Abstract
OBJECTIVE
N-acetylglucosamine/galactosamine (GlycA) and sialic acid (GlycB) moieties of glycosylated serum proteins are nonspecific measures of inflammation, but conclusive data on their relationship with insulin resistance or insulin secretion are missing. Therefore, we aimed to examine the relation of GlycA, GlycB, and C-reactive protein (CRP) to direct measures of insulin sensitivity (insulin sensitivity index [SI]) and insulin secretion (acute insulin response [AIR]).
RESEARCH DESIGN AND METHODS
This study used cross-sectional analyses and included 1,225 participants with and without type 2 diabetes in the Insulin Resistance Atherosclerosis Study (IRAS). SI and AIR were measured using the frequently sampled intravenous glucose tolerance test, and GlycA and GlycB were measured using nuclear magnetic resonance spectroscopy.
RESULTS
GlycA and GlycB had a strong correlation with CRP (r = 0.60 [P < 0.001] and r = 0.46 [P < 0.001], respectively). In a linear regression model with both GlycA and CRP as independent variables, GlycA (β × 1 SD, −0.04 ± 0.02; P < 0.01) and CRP (−0.06 ± 0.02; P < 0.001) were independently associated with SI even after adjusting for demographics, smoking, physical activity, plasma glucose, and BMI. However, neither CRP nor GlycA had an independent relationship with AIR.
CONCLUSIONS
GlycA may complement CRP in evaluating the relationship between inflammation, glucose tolerance, and insulin resistance.
Introduction
C-reactive protein (CRP) concentration, a marker of chronic subclinical inflammation, is related to insulin resistance (1) and has been identified as a risk factor for cardiovascular disease (CVD) (2). CRP concentration may be clinically relevant; it has been shown to improve prediction algorithms for the stratification of individuals according to the risk of future CVD (3).
Glycosylation, the most common posttranslational protein modification, modulates protein function (4,5). Most acute-phase proteins, released from the liver during an inflammatory response, are enzymatically glycosylated and circulate at concentrations high enough to be measurable via proton nuclear magnetic resonance (NMR) spectroscopy (6,7). N-acetylglucosamine/galactosamine (GlycA) and sialic acid (GlycB) moieties of glycosylated serum proteins are nonspecific measures of inflammation. Both GlycA and GlycB have a strong relationship with CRP (7,8). Increased GlycA has been associated with prevalent CVD risk factors including smoking, diabetes, hypertension, dyslipidemia, and obesity (8). Prospectively, GlycA has been associated with incident coronary heart disease and CVD events independent of conventional risk factors in the Women’s Health Study and the Multi-Ethnic Study of Atherosclerosis (MESA), respectively (8,9). In the Women’s Health Study, the relation of GlycA to CVD was comparable to that of CRP (8). However, the association between GlycA and CVD was no longer significant after controlling for CRP (8).
CRP has been established as a risk factor for incident type 2 diabetes (10,11). GlycA also predicts future development of diabetes (12,13), but conclusive data on the relation of GlycA and GlycB to insulin resistance or insulin secretion are missing. It is therefore of interest to determine whether the relation of GlycA and GlycB to insulin resistance and insulin secretion has utility similar or complementary to conventional inflammatory markers such as CRP. Thus, we examined the relation of GlycA, GlycB, and CRP to measures of insulin resistance and insulin secretion in participants of the Insulin Resistance Atherosclerosis Study (IRAS). In the IRAS, a frequently sampled intravenous glucose tolerance test (FSIGTT) was administered in all participants to obtain direct measures of insulin sensitivity and insulin secretion: the insulin sensitivity index (SI) and acute insulin response (AIR), respectively.
Research Design and Methods
Subjects
The design and methods of the IRAS have been described in detail (14). Briefly, the study was conducted at four clinical centers. At centers in Oakland and Los Angeles, California, non-Hispanic whites and African Americans were recruited from Kaiser Permanente, a nonprofit health maintenance organization. Centers in San Antonio, Texas, and San Luis Valley, Colorado, recruited non-Hispanic whites and Hispanics from two ongoing population-based studies (the San Antonio Heart Study and the San Luis Valley Diabetes Study, respectively). A total of 1,625 individuals were enrolled in the IRAS (56% women) from among the 3,416 contacted (response rate of 48%). The examinations began in October 1992 and were completed in April 1994. The IRAS protocol was approved by local institutional review committees, and all participants provided written informed consent.
GlycA and GlycB were measured in 1,489 participants (561 non-Hispanic whites, 429 African Americans, and 499 Hispanics). These participants did not differ from those with missing information on GlycA and GlycB (n = 136) in terms of adiposity, insulin resistance, and plasma concentrations of glucose, lipids, and CRP (P > 0.21 for all comparisons). The present report includes data from 1,225 participants—947 individuals without diabetes and 278 patients with type 2 diabetes who were not taking any glucose-lowering drugs—in order to exclude any potential drug-specific confounding on key outcome measures, including markers of inflammation. Thus, patients with diabetes were newly diagnosed or were treated with diet and/or exercise.
Measurements
Age, sex, ethnicity, smoking, physical activity, menopausal status, and pharmacologic treatment (glucose-lowering agents and estrogen and progesterone medications) were gathered by trained personnel. Anthropometric measurements were carried out using standardized protocols. The IRAS protocol required two visits, approximately 4 h each, 1 week apart. Participants were asked before each visit to fast for 12 h, to abstain from heavy exercise and alcohol for 24 h, and to refrain from smoking the morning of the examination.
