Abstract
Annually, excessive alcohol use accounts for more than $220 billion in economic costs and 80,000 deaths, making excessive alcohol use the third leading lifestyle-related cause of death in the US. Patients with an alcohol-use disorder (AUD) also have an increased susceptibility to respiratory pathogens and lung injury, including a 2–4-fold increased risk of acute respiratory distress syndrome (ARDS). This review investigates some of the potential mechanisms by which alcohol causes lung injury and impairs lung immunity. In intoxicated individuals with burn injuries, activation of the gut-liver axis drives pulmonary inflammation, thereby negatively impacting morbidity and mortality. In the lung, the upper airway is the first checkpoint to fail in microbe clearance during alcohol-induced lung immune dysfunction. Brief and prolonged alcohol exposure drive different post-translational modifications of novel proteins that control cilia function. Proteomic approaches are needed to identify novel alcohol targets and post-translational modifications in airway cilia that are involved in alcohol-dependent signal transduction pathways. When the upper airway fails to clear inhaled pathogens, they enter the alveolar space where they are primarily cleared by alveolar macrophages (AM). With chronic alcohol ingestion, oxidative stress pathways in the AMs are stimulated, thereby impairing AM immune capacity and pathogen clearance. The epidemiology of pneumococcal pneumonia and AUDs is well established, as both increased predisposition and illness severity have been reported. AUD subjects have increased susceptibility to pneumococcal pneumonia infections, which may be due to the pro-inflammatory response of AMs, leading to increased oxidative stress.
Keywords: alcohol, lung immunity, lung injury, gut-liver-lung axis, airway cilia, alveolar macrophage
Introduction
Alcohol-use disorders (AUDs) have long been linked to increased susceptibility to lung infections. In 1789, Dr. Benjamin Rush, the first surgeon general of the United States, observed that individuals with an affinity for alcohol had a higher incidence of pneumonia and tuberculosis (Rush, 1808). In 1885, Sir William Osler reported that alcohol is one of the greatest predisposing factors to the development of pneumonia (Osler, 1892). The Centers for Disease Control estimates that 80,000 annual deaths in the United States are attributed to excessive alcohol ingestion, making it the third leading lifestyle-related cause of death (Mokdad, Marks, Stroup, & Gerberding, 2004). In 2006, excessive alcohol ingestion was responsible for ~$224 billion in healthcare costs, including emergency room and physician office visits (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011). Compared to non-alcoholics, patients with a history of alcohol abuse are twice as likely to develop sepsis, and those with sepsis are twice as likely to develop acute respiratory syndrome (ARDS) (Jong, Hsiue, Chen, Chang, & Chen, 1995; Joshi & Guidot, 2007; Moss, 2005). Alcohol-induced lung injury and immune dysfunction contribute to a higher risk for developing respiratory infections, leading to increased morbidity and mortality in patients with a history of AUDs (Moss, 2005).
This critical review delves into some of the potential mechanisms by which alcohol causes lung injury and impairs lung immunity. With a burn injury, alcohol intoxication exacerbates the pulmonary response through the gut-liver-lung pro-inflammatory response. In the lung, prolonged alcohol exposure causes desensitization of cilia in the upper airway, making motility and pathogen clearance resistant to stimulation due to oxidant stress-related mechanisms. Chronic alcohol ingestion also induces oxidative stress within the microenvironment of the alveolar space, which impairs the capacity of alveolar macrophages to phagocytose and clear bacteria. Translational studies show that when alveolar macrophages are isolated from individuals with AUDs and are stimulated with pneumococcal infections, they exhibit increased pro-inflammatory cytokine production that may promote increased severity of illness. Collectively, these alcohol-associated alterations are likely contributors to deleterious outcomes in the setting of pulmonary infections among people with unhealthy alcohol consumption.
