with SBP ≥ 140 mm Hg and those ≥ 80 years with SBP ≥ 160 mm Hg, pharmacological therapy should begin earlier.
Therapeutic schemes
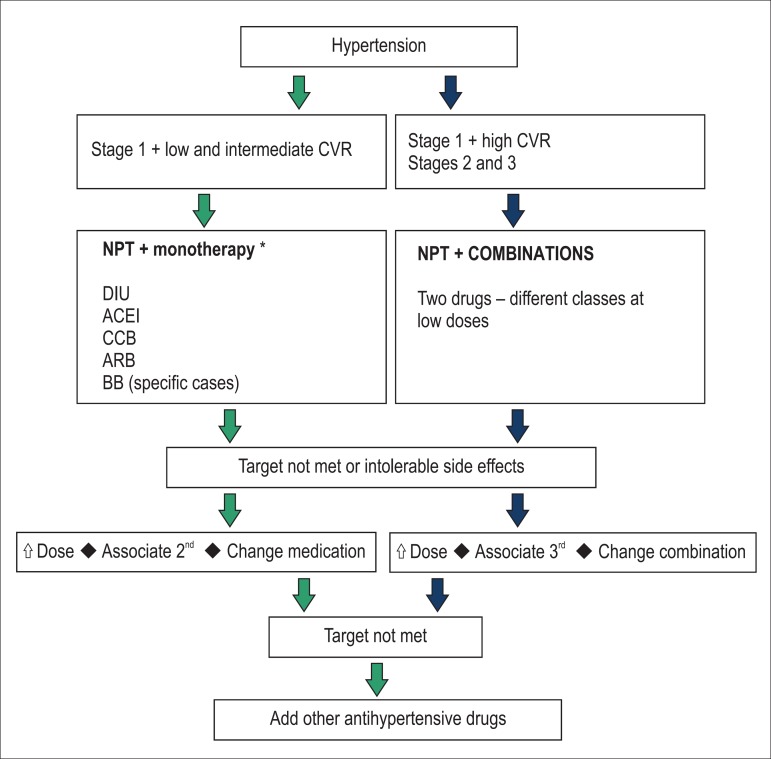
The pharmacological treatment can be performed with one or more drug classes, as required, to meet the BP targets and according to specific situations (Figure 1).
Figure 1.
Flowchart for the treatment of hypertension. CVR: cardiovascular risk; NPT: non-pharmacological treatment; DIU: diuretics; ACEI: angiotensin-convertingenzyme inhibitors; CCB: calcium-channel blockers; ARB: angiotensin-receptor blockers; BB: beta-blockers.
Monotherapy
Monotherapy can be the initial antihypertensive strategy for stage 1 AH patients at low and intermediate CV risk. However, depending on the BP target to be achieved, most patients will require drug combination. The treatment should be individualized, and the initial choice of drug to be used as monotherapy should be based on the following aspects: ability to lower CV morbidity and mortality; predominant pathophysiological mechanism in the patient to be treated; individual characteristics; associated diseases; and socioeconomic conditions.
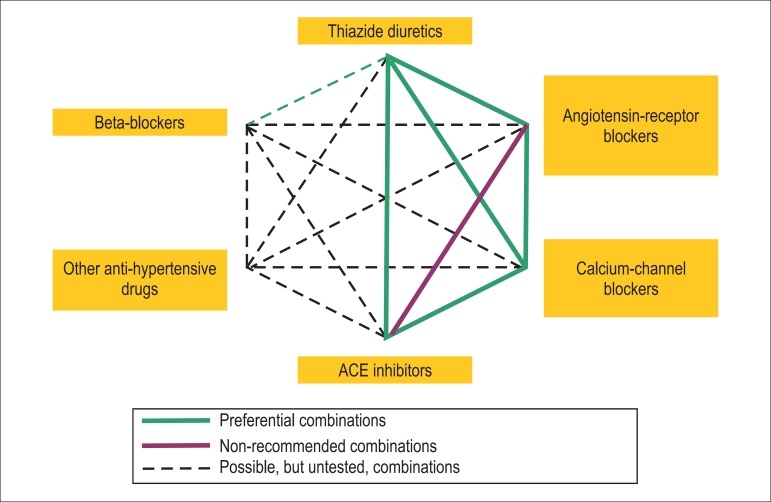
Based on those criteria, the classes of antihypertensive drugs currently preferred for BP control in the initial monotherapy are as follows (Figures 1 and 2):
Figure 2.