A 75-g oral glucose tolerance test was administered to assess glucose tolerance status during the first visit. During the second visit, insulin sensitivity and insulin secretion were determined using an FSIGTT (15,16). Insulin sensitivity, expressed as the SI, was calculated using mathematical modeling methods (MINMOD version 3.0, 1994) (17). Acute insulin response was calculated as the mean plasma insulin concentration 2 and 4 min after the administration of glucose. Laboratory analyses of plasma glucose and insulin took place at the University of Southern California (Los Angeles). Plasma insulin concentration was measured using the dextran-charcoal radioimmunoassay.
CRP was measured by in-house ultrasensitive competitive immunoassay (antibodies and antigens from Calbiochem), with an interassay coefficient of variation of 8.9% (1). GlycA and GlycB were measured using NMR spectroscopy (LipoScience Inc., Raleigh, NC). GlycA and GlycB signals in plasma arise from circulating acute-phase proteins (6,18). The concentration of glycoproteins responsible for the GlycA and GlycB signals was estimated to be ∼13 mg/mL in normal human plasma (18). Fibrinogen, α1-acid glycoprotein, α1-antichymotrypsin, α1-antitrypsin, haptoglobin, and complement C3 contributed significantly to the increase in GlycA in chronic systemic inflammation (18). Blood samples were stored at −70°C until analysis (approximately 18 years later). Ritchie et al. (19) already proved the stability of GlycA in samples stored for more than10 years.
Fasting and 2-h plasma glucose concentrations were used to define categories of glucose tolerance: normal (NGT), fasting glucose <5.6 mmol/L and 2-h glucose <7.8 mmol/L (n = 455); isolated impaired fasting glucose (IFG), fasting glucose 5.6–6.9 mmol/L and 2-h glucose <7.8 mmol/L (n = 188); isolated impaired glucose tolerance (IGT), fasting glucose <5.6 mmol/L and 2-h glucose 7.8–11.0 mmol/L (n = 99); and IFG/IGT, fasting glucose 5.6–6.9 mmol/L and 2-h glucose 7.8–11.0 mmol/L (n = 205). Diabetes was defined as fasting glucose ≥126 mg/dL and/or 2-h glucose ≥200 mg/dL (n = 278). HOMA of insulin resistance (HOMA-IR) was calculated according to Matthew’s formula: fasting insulin (μIU/mL) × fasting glucose (mmol/L) ÷ 22.5. Normal weight, overweight, and obesity were defined as BMI <25, 25–29.9, and ≥30 kg/m2, respectively. Cigarette smoking was categorized as nonsmoker and low- and high-degree smokers (0, 1–9, and ≥10 cigarettes/day, respectively).
Statistical Analyses
The analysis was carried out using SAS statistical software (version 9.2; SAS Institute Inc., Cary, NC). Differences in markers of inflammation in participants categorized by age, sex, ethnicity, smoking, BMI, and glucose tolerance status were determined by one-way ANCOVA. The strength of the relationship between inflammatory markers and between inflammatory markers and other metabolic variables was assessed using Pearson correlation coefficients. Correlation coefficients were compared using the Steiger t test. Multiple linear regression analysis was used to examine the relation of demographic and metabolic variables to each of the inflammatory markers (dependent variable) and to determine the proportion of the variance (R2) that each of the models was able to explain. The relation of markers of inflammation to insulin resistance or BMI (dependent variable) was also examined by multiple linear regression analysis to account for the effect of demographics and other metabolic variables. Log-transformed values of insulin, HOMA-IR, AIR, and CRP were used in all analyses to meet the normality assumptions of the tests. We also used the log transformation of (SI + 1) given that some participants had an SI of zero. We considered a P value <0.050 statistically significant.
Results
Table 1 presents demographic and metabolic characteristics of the three ethnic groups. Hispanics and African Americans had more adiposity and insulin resistance (as measured by fasting insulin concentration, HOMA-IR, and SI), and higher AIR than non-Hispanic whites. Total energy expenditure was higher among Hispanics compared with non-Hispanic whites, but a smaller proportion of Hispanics engaged in vigorous physical activity. Total energy expenditure and vigorous physical activity were similar among African Americans and non-Hispanic whites. Hispanics had higher CRP, GlycA, and GlycB than non-Hispanic whites. However, African Americans had higher CRP, similar GlycA, and lower GlycB.
Table 1.