Alcohol intoxication exacerbates pulmonary response to remote injury: gut-liver-lung axis regulates pulmonary inflammation after intoxication and burn injury
Alcohol intoxication increases both the risk and severity of accidental injury (Smith, Branas, & Miller, 1999; Tulloh & Collopy, 1994). Alcohol use is associated with increased complications and delays the recovery time after injuries such as lacerations (Curtis, Hlavin, Brubaker, Kovacs, & Radek, 2014), fractures (Lauing, Roper, Nauer, & Callaci, 2012), and burns (Silver et al., 2008). Burns are a devastating injury affecting nearly 500,000 people in the US annually, resulting in 40,000 hospitalizations (ABA, 2012). Half of adult burn patients are intoxicated at the time of hospital admission and have increased morbidity, mortality, and socioeconomic costs, relative to their non-intoxicated counterparts (Grobmyer, Maniscalco, Purdue, & Hunt, 1996; Kelley & Lynch, 1992; Silver et al., 2008). Most intoxicated burn patients are considered binge drinkers as opposed to chronic alcoholics (Savola, Niemelä, & Hillbom, 2005), and present with an average blood alcohol content (BAC) of 150 mg/dL (Silver et al., 2008), which is associated with twice the infection risk, greater requirement for surgical procedures, longer stays in the intensive-care unit, and increased need for mechanical ventilation (Brezel, Kassenbrock, & Stein, 1988; Davis et al., 2013; Grobmyer et al., 1996; Silver et al., 2008).
In burn patients, compromised pulmonary function portends a poor prognosis and develops independently of the presence or absence of inhalation injury (Liffner, Bak, Reske, & Sjöberg, 2005), and likely contributes to the poorer outcomes associated with burn injury in AUD patients. The lungs may be particularly susceptible to damage due to their delicate architecture, numerous resident inflammatory cells, and high degree of vascularization. Experimental evidence suggests that the worsened clinical outcomes when alcohol precedes a burn are due to the ability of alcohol to fundamentally alter the pulmonary physiological response to a remote and indirect injury, causing a decrease in lung function (Shults et al., 2015). In a mouse model, a single dose of alcohol (1.1 g/kg) prior to a 15% total body surface area (TBSA) scald burn results in increased pulmonary edema (M. M. Chen, Bird, et al., 2013; M. M. Chen, O’Halloran, Ippolito, Choudhry, & Kovacs, 2015; M. M. Chen, Palmer, et al., 2013; M. M. Chen et al., 2014), neutrophil infiltration (Bird, Morgan, Ramirez, Yong, & Kovacs, 2010; Bird, Zahs, et al., 2010), and susceptibility to infection (Murdoch, Brown, Gamelli, & Kovacs, 2008) relative to either insult alone and despite no direct injury to the lung itself.
Alcohol exacerbates post-burn levels of systemic pro-inflammatory cytokines, such as interleukin-6 (IL-6) (M. M. Chen, Palmer, et al., 2013; Colantoni et al., 2000; Li, Akhtar, Kovacs, Gamelli, & Choudhry, 2011), which can quickly accumulate in the pulmonary vasculature (M. M. Chen, Bird, et al., 2013). Clinically, elevated IL-6 correlates with increased mortality risk in burn patients (Drost et al., 1993; F. L. Yeh, Lin, Shen, & Fang, 1999). Furthermore, the absence of the IL-6 gene or IL-6 blockade confers protection against the increased pulmonary inflammation of the combined insult of alcohol and burn (M. M. Chen, Bird, et al., 2013), suggesting a crucial role for IL-6 in driving this aberrant response. Therefore, the cellular source and impetus for IL-6 production may be key to understanding how alcohol modulates the pulmonary response to remote injury, resulting in subsequent alterations in lung vasculature, lung dysfunction, and susceptibility to infection.