Preferential associations of drugs according to mechanisms of action and synergy. Adapted from Journal of Hypertension 2007, 25:1751-1762.
It is worth noting that DIUs have the greatest evidence of effectiveness regarding CV outcomes, with clear benefits for all types of events. In some situations, the indication of a certain group is reinforced, depending on the existing comorbidity. A BB can be considered the initial drug in certain situations, such as the presence of supraventricular arrhythmias, migraine, HF and CAD, and, in the last two conditions, the BB should be associated with other drugs.47,48
The dosage should be adjusted to provide BP lowering to levels considered adequate for each case (therapeutic targets).1,2,8,79 If the therapeutic objective is not achieved with the initial monotherapy, there are three possible options:
If the result is partial, but with no adverse effect, the dose of the drug used should be increased, and association with an antihypertensive drug of another group should be considered;
When the therapeutic effect expected at the maximum dose recommended is not obtained or in the presence of adverse events, the following is recommended: replace the antihypertensive agent initially used, reduce its dosage, and add another antihypertensive agent of a different class or use another association of drugs;
If the response is inappropriate, three or more drugs should be associated (Figure 1).
Combination of drugs
Most patients will need more than one drug to achieve BP targets. Therefore, patients with stage 1 AH and at high or very high CV risk or with CVD associated and those with stage 2 or 3 AH with or without other CVRF associated should be considered for drug combination (Figure 1). In addition, the association of two drugs at low doses for stage 1 hypertensive patients, even at low or intermediate CV risk, although not preferential, can be considered.
When choosing the drugs to be combined, antihypertensive agents sharing the same mechanism of action should be avoided, except for the association of thiazide DIUs and potassium-sparing DIUs. Loop DIUs should be reserved for individuals with GFR < 30 mL/min or severe edema. Associations with synergistic action provide better results (Figure 2).80
Particularities of the associations
Less tested associations should be reserved for cases requiring a larger number of drugs;
The association of BB and DIU should be performed carefully for patients with glucose metabolism changes, because both drugs contribute to worsen them;
The association of ACEI and ARB is not recommended, because, in addition to showing no benefit in CV outcomes, it increases the risk for adverse effects;33
Studies comparing directly the associations are scarce. A study has shown that the combination of ACEI and CCB, as compared to the association of ACEI and DIU, was more effective in lowering CV morbidity and mortality and the progression of kidney disease, for a similar reduction in BP, mainly in non-obese individuals.81,82
Combinations can be performed freely with separate drugs or in a fixed association (same galenic formulation). If, on the one hand, free combinations allow us to choose the dose of each component, on the other hand, the use of fixed associations favors adherence to treatment, because of the smaller number of tablets.83
If BP control is not attained with two drugs, some decisions can be made:
in case of partial result and no side effect, the dose of the initial combination can be increased, or one more antihypertensive agent of another drug class can be added;
when the target is not achieved at the maximum dose recommended, or if adverse events occur, the combination should be replaced;
if, at maximum doses, BP control is not attained, other antihypertensive drugs should be associated (Figure 1).
If a DIU was not the first choice and is not being used in the association of two drugs, it should be the third drug to be added. Its use potentiates the antihypertensive action of any initial drug.
In cases of resistant AH (lack of BP control with at least three drugs at their maximum doses tolerated, one being a DIU), association of spironolactone is indicated.84-86 Sympatholytic drugs of central action (clonidine) or BBs can be an alternative to the fourth drug to be added, direct vasodilators being reserved for special cases and in association with a DIU and a BB.