Demographic and metabolic variables by ethnicity
| Non-Hispanic whites (n = 477) | African Americans (n = 340) | Hispanics (n = 408) | |
|---|---|---|---|
| Female sex | 50.9 | 57.9† | 58.8† |
| Menopausal status | 36.9 | 42.4 | 44.0 |
| Taking estrogen and/or progesterone | 20.8 | 14.4‡ | 12.3‡ |
| Type 2 diabetes | 21.2 | 28.5† | 19.6 |
| Smokers | 12.6 | 16.8 | 22.4§ |
| Age (years) | 56.2 (49–64) | 55.2 (48–62) | 54.4 (47–62)‡ |
| BMI (kg/m2) | 28.2 (24.5–31.1) | 30.3 (26.3 – 33.2)§ | 29.2 (25.6–31.7)† |
| Waist circumference (cm) | 92.0 (83.1–100.4) | 93.5 (84.5–101.3) | 92.4 (83.3–100.0) |
| Total energy expenditure (kcal/kg/year) | 14,602 (12,853–15,458) | 14,324 (12,651–15,026) | 15,098 (13,104–16,346)† |
| Vigorous activity per week | |||
| <1 time | 16.9 | 13.6 | 19.5 |
| 1 time | 6.0 | 3.0 | 4.0 |
| >1 time | 16.1 | 11.2 | 9.7‡ |
| Fasting glucose (mg/dL) | 109.4 (92–113) | 114.7 (95–122)† | 106.2 (91–110) |
| 2-h glucose (mg/dL) | 154.6 (105–177) | 165.7 (106–202)† | 153.3 (102–182) |
| Fasting insulin (μU/mL)* | 12.6 (8.0–18.0) | 15.2 (10.5–22.0)§ | 15.3 (10.0–23.0)§ |
| HOMA-IR* | 3.29 (1.97–5.16) | 4.18 (2.48–6.42)§ | 3.93 (2.41–6.36)§ |
| SI (× 10−4 min–1 · μU−1· mL−1)* | 2.64 (0.75–3.06) | 2.18 (1.51–2.92)§ | 2.25 (1.49–3.32)§ |
| AIR (μU/mL)* | 35.9 (20.5–62.2) | 44.3 (22.6–83.1)§ | 50.9 (28.5–88.2)§ |
| CRP (mg/L)* | 1.70 (0.72–3.53) | 2.25 (0.95–5.53)§ | 2.32 (1.17–5.10)§ |
| GlycA (μmol/L) | 349.5 (305.3–392.7) | 359.5 (304.3–400.8) | 365.5 (317.3–404.6)‡ |
| GlycB (μmol/L) | 85.8 (67.2–101.2) | 81.6 (63.1–96.6)† | 99.6 (76.6–120.2)§ |
Data are percentages or mean (25th–75th percentiles).
*Values log-transformed then back-transformed
P for test of difference between minority populations and non-Hispanic whites; †P < 0.05; ‡P < 0.01; §P < 0.001.
CRP, GlycA, and GlycB by age, sex, ethnicity, BMI, and glucose tolerance categories are shown in Supplementary Fig. 1. In addition to the ethnic differences in CRP, GlycA, and GlycB, as presented above, none of these inflammatory markers was related to age, but all three were higher among women compared with men (P < 0.001 for all three markers). All three markers of subclinical inflammation were elevated in isolated IGT, IFG/IGT, and type 2 diabetes compared with NGT (P < 0.001 for all comparisons), but none was increased in isolated IFG. Also, there was a linear increase of all three markers by BMI category.
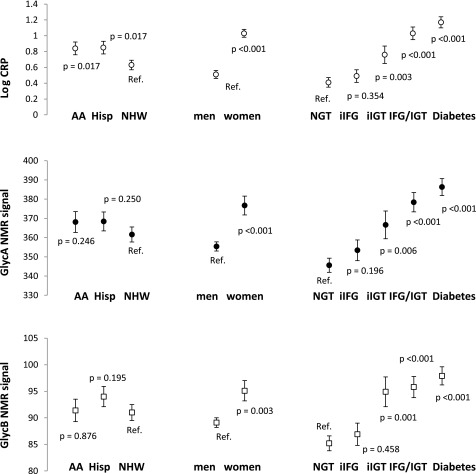
We generated three models that had each inflammatory marker as the dependent variable and age, sex, ethnicity, clinic, current smoking, and glucose tolerance categories as independent variables (Fig. 1). There were no ethnic differences in GlycA and GlycB, but CRP was higher among both Hispanics (P = 0.017) and African Americans (P = 0.017). All three inflammatory markers were higher among women compared with men (P < 0.01 for all three markers), and in isolated IGT compared with NGT (P < 0.01 for all three markers). However, none of the inflammatory markers was associated with isolated IFG. CRP levels and GlycA were also elevated in type 2 diabetes compared with isolated IGT (P < 0.001 and P = 0.015, respectively), but GlycB was not increased.
Figure 1.
CRP levels and GlycA and GlycB NMR signals (dependent variables). Age, sex, ethnicity, clinic, current smoking, and glucose tolerance categories were included as independent variables in all three models. AA, African American; Hisp, Hispanic; iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance; NHW, non-Hispanic white; Ref., reference category.
Pearson correlation coefficients relating markers of subclinical inflammation to relevant metabolic variables are shown in Supplementary Table 1. GlycA and GlycB were highly correlated (r = 0.74; P < 0.001). GlycA (r = 0.60; P < 0.001) and GlycB (r = 0.46; P < 0.001) had a strong relationship with CRP. All three inflammatory markers had direct relationships with measures of adiposity, plasma glucose concentrations, and insulin resistance, and inverse correlations with SI and total energy expenditure. Correlations involving measures of adiposity and insulin resistance were relatively strong. The relation of CRP to SI (r = −0.41; P < 0.001) was stronger than that of GlycA (r = −0.33, P < 0.001) and GlycB (r = −0.29; P < 0.001). All three inflammatory markers were more related to 2-h glucose than to fasting glucose (P < 0.001 for all comparisons), and none was related to AIR after adjusting for SI. There was no significant ethnic interaction for the relation of CRP, GlycA, and GlycB to measures of adiposity, plasma glucose, and insulin resistance/sensitivity (P for interaction >0.05). GlycA had a more robust correlation with CRP, plasma glucose, and measures of adiposity and insulin resistance than GlycB (P < 0.05 for all comparisons).