The high baseline bacterial content of the intestines represents an enormous potential reservoir for systemic infections and sepsis after injury (Bahrami, Redl, Yao, & Schlag, 1996; Deitch, 2001; Hassoun et al., 2001). Animal studies demonstrate that post-burn intestinal permeability is exacerbated when intoxication precedes the injury (Choudhry, Fazal, Goto, Gamelli, & Sayeed, 2002; Kavanaugh et al., 2005), leading to greater translocation of bacteria and bacterial products, such as lipopolysaccharide (LPS), into the lymphatic (Baron et al., 1994; Deitch, Maejima, & Berg, 1985; Zahs, Bird, Ramirez, Choudhry, & Kovacs, 2013) and portal (Deitch, 1990) systems. This occurs secondary to alcohol’s potentiating effect on bradykinin signaling, thereby enhancing post-burn third spacing of fluid which, together with catecholamine-mediated splanchnic vasoconstriction, leads to ischemic injury in the bowel (M. M. Chen et al., 2015). Portal blood then carries the bacterial products to the body’s largest tissue-fixed macrophage population, the Kupffer cells of the liver. Kupffer cells continuously sample portal blood for foreign antigens and orchestrate the hepatic response to injury (Wisse, 1974). Kupffer cells can become activated when LPS binds to toll-like receptor 4 (TLR4), an interaction dependent on its co-receptor, CD14 (Su et al., 2002). Even a single dose of alcohol may sensitize Kupffer cells to LPS via a variety of mechanisms, including changes in CD14 expression (Enomoto et al., 1998), lipid rafts (Dolganiuc, Bakis, Kodys, Mandrekar, & Szabo, 2006), and mitogen-activated protein kinases (MAPKs) (Kishore, Hill, McMullen, Frenkel, & Nagy, 2002; Kishore, McMullen, & Nagy, 2001), the functional consequence of which is increased TLR4 signaling and cytokine production. At low levels, cytokines, such as IL-6, are beneficial to hepatocyte survival, but exposure to levels above an acceptable threshold can be detrimental (Jin et al., 2006). When alcohol precedes a burn, there is both increased stimulus for and sensitivity to TLR4 signaling, leading to harmful levels of IL-6 relative to either insult alone. Accordingly, the combined insult has been shown to result in greater hepatic damage as evidenced by elevated serum transaminase levels, hepatic triglycerides, and liver-weight to bodyweight ratio (M. M. Chen et al., 2014, 2015; M. M. Chen, Palmer, et al., 2013). Using the same model of intoxication and burn in which mice are deficient in TLR4 but not TLR2, IL-6 production and organ damage are attenuated after alcohol intoxication and burn injury compared to wild-type mice (Bird, Zahs, et al., 2010).
The interaction between the intestinal microbiome and the liver is known as the gut-liver axis and is thought to regulate a myriad of human diseases (Compare et al., 2012; Seo & Shah, 2012; Szabo & Bala, 2010; Tabibian, O’Hara, & Larusso, 2012; Volta, Caio, Tovoli, & De Giorgio, 2013). Because alcohol can increase post-burn intestinal damage while simultaneously augmenting the hepatic IL-6 response to intestine-derived LPS (M. M. Chen et al., 2014), the gut-liver axis may play an especially prominent role in how alcohol drives post-burn pulmonary inflammation. Supporting this idea are findings that sterilization of the gut before intoxication and burn or pharmacologic restoration of intestinal permeability after injury decreases bacterial translocation and subsequent hepatic damage and IL-6 production (M. M. Chen et al., 2014). Interestingly, interrupting the crosstalk between the gut and liver after intoxication and burn corresponds to diminished pulmonary inflammation as well (M. M. Chen et al., 2014). Similarly, antecedent depletion of Kupffer cells leads to decreased systemic IL-6 and improved pulmonary parameters after intoxication and burn despite no effect on the alveolar macrophage (AM) population (M. M. Chen, O’Halloran, Shults, & Kovacs, in press), again highlighting the role of the gut-liver axis in driving pulmonary inflammation in this setting.
These findings do not exclude the importance of direct effects of alcohol on the lung, including impairment of AM phagocytosis (Karavitis, Murdoch, Deburghgraeve, Ramirez, & Kovacs, 2012; Murdoch et al., 2008) and pulmonary neutrophil sequestration (Murdoch, Karavitis, Deburghgraeve, Ramirez, & Kovacs, 2011). Rather, they suggest that the full effects of alcohol on the pulmonary response to remote injury may be multifactorial and involve effects on multiple organ systems.
Alcohol-mediated ciliated airway dysfunction
Ciliated airway cells clear inhaled particles from the lung, thus acting as the first line of defense against inhaled pathogens. During alcohol ingestion, these ciliated cells are uniquely exposed to vapor-phase alcohol during breathing. The high alcohol blood concentration in the bronchial circulation is directly exposed to the ciliated airways. This occurs because, during alcohol ingestion, alcohol is off-gassed from the bronchial circulation of the conducting airways into the exhaled breath, resulting in alcohol flux through the airway epithelium into the exhaled air (George, Hlastala, Souders, & Babb, 1996). Indeed, this is the mechanism for delivery of alcohol into the airways, which is the basis for the Breathalyzer™ test. Importantly, brief low-dose alcohol exposure has very different effects on airway cilia than prolonged high-dose alcohol exposure. These bimodal effects of alcohol on airway cilia function share related but different mechanisms.
