Introduction
Individuals with prediabetes, namely impaired glucose tolerance (IGT) and/or impaired fasting glucose (IFG) are at increased risk of developing type 2 diabetes mellitus (T2DM) and cardiovascular disease. However, only 30–40% of those with IFG/IGT eventually develop T2DM [1–3]. In fact, about 40% of individuals who develop T2DM after 10 years have NGT at baseline [3] indicating that the conventional fasting and 2-h oral glucose tolerance test (OGTT) do not recognize most high-risk individuals.
Recently, population-based studies have consistently shown that 1-h post-load glucose (PG) levels may be a better predictor of T2DM and associated complications [4–7]. Furthermore, NGT individuals with elevated 1-h PG>8.6 mmol/l were found to be more insulin resistant, have worse beta-cell function and an atherogenic profile similar to those with prediabetes [8,9]. These results were from a research setting using well-defined cohorts. There is sparse information regarding the performance of a 1-h OGTT in a health care setting where the population may be more heterogeneous. Therefore, we investigated the pathophysiological associations of 1-h IGT with dysglycemic conditions in a cohort of outpatients undergoing screening for T2DM.
Methods
This cross-sectional analysis was performed in 236 patients referred to the New York University Langone Diabetes and Endocrine Associates between 2010 and 2015 for T2DM screening due to high HbA1c values (≥5.7%). After an overnight fast, plasma glucose and insulin concentrations were measured fasting, 1-h and 2-h after a standard 75-g OGTT. Based on the fasting and 2-h plasma glucose concentrations, subjects were assigned to one of three glycemic categories: (1) NGT: FPG <5.6 mmol/l and 2-h glucose <7.8 mmol/l; (2) prediabetes (IFG: ≥5.6–<6.9mmol/l) and/or (IGT) 2-h glucose ≥7.8–<11.1 mmol/l; and (3) T2DM (FPG ≥ 7.0 mmol/l and/or 2-h glucose ≥ 11.1 mmol/l) [10]. Individuals in the NGT category were further classified based on their 1-h OGTT results as either NGT1h-normal (≤8.6mmol/l) or NGT1h-high (>8.6 mmol/l).
Other data collected included demographics, body weight, height, and family history of diabetes. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). All biochemical tests (HbA1c, glucose and insulin measurements) were processed in an accredited laboratory (NYU Langone Clinical Laboratory). Whole body insulin sensitivity was calculated using Matsuda’s insulin sensitivity index; formula: (104/square root of (fasting glucose * insulin) * (mean OGTT glucose * mean OGTT insulin)), with mean glucose and insulin calculated from values at fasting, 1 and 2h of the OGTT [11]. Insulin secretion [12] was estimated from the ratio of the total area under the curve (AUC) for insulin (pmol/l) and for glucose (mmol/l) using the trapezoidal rule (AUCins/glu). Beta-cell function was then calculated using the oral disposition index as Matsuda’s insulin sensitivity index multiplied by AUCins/glu [13]. This study was approved by the New York University Langone Medical Center Institutional Review Board.
Statistical analysis
Continuous values were compared across glycemic categories using one-way analysis of variance (ANOVA) with Bonferroni post hoc corrections for normally distributed variables, and Kruskal–Wallis test with Dunn’s post hoc corrections for non-normally distributed variables. Polytomous logistic regression was used to determine the association of each glycemic category (NGT1h-high, prediabetes and T2DM) compared with NGT1h-normal (reference category) for DIo, HOMA-IR, and other covariates. The variables were centered on the mean to estimate the odds ratio (OR) per standard deviation (SD) change. Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, version 23.0. Armonk, NY: IBM Corp).
Results
The mean age was 55.7 ± 12.8 years, and 159 (69.1%) were female. Based on the fasting and 2-h OGTT glucose concentrations, 128 (55.7%) had NGT, 82 (34.7%) had prediabetes (IFG: 38 (16.1%); IGT: 18 (7.6%), and IFG+IGT: 26 (11.0%)), and 20 (8.7%) had T2DM.
Abnormalities in 1-h PG>8.6 mmol/l were observed in individuals with prediabetes (IFG: 60.5%; IGT 94.4%; IFG+IGT: 88.5%), and T2DM (90.0%). Overall, patients with prediabetes and T2DM had significantly higher FPG, 1-h and 2-h PG concentrations (P<0.0001). Among the 128 patients with NGT, those with NGT1h-high (n=37) were significantly older (60.3±10.4 years vs.51.9±12.2 ; P<0.0001), and had higher fasting (5.0±0.4 vs.4.8±0.4mmol/l; P=0.027) and 2-h PG concentrations (6.1±1.1 vs 5.1±1.1 mmol/l; P<0.0001). HbA1c levels were higher in patients with prediabetes (6.2±0.3%) than those with NGT, regardless of whether they had NGT1h-normal (5.9±0.3%; p<0.05) or NGT1h-high (5.9±0.3%; P<0.05). However, there were no significant differences in HbA1c levels between the T2DM and other groups (6.0±0.5%; P>0.05).