References
- 1.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275(20):1571–1576. [PubMed] [Google Scholar]
- 2.Padwal R, Straus SE, McAlister FA. Evidence based management of hypertension: Cardiovascular risk factors and their impact on decision to treat hypertension: an evidence-based review. BMJ. 2001;322(7292):977–980. doi: 10.1136/bmj.322.7292.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014;16(1):14–26. doi: 10.1111/jch.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daskalopoulou SS, Rabi DM, Zarnke KB, Dasgupta K, Nerenberg K, Cloutier L, et al. The 2015 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2015;31(5):549–568. doi: 10.1016/j.cjca.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. nullErratum in Lancet. 2003;361(9362):1060. [DOI] [PubMed] [Google Scholar]
- 6.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single-drug therapy for hypertension in men: a comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328(13):914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 7.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32(12):2285–2295. doi: 10.1097/HJH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 8.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 2. Effects at different baseline and achieved blood pressure levels overview and meta-analyses of randomized trials. J Hypertens. 2014;32(12):2296–2304. doi: 10.1097/HJH.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 9.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk overview and meta-analyses of randomized trials. J Hypertens. 2014;32(12):2305–2314. doi: 10.1097/HJH.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 10.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs -- overview and meta-analyses. J Hypertens. 2015;33(2):195–211. doi: 10.1097/HJH.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 11.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J Hypertens. 2015;33(7):1321–1341. doi: 10.1097/HJH.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 12.Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, et al. Health outcomes associated with antihypertensive therapies used as first-line agents: a systematic review and meta-analysis. JAMA. 1997;277(9):739–745. [PubMed] [Google Scholar]
- 13.Wright JM, Lee CH, Chamber GK. Systematic review of antihypertensive therapies: does the evidence assist in choosing a first-line drug? CMAJ. 1999;161(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- 14.SHEP-Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265(24):3255–3264. [PubMed] [Google Scholar]
- 15.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcome in high-risk hypertensive patients to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;228(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 16.Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes. UKPDS 39. UK Prospective Diabetes Study Group BMJ. 1998;317(7160):713–720. [PMC free article] [PubMed] [Google Scholar]
- 17.Neal B, MacMahon S, Chapman N, Blood Pressure Lowering Trialist's Collaboration Effects of ACE inhibitors, calcium antagonists and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomized trials. Blood Pressure Lowering Trialist's Collaboration. Lancet. 2000;356(9246):1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 18.Hansson L, Lindholm LH, Ekborn T, Dahlöf B, Lanke J, Scherstén B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity. The Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354(9192):1129–1133. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 19.Stassen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic hypertension in Europe (SYST-EUR) Lancet. 1997;350(9080):757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 20.Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomized to double-blind treatment with long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000;356(9227):366–372. doi: 10.1016/S0140-6736(00)02527-7. nullErratum in Lancet. 2000;356(9228):514. [DOI] [PubMed] [Google Scholar]
- 21.Hansson L, Hedner T, Lund-Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, et al. Randomized trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazen (NORDIL) study. Lancet. 2000;356(9227):359–365. doi: 10.1016/s0140-6736(00)02526-5. [DOI] [PubMed] [Google Scholar]
- 22.Pahor M, Psaty BM, Alderman MH, Applegate WB, Williamson JD, Cavazzini C, et al. Health outcomes associated with calcium antagonists compared with other first line antihypertensive therapies: a meta-analysis of randomised controlled trials. Lancet. 2000;356(9246):1949–1954. doi: 10.1016/S0140-6736(00)03306-7. [DOI] [PubMed] [Google Scholar]