Alcohol modifies airway cilia in two ways. Brief exposure to moderate concentrations of alcohol stimulates cilia to beat faster through a nitric oxide-dependent mechanism (Sisson, Pavlik, & Wyatt, 2009). Conversely, prolonged alcohol exposure causes desensitization of cilia, making motility resistant to stimulation, a process known as alcohol-induced ciliary dysfunction (AICD), through a mechanism related to oxidant stress (Simet, Pavlik, & Sisson, 2013a; Wyatt, Gentry-Nielsen, Pavlik, & Sisson, 2004). While the mechanisms of alcohol-driven cilia stimulation and AICD are known to involve dysregulation of key cilia kinases and phosphatases that regulate motility, the upstream triggers of these post-translational processes are unknown. One mechanism may be through post-translational modifications in key kinases and phosphatases that regulate the effects of alcohol on airway ciliary control. Specifically, studies have focused on alcohol’s ability to modulate phosphorylation and S-nitrosylation of target molecular regulatory pathways in airway cilia, thereby providing insight into the differential effects of brief versus prolonged alcohol exposure on the ciliated airway epithelium.
Two approaches to explore protein changes in alcohol-exposed isolated bovine tracheal cilia were developed. 1) 2-dimensional gel analysis of ciliary proteins briefly exposed to modest concentrations of alcohol was performed. The alcohol-modified ciliary protein blots were probed with phospho-antibodies, and mass spectroscopy was used to detect unique alcohol-driven phosphorylated protein sites. 2) The formation of S-nitrosylated ciliary proteins was separately examined with mass spectroscopy to identify post-translationally modified ciliary proteins after prolonged alcohol exposure and experiments to mimic AICD.
Brief alcohol exposure increases phosphorylation of heat shock protein 90 (HSP90) in isolated demembranated bovine tracheal cilia
Using the first approach, brief alcohol exposure (1 h with 100-mM ethanol) significantly stimulated serine/threonine phosphorylation of only 6 out of over 600 (1%) ciliary proteins. Most of the 6 were structural proteins, but conspicuous among that group was the striking phosphorylation of HSP90. Functional experiments were performed to confirm that HSP90 is required for alcohol to stimulate cilia via a chaperone and translocation mechanism, likely involving intraflagellar transport (Simet, Pavlik, & Sisson, 2013b).
Prolonged alcohol exposure causes a 20-fold increase of S-nitrosylation of protein phosphatase 1 (PP1) in isolated demembranated bovine tracheal cilia
With prolonged alcohol exposure (24 h with 100-mM ethanol), S-nitrosylation was decreased in 158 of 626 (25%) ciliary proteins but increased in 121 of 626 (19%) ciliary proteins. Of these proteins, 10 unique S-nitrosylation sites, corresponding to 6 unique proteins, demonstrated a >20-fold increase in S-nitrosylation by alcohol exposure. Four of these unique sites corresponded to different residues of PP1. Preliminary functional cilia experiments demonstrated that prolonged alcohol exposure of ciliated cells upregulated PP1 activity and blocked the cilia desensitization seen in AICD (Price, Pavlik, Sisson, & Wyatt, 2015).
Alcohol-induced alveolar macrophage (AM) oxidative stress and dysfunction
When the upper airway fails to clear inhaled pathogens, they enter the alveolar space where they are primarily cleared by AMs. In the alveolar space, AM surveillance is the first line of defense in cellular immunity because the AMs ingest and clear pathogens as well as release cytokines and chemokines to recruit other immune cells (Aderem & Underhill, 1999). Although the molecular mechanisms are poorly understood, oxidative stress results in impaired phagocytosis and diminished pathogen clearance. Alcohol induces an oxidized microenvironment within the lung. In a sampling of the alveolar epithelial lining fluid (ELF) of otherwise healthy patients with a history of AUDs, chronic alcohol ingestion depleted the critical antioxidant glutathione (GSH) and caused corresponding oxidation of the GSH pool to glutathione disulfide (GSSG), resulting in oxidation of the GSH/GSSG redox potential (Liang, Yeligar, & Brown, 2012; Moss et al., 2000; F. L. Yeh et al., 1999). GSH and oxidation of the GSH/GSSG potential were similarly depleted in the exhaled breath condensate (EBC) of alcoholic subjects (M. Y. Yeh, Burnham, Moss, & Brown, 2008). Experimental animal models of chronic alcohol ingestion demonstrated similar oxidation of the lung microenvironment. GSH levels were abrogated in the lungs and bronchoalveolar lavage (BAL) fluid of ethanol-fed rats (Holguin, Moss, Brown, & Guidot, 1998) and mice (Yeligar, Harris, Hart, & Brown, 2014). Collectively, these studies indicate that chronic alcohol abuse alters GSH homeostasis in the lung, leading to an increasingly oxidized pulmonary microenvironment.