We used multiple linear regression analysis to examine the effect of menopausal status and estrogen/progesterone drugs on subclinical inflammation in women. Menopausal status was not associated with CRP (β = 0.08 [95% CI −0.13, 0.30]), GlycA (5.56 [−8.85, 19.97]), or GlycB (0.85 [−4.75, 6.46]) after adjusting for age, estrogen/progesterone drugs, ethnicity, clinic, smoking, physical activity, 2-h glucose, BMI, and SI. However, taking estrogen and/or progesterone was independently related to all three: CRP (β = 0.64 [95% CI 0.47, 0.80]; P < 0.001), GlycA (16.76 [5.45, 28.07]; P = 0.004), and GlycB (4.54 [0.14, 8.94]; P = 0.043).
The relation of demographics, estrogen/progesterone drugs, cigarette smoking, physical activity, 2-h glucose, BMI, and SI (independent variables) to GlycA, GlycB, or CRP (dependent variable) was further examined by multiple linear regression analysis (Table 2). Sex, estrogen/progesterone drugs, smoking more than 10 cigarettes a day, >1 episode of vigorous physical activity per week, BMI, and SI had a strong independent association with CRP, GlycA, and GlycB. Other independent relationships (Hispanic ethnic origin and 2-h glucose with CRP) were relatively weak. These models explained 34.0, 24.1, and 28.5% of the variability of CRP, GlycA, and GlycB, respectively. In similar analyses limited to premenopausal women aged 40 to 49 years who were not taking estrogen/progesterone drugs and age-matched men, CRP (β = 0.22 [95% CI 0.02, 0.42]; P = 0.028) and GlycA (21.0 [6.06, 36.0]; P = 0.006) were elevated in women. However, GlycB was not significantly different (3.75 [−2.88, 10.37]; P = 0.267).
Table 2.
Multiple linear regression analysis with GlycA or GlycB NMR signals or CRP levels as the dependent variable
| Log CRP | GlycA | GlycB | |
|---|---|---|---|
| Intercept | 0.42 (0.26, 0.58)‡ | 333.03 (321.72, 344.34)‡ | 79.04 (74.59, 83.49)‡ |
| Age (× 1 SD) | 0.05 (−0.01, 0.10) | 1.21 (−2.54, 4.95) | 1.37 (−0.11, 2.84) |
| Sex§ | |||
| Women not taking hormones vs. men | 0.24 (0.12, 0.36)‡ | 24.08 (15.85, 32.31)‡ | 4.24 (1.01, 7.48)* |
| Women taking hormones vs. men | 0.89 (0.73, 1.04)‡ | 41.30 (30.70, 51.86)‡ | 9.30 (5.14, 13.46)‡ |
| Ethnicity | |||
| African American vs. NHW | 0.09 (−0.06, 0.25) | −0.58 (−11.12, 9.95) | −2.14 (−6.28, 2.00) |
| Hispanic vs. NHW | 0.23 (0.07, 0.39)† | 6.33 (−4.65, 17.30) | 2.78 (−1.53, 7.10) |
| Smoking (cigarettes/day) | |||
| 1–9 vs. none | −0.08 (−0.31, 0.15) | 6.35 (−9.73, 22.43) | −0.65 (−6.97, 5.67) |
| ≥10 vs. none | 0.32 (0.16, 0.49)‡ | 35.12 (23.61, 46.64)‡ | 11.32 (6.79, 15.84)‡ |
| Vigorous activity (times/week) | |||
| 1 vs. <1 | 0.02 (−0.15, 0.19) | 1.78 (−9.75, 13.30) | −1.31 (−5.85, −3.22) |
| >1 vs. <1 | −0.17 (−0.29, −0.05)† | −10.16 (−18.41, −1.92)* | −4.03 (−7.28, −0.79)* |
| 2-h glucose (× 1 SD) | 0.11 (0.04, 0.17)† | 3.48 (−0.94, 7.89) | 1.14 (−0.59, 2.88) |
| BMI (× 1 SD) | 0.35 (0.28, 0.41)‡ | 14.97 (10.63, 19.31)‡ | 5.01 (3.30, 6.71)‡ |
| Log SI (× 1 SD) | −0.21 (−0.29, −0.14)‡ | −13.75 (−18.77, −8.72)‡ | −4.30 (−6.28, −2.32)‡ |
| R2 for the model (%) | 34.0 | 24.1 | 28.5 |
Data are β (95% CI) unless otherwise indicated. NHW, non-Hispanic white.
*P < 0.05;
†P < 0.01;
‡P < 0.001;
§Hormones indicate estrogen and/or progesterone medications.
Because of the strong relationship that CRP, GlycA, and GlycB had with measures of insulin resistance and adiposity, we further assessed the relation of each inflammatory marker (independent variable) to SI or BMI (dependent variable) after adjusting for demographic variables, estrogen/progesterone drugs, smoking, physical activity, 2-h glucose, and BMI (or SI) (Table 3). All three inflammatory markers were independently related to both SI and BMI (models 1–4). In a model that had both CRP and GlycA as independent variables (model 5), CRP and GlycA had an independent relationship with SI, but only CRP had an independent association with BMI.