- 23.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, ASCOT Investigators et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicenter randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 24.Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. Effect of angiotensin converting enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality on hypertension: the Captopril Prevention Project (CAPPP) randomized trial. Lancet. 1999;353(9489):611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 26.PROGRESS Collaborative Group Randomized trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;354(9287):1033–1041. doi: 10.1016/S0140-6736(01)06178-5. nullErratum in Lancet. 2001;358(9292):1556. [DOI] [PubMed] [Google Scholar]
- 27.Dahlof B, Devereux R, Kjeldsen S, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the losartan intervention or endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 28.Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, et al. LIFE Study Group Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002;359(9311):1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 29.Julius S, Kejdelsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients in high cardiovascular risk treated with regimens based on valsartan and amlodipine: the VALUE radomised trial. Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 30.Julius S, Weber MA, Kjeldsen SE, McInnes GT, Zanchetti A, Brunner HR, et al. The Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) trial: outcomes in patients receiving monotherapy. Hypertension. 2006;48(3):385–391. doi: 10.1161/01.HYP.0000236119.96301.f2. [DOI] [PubMed] [Google Scholar]
- 31.Ogihara T, Nakao K, Fukui T, Fukiyama K, Ueshima K, Oba K, et al. Candesartan Antihypertensive Survival Evaluation in Japan Trial Group Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trial. Hypertension. 2008;51(2):393–398. doi: 10.1161/HYPERTENSIONAHA.107.098475. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H, Kanno Y, Efficacy of Candesartan on Outcome in Saitama Trial Group Effects of candesartan on cardiovascular outcomes in Japanese hypertensive patients (E-COST) Hypertens Res. 2005;28(4):307–314. doi: 10.1291/hypres.28.307. nullErratum in Hypertens Res. 2005;28(6):553. [DOI] [PubMed] [Google Scholar]
- 33.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, ONTARGET Investigators et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 34.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665–b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet. 2004;364(9446):1684–1689. doi: 10.1016/S0140-6736(04)17355-8. nullErratum in Lancet. 2005;365(9460):656. [DOI] [PubMed] [Google Scholar]
- 36.Lindholm LH, Calberg B, Samuelsson O. Should beta blocker remain a first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366(9496):1545–1553. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 37.Bradley H, Wiysonge CS, Volmink JA, Mayosi BM, Opie LH. How strong is the evidence for the use of beta-blockers as first-line therapy for hypertension? Systematic review and meta-analysis. J Hypertens. 2006;24(11):2131–2141. doi: 10.1097/01.hjh.0000249685.58370.28. [DOI] [PubMed] [Google Scholar]
- 38.Parving HH, Brenner BM, McMurray JJV, Zeeuw D, Haffner SM, Solomon SD, ALTITUDE Investigators et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 39.The hypertension optimal treatment study (the HOT study) Blood Press. 1993;2(1):62–68. doi: 10.3109/08037059309077529. [DOI] [PubMed] [Google Scholar]
- 40.Vongpatanasin W, Kario K, Atlas SA, Victor RG. Central sympatholitic drugs. J Clin Hypertens (Greenwich) 2011;13(9):658–661. doi: 10.1111/j.1751-7176.2011.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan NM, Victor RG. Clinical hypertension. 11th ed. China: Wolters Kluwer; 2015. [Google Scholar]
- 42.Wagner ML, Walters AS, Coleman RG, Hening WA, Grasing K, Chokroverty S. Randomized double-blind, placebo-controlled study of clonidine in restless legs syndrome. Sleep. 1996;19(1):52–58. doi: 10.1093/sleep/19.1.52. [DOI] [PubMed] [Google Scholar]
- 43.Bond WS. Psychiatric indications for clonidine. J Clin Psychopharmacol. 1986;6(2):81–87. [PubMed] [Google Scholar]
- 44.Pandya KJ, Raubertas RF, Flynn PJ, Hynes HE, Rosenbluth RJ, Kirshner JJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med. 2000;132(10):788–793. doi: 10.7326/0003-4819-132-10-200005160-00004. [DOI] [PubMed] [Google Scholar]