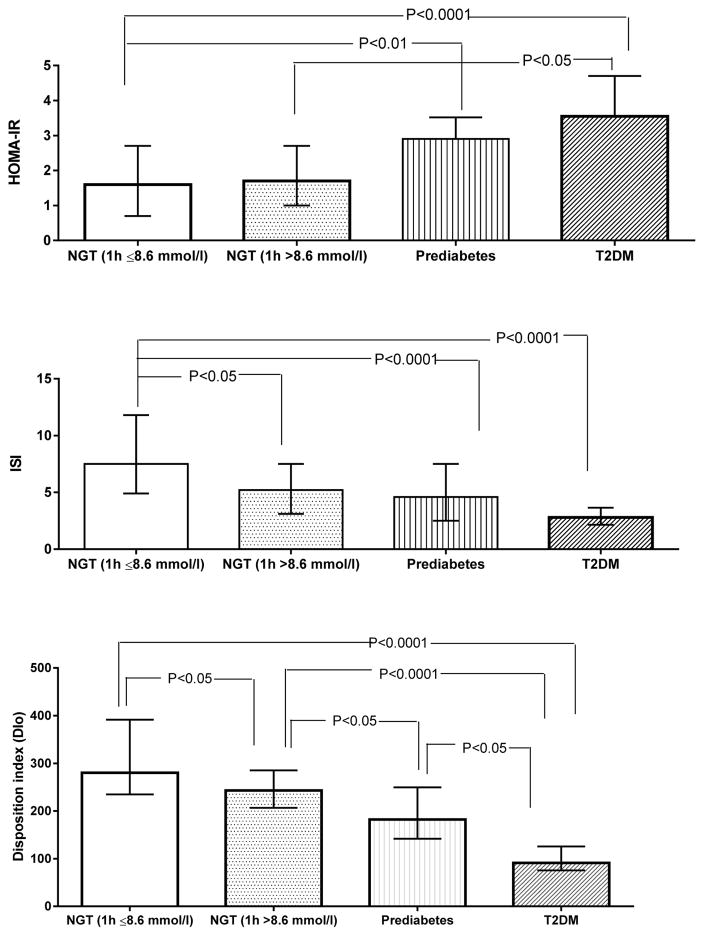
There was a descending trend in insulin sensitivity and beta-cell function as glycemia worsened. The levels of ISI was 27% (7.5 vs. 5.5; P=0.007) and DIo was 12% (280.3 vs. 247.5; P=0.001) lowered in individuals with NGT1h-high than in those with NGT1h-normal. As expected, the levels of DIo and ISI was significantly lower in prediabetic and T2DM compared with NGT-groups (P<0.0001). The levels of, DIo was further decreased in the T2DM group compared with the prediabetic group (P<0.05) (see Figure). Polytomous logistic regression showed a graded association between dysglycemia and DIo (NGT1h-high: OR [95%CI]: 0.42[0.24–0.74]), prediabetes (0.10[0.05–0.21] and T2DM (0.001[0.003–0.039]) in this cohort. The magnitude of association was not attenuated after adjusting for age, BMI and sex (NGT 1h-high: 0.34[0.18–0.65]; prediabetes: 0.10[0.05–0.20] and T2DM: 0.01[0.002–0.039]. Similarly, there was a moderate association between dysglycemia and ISI (NGT1h-high: 0.53[0.31–0.89]; prediabetes: 0.41[0.26–0.65] and T2DM: 0.38[0.17–0.87]).
Figure 1.
Insulin resistance (HOMA-IR) insulin sensitivity (ISI), and beta-cell function (DIo) during the OGTT according to glycemic status. NGT (1 h B 8.6 mmol/l): Normal Glucose Tolerance with normal levels of 1 h plasma glucose; NGT (1 h[8.6 mmol/l): NGT with 1-h OGTT glucose[8.6 mmol/l; Prediabetes: Individuals with impaired fasting glucose and/or impaired glucose tolerance and T2DM: type 2 diabetes.
Discussion
The results of the present analyses indicates that 1-h PG >8.6 mmol/l during an OGTT may provide a useful tool to identify individuals with NGT who are at increased risk of T2DM. Individuals with NGT1h-high had reduced insulin sensitivity and beta-cell function compared with NGT1h-normal. The levels of ISI were comparable to those with prediabetes, although the latter had worse beta-cell function. These findings were corroborated by other trials using hyperinsulinemic clamp assays [8, 9]. Recently, Fiorentino et al [14] demonstrated that NGT1h-high was a stronger predictor of diabetes than IFG or NGT1h-normal and exhibited decreased insulin sensitivity and beta-cell function with the euglycemic hyperinsulinemic clamp method. Our observations are similar using routinely measurable index of beta cell function. Furthermore, our study cohort was derived from a real-life clinical practice and the analysis also included those with T2DM. Our subjects were older and were not required to have one parent with T2DM as the Eugene Study in the Fiorentino analysis.