Table 3.
Multiple linear regression analysis relating GlycA and GlycB NMR signals and CRP concentrations to BMI or SI
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Log SI as the dependent variable* | |||||
| Intercept | 0.82 (0.75, 0.90)‡ | 0.81 (0.73, 0.88)‡ | 0.80 (0.73, 0.87)‡ | 0.81 (0.73, 0.88)‡ | 0.80 (0.72, 0.87)‡ |
| Age (× 1 SD) | −0.05 (−0.07, −0.02)‡ | −0.04 (−0.06, −0.02)‡ | −0.04 (−0.07, −0.02)‡ | −0.04 (−0.07, −0.02)‡ | −0.04 (−0.06, −0.02)‡ |
| Sex | |||||
| Women not taking hormones vs. men | 0.13 (0.07, 0.18)‡ | 0.14 (0.08, 0.19)‡ | 0.15 (0.09, 0.20)‡ | 0.13 (0.08, 0.18)‡ | 0.15 (0.09, 0.20)‡ |
| Women taking hormones vs. men | 0.05 (−0.02, 0.12) | 0.12 (0.05, 0.19)§ | 0.09 (0.02, 0.16)† | 0.07 (0.003, 0.14)‖ | 0.12 (0.05, 0.19)‡ |
| Ethnicity | |||||
| African American vs. NHW | −0.11 (−0.18, −0.04)§ | −0.10 (−0.17, −0.03)§ | −0.11 (−0.18, −0.04)§ | −0.11 (−0.18, −0.04)§ | −0.10 (−0.17, −0.03)§ |
| Hispanic vs. NHW | −0.06 (−0.13, 0.01) | −0.04 (−0.11, 0.03) | −0.05 (−0.12, 0.02) | −0.05 (−0.12, 0.02) | −0.04 (−0.11, 0.03) |
| Smoking (cigarettes) | |||||
| 1–9 vs. none | −0.01 (−0.12, 0.09) | −0.02 (−0.12, 0.08) | −0.01 (−0.11, 0.10) | −0.01 (−0.12, 0.09) | −0.01 (−0.11, 0.09) |
| ≥10 vs. none | −0.03 (−0.11, 0.04) | −0.01 (−0.08, 0.07) | 0.00 (−0.07, 0.08) | −0.01 (−0.08, 0.07) | 0.01 (−0.06, 0.08) |
| Vigorous activity (times/week) | |||||
| 1 vs. <1 | 0.08 (0.003, 0.15)‖ | 0.07 (−0.003, 0.14) | 0.08 (0.004, 0.15)‖ | 0.07 (−0.001, 0.15) | 0.07 (−0.003, 0.14) |
| >1 vs. <1 | 0.08 (0.03, 0.13)§ | 0.07 (0.01, 0.12)‖ | 0.07 (0.01, 0.12)‖ | 0.07 (0.02, 0.12)‖ | 0.06 (0.01, 0.11)‖ |
| 2-h glucose (× 1 SD) | −0.25 (−0.27, −0.22)‡ | −0.23 (−0.26, −0.21)‡ | −0.24 (−0.26, −0.21)‡ | −0.24 (−0.27, −0.22)‡ | −0.23 (−0.26, −0.21)‡ |
| BMI (× 1 SD) | −0.22 (−0.24, −0.19)‡ | −0.19 (−0.21, −0.16)‡ | −0.20 (−0.22, −0.17)‡ | −0.20 (−0.23, −0.18)‡ | −0.18 (−0.21, −0.16)‡ |
| Log CRP (× 1 SD) | – | −0.08 (−0.11, −0.05)‡ | – | – | −0.06 (−0.09, −0.03)‡ |
| GlycA (× 1 SD) | – | – | −0.07 (−0.10, −0.05)‡ | – | −0.04 (−0.07, −0.01)§ |
| GlycB (× 1 SD) | – | – | – | −0.06 (−0.09, −0.03)‡ | – |
| R2 for the model (%) | 47.9 | 49.4 | 48.8 | 48.7 | 49.7 |
| BMI as the dependent variable† | |||||
| Intercept | 28.75 (27.88, 29.62)‡ | 29.22 (28.39, 30.06)‡ | 289.12 (28.26, 29.98)‡ | 29.08 (28.22, 29.95)‡ | 29.29 (28.44, 30.12)‡ |
| Age (× 1 SD) | −0.80 (−1.08, −0.51)‡ | −0.79 (−1.07, −0.52)‡ | −0.79 (−1.06, −0.51)‡ | −0.82 (−1.10, −0.54)‡ | −0.79 (−1.06, −0.52)‡ |
| Sex | |||||
| Women not taking hormones vs. men | 1.66 (1.04, 2.29)‡ | 1.12 (0.52, 1.73)‡ | 1.25 (0.63, 1.88)‡ | 1.48 (0.86, 2.10)‡ | 1.05 (0.44, 1.66)‡ |
| Women taking hormones vs. men | 0.50 (−0.32, 1.31) | −0.87 (−1.69, −0.06)‖ | −0.12 (−0.94, 0.70) | 0.19 (−0.62, 1.00) | −0.92 (−1.74, −0.11)‖ |
| Ethnicity | |||||
| African American vs. NHW | 0.62 (−0.19, 1.43) | 0.45 (−0.33, 1.22) | 0.60 (−0.19, 1.40) | 0.67 (−0.13, 1.47) | 0.46 (−0.32, 1.23) |
| Hispanic vs. NHW | 0.33 (−0.51, 1.17) | −0.04 (−0.85, 0.77) | 0.23 (−0.60, 1.06) | 0.23 (−0.60, 1.07) | −0.04 (−0.85, 0.77) |
| Smoking (cigarettes) | |||||
| 0–9 vs. none | −1.04 (−2.28, 0.19) | −0.83 (−0.201, 0.35) | −1.10 (−2.31, 0.11) | −0.99 (−2.21, 0.22) | −0.87 (−2.04, 0.08) |
| ≥10 vs. none | −1.17 (−2.05, −0.28)‖ | −1.55 (−2.39, −0.70)‡ | −1.63 (−2.51, −0.75)‡ | −1.49 (−2.37, −0.61)‡ | −1.65 (−2.50, −0.79)‡ |
| Vigorous activity (times/week) | |||||