- 45.Fedorak RN, Field M, Chang EB. Treatment of diabetic diarrhea with clonidine. Ann Intern Med. 1985;102(2):197–199. doi: 10.7326/0003-4819-102-2-197. [DOI] [PubMed] [Google Scholar]
- 46.Esler M, Dudley F, Jennings G, Debinski H, Lambert G, Jones P, et al. Increased sympathetic nervous activity and the effects of its inhibition with clonidine in alcoholic cirrohosis. Ann Intern Med. 1992;116(6):446–455. doi: 10.7326/0003-4819-116-6-446. [DOI] [PubMed] [Google Scholar]
- 47.Helfand M, Peterson K, Dana T. Drug class review on beta adrenergic blockers: Final Report. [2015 Jan 10]. Internet. Available from: http://www.ohsu.edu/drugeffectiveness/reports/final.cfm. [PubMed] [Google Scholar]
- 48.López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, et al. Task Force on Beta-Blockers of the European Society of Cardiology Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. 2004;25(15):1341–1362. doi: 10.1016/j.ehj.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Dulin B, Abraham WT. Pharmacology of carvedilol. Am J Cardiol. 2004;93(9A):3B–6B. doi: 10.1016/j.amjcard.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen ME, Cockcroft JR. The vasodilatory beta-blockers. Curr Hypertens Rep. 2007;9(4):269–277. doi: 10.1007/s11906-007-0050-2. [DOI] [PubMed] [Google Scholar]
- 51.Elliott WJ, Ram CV. Calcium channel blockers. J Clin Hypertens (Greenwich) 2011;13(9):687–689. doi: 10.1111/j.1751-7176.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Messerli FH. Calcium antagonists in hypertension: from hemodynamics to outcomes. Pt 2Am J Hypertens. 2002;15(7):94S–97S. doi: 10.1016/s0895-7061(02)02950-3. [DOI] [PubMed] [Google Scholar]
- 53.Elliott WJ, Bandari A. The role of calcium antagonists in stroke prevention. J Clin Hypertens (Greenwich) 2005;7(4 Suppl 1):5–8. doi: 10.1111/j.1524-6175.2005.04446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nathan S, Pepine CJ, Bakris GL. Calcium antagonists: effects on cardio-renal risk in hypertensive patients. Hypertension. 2005;46(4):637–642. doi: 10.1161/01.HYP.0000184541.24700.c7. [DOI] [PubMed] [Google Scholar]
- 55.Oparil S, Bakir SE. Calcium antagonists in cardiovascular disease: clinical evidence from morbidity and mortality trials. Drugs. 2000;59 Spec(2):25–37. [PubMed] [Google Scholar]
- 56.Rollins G. Calcium antagonist and beta blocker regimens found equally effective in hypertensive patients with coronary artery disease. Rep Med Guidel Outcomes Res. 2004;15(2):5–6. [PubMed] [Google Scholar]
- 57.Sindone A, Erlich J, Perkovic V, Suranyi M, Newman H, Lee C, et al. ACEIs for cardiovascular risk reduction--have we taken our eye off the ball. Aust Fam Physician. 2013;42(9):634–638. [PubMed] [Google Scholar]
- 58.Vejakama P, Thakkinstian A, Lertrattananon D, Ingsathit A, Ngarmukos C, Attia J. Reno-protective effects of renin-angiotensin system blockade in type 2 diabetic patients: a systematic review and network meta-analysis. Diabetologia. 2012;55(3):566–578. doi: 10.1007/s00125-011-2398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baram M, Kommuri A, Sellers SA, Cohn JR. ACE inhibitor-induced angioedema. J Allergy Clin Immunol Pract. 2013;1(5):442–445. doi: 10.1016/j.jaip.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Ryan MJ, Tuttle KR. Elevations in serum creatinine with RAAS blockade: why isn't it a sign of kidney injury? Curr Opin Nephrol Hypertens. 2008;17(5):443–449. doi: 10.1097/MNH.0b013e32830a9606. [DOI] [PubMed] [Google Scholar]
- 61.Polifka JE. Is there an embryopathy associated with first-trimester exposure to angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists? A critical review of the evidence. Birth Defects Res A Clin Mol Teratol. 2012;94(8):576–598. doi: 10.1002/bdra.23027. [DOI] [PubMed] [Google Scholar]
- 62.Laube GF, Kemper MJ, Schubiger G, Neuhaus TJ. Angiotensin-converting enzyme inhibitor fetopathy: long-term outcome. Arch Dis Child Fetal Neonatal Ed. 2007;92(5):F402–F403. doi: 10.1136/adc.2006.101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, et al. CHARM Investigators and Committees Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362(9386):759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 64.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 65.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Collaborative Study Group Renoprotective effect of the angiotensin receptor antagonist irbersartan in patients with nephropathy due to type 2 diabetes. N Eng J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 66.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group The effect of irbersartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 67.Müller DN, Derer W, Dechend R. Aliskiren-mode of action and preclinical data. J Mol Med (Berl) 2008;86(6):659–662. doi: 10.1007/s00109-008-0330-6. [DOI] [PubMed] [Google Scholar]