Our findings suggest that preventive measures may be more effective in those with subtle abnormalities in beta-cell function before achieving critical thresholds for IFG or IGT [15]. In accordance with this hypothesis, the US Diabetes Prevention Program demonstrated that lifestyle intervention was significantly greater among individuals with lower glucose concentrations and better beta-cell function at baseline [16]. Furthermore, a recent prevention study among Asian Indians with prediabetes showed that baseline and improvement in beta-cell function are important for regression of prediabetes to NGT [17].
The cross-sectional design and small sample size are limitations of the present study. Our findings provide evidence that 1-h OGTT was able to discriminate those at greater risk of developing T2DM. Prevention studies have focused on prediabetic individuals. Our findings, however, suggest that those with an earlier abnormality, namely an elevated 1-hour level, already have compromised beta-cell function and should be identified well before reaching the prediabetic state in the dysglycemic continuum as currently defined by traditional criteria. Because approximately one-third of patients with NGT have 1-h PG>8.6 mmol/l, this opens up “new target subgroup” to be identified for initiating preventative measures.
Acknowledgments
Funding: This study was funded by CTSI grant number1UL1RR029893 (NCRR, NIH and the Schuman Foundation) and partly by NIH-K24-NR012226.
We thank David St-Jules for proof-reading the manuscript and Elong Zhang for his statistical assistance during the preparation of the manuscript.
Footnotes
Conflict of interest: None
References
- 1.Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, Yazdi H, Booker L. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes research and clinical practice. 2007;78(3):305–312. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, Haffher SM, Pettitt DJ, Sorkin JD, Muller DC. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46(4):701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabetic medicine : a journal of the British Diabetic Association. 2002;19(9):708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes care. 2009;32(2):281–286. doi: 10.2337/dc08-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Succurro E, Marini MA, Arturi F, Grembiale A, Lugara M, Andreozzi F, Sciacqua A, Lauro R, Hribal ML, Perticone F, Sesti G. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis. 2009;207(1):245–249. doi: 10.1016/j.atherosclerosis.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Succurro E, Arturi F, Lugara M, Grembiale A, Fiorentino TV, Caruso V, Andreozzi F, Sciacqua A, Hribal ML, Perticone F, Sesti G. One-hour postload plasma glucose levels are associated with kidney dysfunction. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(11):1922–1927. doi: 10.2215/CJN.03240410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman M, Chetrit A, Roth J, Dankner R. Dysglycemia and long-term mortality: observations from the Israel study of glucose intolerance, obesity and hypertension. Diabetes/metabolism research and reviews. 2015;31(4):368–375. doi: 10.1002/dmrr.2618. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi C, Miccoli R, Trombetta M, Giorgino F, Frontoni S, Faloia E, Marchesini G, Dolci MA, Cavalot F, Cavallo G, Leonetti F, Bonadonna RC, Del Prato S Investigators G. Elevated 1-hour postload plasma glucose levels identify subjects with normal glucose tolerance but impaired beta-cell function, insulin resistance, and worse cardiovascular risk profile: the GENFIEV study. The Journal of clinical endocrinology and metabolism. 2013;98(5):2100–2105. doi: 10.1210/jc.2012-3971. [DOI] [PubMed] [Google Scholar]
- 9.Marini MA, Succurro E, Frontoni S, Mastroianni S, Arturi F, Sciacqua A, Lauro R, Hribal ML, Perticone F, Sesti G. Insulin sensitivity, beta-cell function, and incretin effect in individuals with elevated 1-hour postload plasma glucose levels. Diabetes Care. 2012;35(4):868–872. doi: 10.2337/dc11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 12.Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. European journal of endocrinology / European Federation of Endocrine Societies. 2004;150(2):97–104. doi: 10.1530/eje.0.1500097. [DOI] [PubMed] [Google Scholar]
- 13.Ram J, Snehalatha C, Selvam S, Nanditha A, Shetty AS, Godsland IF, Johnston DG, Ramachandran A. The oral disposition index is a strong predictor of incident diabetes in Asian Indian prediabetic men. Acta Diabetol. 2015 doi: 10.1007/s00592-015-0718-z. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino TV, Marini MA, Andreozzi F, Arturi F, Succurro E, Perticone M, Sciacqua A, Letizia Hribal M, Perticone F, Sesti G. One-hour post-load hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. The Journal of clinical endocrinology and metabolism. 2015:jc20152573. doi: 10.1210/jc.2015-2573. [DOI] [PubMed] [Google Scholar]
- 15.Bergman M. Inadequacies of current approaches to prediabetes and diabetes prevention. Endocrine. 2013;44(3):623–633. doi: 10.1007/s12020-013-0017-9. [DOI] [PubMed] [Google Scholar]
- 16.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R, Murphy MB, Shamoon H. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanditha A, Ram J, Snehalatha C, Selvam S, Priscilla S, Shetty AS, Arun R, Godsland IF, Johnston DG, Ramachandran A. Early improvement predicts reduced risk of incident diabetes and improved cardiovascular risk in prediabetic asian Indian men participating in a 2-year lifestyle intervention program. Diabetes care. 2014;37(11):3009–3015. doi: 10.2337/dc14-0407. [DOI] [PubMed] [Google Scholar]