| 1 vs. <1 | 0.11 (−0.78, 0.99) | 0.03 (−0.82, 0.88) | 0.08 (−0.79, 0.95) | 0.14 (−0.73, 1.02) | 0.02 (−0.82, 0.87) |
| >1 vs. <1 | −0.77 (−1.40, −0.14)‖ | −0.45 (−1.06, 0.16) | −0.59 (−1.21, 0.04) | −0.62 (−1.24, 0.01) | −0.77 (−1.40, −0.14)‖ |
| 2-h glucose (× 1 SD) | −0.08 (−0.42, 0.26) | −0.24 (−0.57, 0.09) | −0.12 (−0.46, 0.21) | −0.11 (−0.45, 0.22) | −0.24 (−0.57, 0.09) |
| Log SI (× 1 SD) | −3.00 (−3.35, −2.65)‡ | −2.42 (−2.77, −2.07) ‡ | −2.69 (−3.04, −2.34)‡ | −2.78 (−3.13, −2.43)‡ | −2.39 (−2.74, −2.03)‡ |
| Log CRP (× 1 SD) | – | 1.73 (1.41, 2.03)‡ | – | – | 1.57 (1.21, 1.93)‡ |
| GlycA (× 1 SD) | – | – | 1.06 (0.75, 1.36)‡ | – | 0.30 (−0.04, 0.65) |
| GlycB (× 1 SD) | – | – | – | 0.94 (0.62, 1.26)‡ | – |
| R2 for the model (%) | 31.3 | 37.5 | 33.8 | 33.1 | 37.6 |
Data are β (95% CI) unless otherwise indicated.
*Basic model: age, sex, ethnicity, clinic, smoking, 2-h glucose, and BMI;
†Basic model: age, sex, ethnicity, clinic, smoking, 2-h glucose, and SI;
‖P < 0.05;
§P < 0.01;
‡P < 0.001. NHW, non-Hispanic white.
Conclusions
Our study has the following findings: 1) Adiposity and SI have independent relationships with CRP concentration and GlycA and GlycB NMR signals. 2) Both CRP and GlycA demonstrate a statistically independent relation to SI, suggesting that GlycA may reflect an inflammatory pathway distinct from the pathway related to CRP. 3) All three inflammatory markers are more related to 2-h glucose than to fasting glucose. 4) GlycB has weaker relationships with CRP and measures of insulin resistance and adiposity than GlycA.
CRP and GlycA have an independent relationship with insulin resistance (Table 3, model 5), but not with insulin secretion (Supplementary Table 1). The association of inflammatory markers with insulin resistance has been demonstrated in cross-sectional studies (1), and results of prospective studies indicate a role of inflammatory processes in the pathophysiology of type 2 diabetes and CVD (1–3,11,20–22). These findings have led to attempts to improve metabolic disease by way of anti-inflammatory interventions (22); however, the exact mechanisms linking inflammation and metabolic disease are still incompletely understood (20). Our results also indicate that the relation of CRP to insulin resistance is stronger than that of GlycA and GlycB.
GlycA, a composite signal that can be detected by NMR spectroscopy, represents a subset of acute-phase reactants, including α1-acid glycoprotein, haptoglobin, α1-antitrypsin, α1-antichymotrypsin, and transferrin (7), and has recently been characterized as an inflammatory biomarker with analytic and clinical attributes that may complement or provide advantages over existing clinical markers of systemic inflammation (7). CRP, an acute-phase protein synthesized by the liver mainly as a result of interleukin-6 stimulation, also undergoes glycosylation with glycan attachments, which may vary under acute inflammatory conditions (23), but CRP contributes only negligibly to the GlycA signal (7). In a prospective study of initially healthy women, baseline GlycA was associated with incident CVD, consistent with a possible role for protein glycans in inflammation and CVD (8). The association between GlycA and CVD events was comparable to that of CRP, and was attenuated after adjusting for CRP. Interestingly, an analysis of follow-up time in this long-term study revealed a relation of GlycA to incident CVD that was independent of CRP in the first 6 years of the study, but not thereafter (median follow-up 17.2 years).