- 68.Danser AH. Pro)renin receptors: are they biologically relevant? Curr Opin Nephrol Hypertens. 2009;18(1):74–78. doi: 10.1097/MNH.0b013e3283196aaf. [DOI] [PubMed] [Google Scholar]
- 69.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57(12):3297–3306. doi: 10.2337/db08-0805. nullErratum in Diabetes. 2009;58(3):770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Musini VM, Fortin PM, Bassett K, Wright JM. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD007066–CD007066. doi: 10.1002/14651858.CD007066.pub2. [DOI] [PubMed] [Google Scholar]
- 71.Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689–694. doi: 10.1161/HYPERTENSIONAHA.110.161505. [DOI] [PubMed] [Google Scholar]
- 72.Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110–1117. doi: 10.1161/HYPERTENSIONAHA.112.191106. [DOI] [PubMed] [Google Scholar]
- 73.Turnbull F, Neal B, Pfeffer M, Kostis J, Algert C, Woodward M, et al. Blood Pressure Lowering Treatment Trialists' Collaboration Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25(5):951–958. doi: 10.1097/HJH.0b013e3280bad9b4. nullErratum in J Hypertens. 2007;25(7):1524. [DOI] [PubMed] [Google Scholar]
- 74.Reboldi G, Angeli F, Cavallini C, Gentile G, Mancia G, Verdecchia P. Comparison between angiotensinconverting enzyme inhibitors and angiotensin receptor blockers on the risk of myocardial infarction, stroke and death: a meta-analysis. J Hypertens. 2008;26(7):1282–1289. doi: 10.1097/HJH.0b013e328306ebe2. [DOI] [PubMed] [Google Scholar]
- 75.vanVark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33(16):2088–2097. doi: 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, et al. Valsartan in Acute Myocardial Infarction Trial Investigators Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–1906. doi: 10.1056/NEJMoa032292. nullErratum in N Engl J Med. 2004/01/08;350(2):203. [DOI] [PubMed] [Google Scholar]
- 77.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. ONTARGET investigators Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 78.Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta-analysis of randomised clinical trials. Diabetes Metab. 2004;30(6):487–496. doi: 10.1016/s1262-3636(07)70146-5. [DOI] [PubMed] [Google Scholar]
- 79.Wright Jr JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. SPRINT Research Group A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11 000 participants from 42 trials. Am J Med. 2009;122(3):290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 81.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. ACCOMPLISH Trial Investigators Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 82.Bakris GL, Sarafi PA, Weir MR, Dahlof B, Pitt B, Jamerson K, et al. ACCOMPLISH Trial investigators Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a pre-specified secondary analysis of a randomized controlled trial. Lancet. 2010;375(9721):1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- 83.Corrao G, Parodi A, Zambon A, Heiman F, Filippi A, Cricelli C, et al. Reduced discontinuation of antihypertensive treatment by two-drug combination as first step: evidence from daily life practice. J Hypertens. 2010;28(7):1584–1590. doi: 10.1097/HJH.0b013e328339f9fa. [DOI] [PubMed] [Google Scholar]
- 84.Vaclavik J, Sediak R, Plachy M, Navratil K, Plasek J, Jarkovsky J, et al. Addition of spirolactone in patients with resistant hypertension (ASPIRANT): a randomized double-blind, placebo-controlled trial. Hypertension. 2011;57(6):1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. nullErratum in Hypertension. 2015;65(2):e7. [DOI] [PubMed] [Google Scholar]
- 85.Bobrie G, Frank M, Azizi M, Peyrard S, Boutouyrie P, Chatellier G, et al. Sequential nophron blockade vs. sequential renin-angiotensin system blockade in resistant hypertension: a prospective, randomized, open blinded endpoint study. J Hypertens. 2012;30(8):1656–1664. doi: 10.1097/HJH.0b013e3283551e98. [DOI] [PubMed] [Google Scholar]
- 86.Dahal K, Kunwar S, Rijal J, Alqatahni F, Panta R, Ishak N, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28(11):1376–1385. doi: 10.1093/ajh/hpv031. [DOI] [PubMed] [Google Scholar]