We also found that all three inflammatory markers are more related to 2-h glucose than to fasting glucose. In multivariate analyses, there is a closer relationship of CRP to glycemia compared with that of GlycA and GlycB (Table 2). Taking these data together, one might speculate that CRP and GlycA reflect distinct inflammatory processes, which may affect insulin resistance and subsequently incident type 2 diabetes. Further research, including “omics”-based analyses, may help identify targets involved in this pathophysiological cascade; those targets may be amenable to therapeutic intervention. Impaired insulin secretion, by contrast, may not contribute as much as insulin resistance, or its contribution may be missed by epidemiology, because proteins reflecting the metabolism of large organs (such as liver and adipose tissue) may yield levels of biomarkers sufficiently high to be detectable in serum, whereas markers reflecting solely islet cell inflammation (and hence affecting insulin secretion) might not reach detectable serum concentrations (20).
CRP, GlycA, and GlycB are associated with adiposity even after adjusting for insulin resistance and glucose tolerance. A decrease of CRP levels with lifestyle interventions (weight loss and physical activity) has previously been demonstrated in individuals with and without diabetes (24–26). The CRP concentration decrease with weight loss may be related in part to the regulation of insulin resistance and the inflammatory response by macrophages and T cells in adipose tissue (27,28). Our results suggest that BMI has a relationship with all three inflammatory markers that is at least as robust as that of waist circumference (Supplementary Table 1). This suggests that the production of CRP and glycosylation of acute-phase proteins by the liver is determined by overall adiposity rather than visceral adiposity in particular, unlike lipoproteins and insulin resistance.
Several studies have reported ethnic differences in CRP concentration, but only among women (29–31). In these studies, results were adjusted for demographics, cardiovascular risk factors, and/or adiposity, but none of them took into consideration the effect of insulin resistance. In another study that took into consideration insulin sensitivity determined by an FSIGTT (32), there was no excess CRP concentration in African American women. In the IRAS, both African Americans and Hispanics have higher CRP levels than non-Hispanic whites. However, only Hispanic ethnic origin is associated with an increased CRP concentration independent of smoking, adiposity, and insulin resistance. Our results also indicate that there are no significant ethnic differences in GlycA and GlycB after adjusting for multiple risk factors, including insulin resistance (Table 2).
Smokers, women, and sedentary individuals tend to have elevated CRP (33–38). In European studies, CRP did not differ between men and women after taking into consideration the effect of estrogen on CRP (39,40). This suggests that no sex-specific cut point for CRP is indicated to assess CVD risk (40). However, higher CRP has been described in women not taking estrogen in U.S. populations (33–35). In the IRAS, both female sex and the intake of estrogen/progesterone drugs (along with smoking and a lack of vigorous physical activity) are independently associated with elevated CRP. Female sex, estrogen/progesterone drugs, smoking, and lack of vigorous physical activity are also independently associated with elevated GlycA and GlycB.
Strengths of this study include 1) a large, well-described, multiethnic population, 2) a direct measure of insulin resistance and insulin secretion, and 3) use of an accurate methodology for the assessment of inflammatory markers and insulin sensitivity. Limitations of the study include 1) the cross-sectional nature of the analysis, making conclusions related to cause and effect difficult, and 2) the absence of upstream markers of inflammation (such as interleukin-6). Interleukin-6 was not examined because our study is not designed to evaluate mechanisms.
In summary, GlycA was related to insulin resistance and other features of the metabolic syndrome, independent of CRP, indicating that GlycA may represent an inflammatory pathway distinct from the CRP-related pathway. Further research in the field may help clarify our understanding of the inflammatory pathophysiology of type 2 diabetes and CVD, and the ability of GlycA to improve prediction models for CVD.
Supplementary Material
Article Information
Funding. This work was supported by the National Heart, Lung, and Blood Institute (grants HL-47887, HL-47889, HL-47890, HL-47892, HL-47902, HL-55208, and R01-HL-58329) and the General Clinic Research Centers Program (grants NCRR GCRC, M01 RR431, and M01 RR01346).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.L. and A.F. analyzed and interpreted data, drafted the manuscript, and gave final approval to the version to be published. A.F. critically revised the manuscript for important intellectual content. A.J.H. interpreted data, critically revised the manuscript for important intellectual content, and gave final approval to the version to be published. M.J.R. and A.E. acquired data, critically revised the manuscript for important intellectual content, and gave final approval to the version to be published. S.M.H. conceived and designed the study, drafted the manuscript, interpreted data, critically revised the manuscript for important intellectual content, and gave final approval to the version to be published. C.L. and A.F. are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-1569/-/DC1.
C.L. and A.F. contributed equally to this study.
Retired.
References
- 1.Festa A, D’Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102:42–47 [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347:1557–1565 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation 2008;118:2243–2251, 4p following 2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell 2006;126:855–867 [DOI] [PubMed] [Google Scholar]
- 5.Lauc G. Sweet secret of the multicellular life. Biochim Biophys Acta 2006;1760:525–526 [DOI] [PubMed] [Google Scholar]
- 6.Bell JD, Brown JC, Nicholson JK, Sadler PJ. Assignment of resonances for “acute-phase” glycoproteins in high resolution proton NMR spectra of human blood plasma. FEBS Lett 1987;215:311–315 [DOI] [PubMed] [Google Scholar]
- 7.Otvos JD, Shalaurova I, Wolak-Dinsmore J, et al. . GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem 2015;61:714–723 [DOI] [PubMed] [Google Scholar]
- 8.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc 2014;3:e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR Jr. Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clin Chem 2016;62:1020–1031 [DOI] [PubMed] [Google Scholar]
- 10.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 11.Festa A, D’Agostino R Jr, Tracy RP, Haffner SM; Insulin Resistance Atherosclerosis Study . Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 2002;51:1131–1137 [DOI] [PubMed] [Google Scholar]
- 12.Akinkuolie AO, Pradhan AD, Buring JE, Ridker PM, Mora S. Novel protein glycan side-chain biomarker and risk of incident type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2015;35:1544–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connelly MA, Gruppen EG, Wolak-Dinsmore J, et al. . GlycA, a marker of acute phase glycoproteins, and the risk of incident type 2 diabetes mellitus: PREVEND study. Clin Chim Acta 2016;452:10–17 [DOI] [PubMed] [Google Scholar]
- 14.Wagenknecht LE, Mayer EJ, Rewers M, et al. . The Insulin Resistance Atherosclerosis Study (IRAS) objectives, design, and recruitment results. Ann Epidemiol 1995;5:464–472 [DOI] [PubMed] [Google Scholar]
- 15.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86 [DOI] [PubMed] [Google Scholar]
- 16.Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes 1993;42:250–256 [DOI] [PubMed] [Google Scholar]
- 17.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986;23:113–122 [DOI] [PubMed] [Google Scholar]
- 18.Smith KD, Pollacchi A, Field M, Watson J. The heterogeneity of the glycosylation of alpha-1-acid glycoprotein between the sera and synovial fluid in rheumatoid arthritis. Biomed Chromatogr 2002;16:261–266 [DOI] [PubMed] [Google Scholar]
- 19.Ritchie SC, Würtz P, Nath AP, et al. . The biomarker GlycA is associated with chronic inflammation and predicts long-term risk of severe infection. Cell Syst 2015;1:293–301 [DOI] [PubMed] [Google Scholar]
- 20.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Bao W, Liu J, et al. . Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2013;36:166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 2014;13:465–476 [DOI] [PubMed] [Google Scholar]
- 23.Gornik O, Lauc G. Glycosylation of serum proteins in inflammatory diseases. Dis Markers 2008;25:267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belalcazar LM, Reboussin DM, Haffner SM, et al.; Look AHEAD Research Group . A 1-year lifestyle intervention for weight loss in individuals with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change: from the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care 2010;33:2297–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haffner S, Temprosa M, Crandall J, et al.; Diabetes Prevention Program Research Group . Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54:1566–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito K, Pontillo A, Di Palo C, et al. . Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289:1799–1804 [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Barnes GT, Yang Q, et al. . Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Ghosh S, Perrard XD, et al. . T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007;115:1029–1038 [DOI] [PubMed] [Google Scholar]
- 29.Carroll JF, Fulda KG, Chiapa AL, et al. . Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring) 2009;17:1420–1427 [DOI] [PubMed] [Google Scholar]
- 30.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al.; SWAN Investigators . Ethnic differences in C-reactive protein concentrations. Clin Chem 2008;54:1027–1037 [DOI] [PubMed] [Google Scholar]
- 31.Albert MA, Glynn RJ, Buring J, Ridker PM. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study). Am J Cardiol 2004;93:1238–1242 [DOI] [PubMed] [Google Scholar]
- 32.Hyatt TC, Phadke RP, Hunter GR, Bush NC, Muñoz AJ, Gower BA. Insulin sensitivity in African-American and white women: association with inflammation. Obesity (Silver Spring) 2009;17:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford ES, Giles WH, Myers GL, Mannino DM. Population distribution of high-sensitivity C-reactive protein among US men: findings from National Health and Nutrition Examination Survey 1999–2000. Clin Chem 2003;49:686–690 [DOI] [PubMed] [Google Scholar]
- 34.Khera A, McGuire DK, Murphy SA, et al. . Race and gender differences in C-reactive protein levels. J Am Coll Cardiol 2005;46:464–469 [DOI] [PubMed] [Google Scholar]
- 35.Albert MA, Ridker PM. C-reactive protein as a risk predictor: do race/ethnicity and gender make a difference? Circulation 2006;114:e67–e74 [DOI] [PubMed] [Google Scholar]
- 36.Fröhlich M, Sund M, Löwel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95). Eur Heart J 2003;24:1365–1372 [DOI] [PubMed] [Google Scholar]
- 37.Kuller L, Tracy R, Shaten J, Meilahn EN. Relationship of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol 1996;144:537–547 [DOI] [PubMed] [Google Scholar]
- 38.Ohsawa M, Okayama A, Nakamura M, et al. . CRP levels are elevated in smokers but unrelated to the number of cigarettes and are decreased by long-term smoking cessation in male smokers. Prev Med 2005;41:651–656 [DOI] [PubMed] [Google Scholar]
- 39.Imhof A, Fröhlich M, Loewel H, et al. . Distributions of C-reactive protein measured by high-sensitivity assays in apparently healthy men and women from different populations in Europe. Clin Chem 2003;49:669–672 [DOI] [PubMed] [Google Scholar]
- 40.Ledue TB, Rifai N. Preanalytic and analytic sources of variations in C-reactive protein measurement: implications for cardiovascular disease risk assessment. Clin Chem 2003;49:1258–1271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.