The AM is acutely affected by chronic alcohol ingestion and the oxidized lung microenvironment that results from alcohol abuse. Through multifactorial mechanisms, alcohol stimulates oxidative stress within the AM, resulting in impaired phagocytic capacity and decreased bacterial clearance. The mechanisms involved in alcohol-induced AM oxidative stress include altered GSH/GSSG redox status, decreased intracellular zinc, attenuated nuclear factor erythroid 2 [NF-E2]-related factor 2 (Nrf2), diminished granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor beta (GM-CSFRβ), depleted peroxisome proliferator-activated receptor gamma (PPARγ), enhanced NADPH oxidases (Nox), and increased transforming growth factor beta-1 (TGFβ1). Experimental manipulations of each of these mechanisms attenuated alcohol-induced oxidative stress and improved AM immune function.
AMs depend on the GSH in the ELF pool for cellular uptake and protection against the oxidative stress generated during immune responses (Liang, Harris, & Brown, 2014). During chronic alcohol ingestion, AMs themselves exhibit alterations in GSH/GSSG redox status. As observed in the ELF, AMs isolated from ethanol-fed mice had decreased GSH levels, increased GSSG, and increased oxidation of the GSH/GSSG redox potential. This was associated with compromised in vitro fluorescent Staphylococcus aureus (S. aureus) internalization and impaired in vivo clearance of Klebsiella pneumoniae (K. pneumoniae) (Yeligar et al., 2014). These studies demonstrated that alcohol-induced alterations in AM GSH/GSSG redox status resulted in impaired AM phagocytic capacity and bacterial clearance.
Zinc deficiency and down-regulation of Nrf2, GM-CSFRβ, and PPARγ are additional mechanisms that drive AM oxidative stress during chronic alcohol ingestion. AM isolated from alcoholic subjects had decreased intracellular zinc levels, and ex vivo zinc treatments restored AM capacity to bind and internalize in vitro fluorescent S. aureus (Mehta, Yeligar, Elon, Brown, & Guidot, 2013). Additionally, ethanol-fed female mice had decreased AM zinc levels, zinc transporter expression, and bacterial clearance (Konomi, Harris, Ping, Gauthier, & Brown, 2015). Nrf2, a zinc-dependent protein essential for antioxidant defenses, was also depleted by chronic alcohol ingestion in rat AMs (Staitieh, Fan, Neveu, & Guidot, 2015), causing impaired in vivo lung bacterial clearance of K. pneumoniae (Mehta et al., 2011). GM-CSFRβ, which initiates intracellular signaling cascades responsible for AM function including phagocytosis, was decreased in AM from ethanol-fed rats (Joshi et al., 2005, 2006; Joshi, Mehta, Jabber, Fan, & Guidot, 2009) and alcoholic subjects (Mehta et al., 2013) could be restored by GM-CSF treatments. Further, alcohol-induced AM depletions in PPARγ, which is associated with augmented oxidative stress, and treatment with PPARγ ligands reversed impaired phagocytic function and bacterial clearance capacity (Yeligar, Mehta, Harris, Brown, & Hart, 2015). Collectively, these studies indicate that diminutions in intracellular zinc, Nrf2, GM-CSFRβ, and PPARγ result in an oxidized lung redox status and subsequent AM dysfunction.
Reactive oxygen species (ROS) play important roles in disease pathogenesis due to their involvement in complex physiological processes (Thannickal & Fanburg, 2000). Nox proteins, which are membrane-associated enzymes that use NADPH as an electron donor to catalyze the reduction of molecular oxygen to superoxide and hydrogen peroxide (H2O2) (D. I. Brown & Griendling, 2009), are major sources of ROS in the lungs under physiologic conditions (Piotrowski & Marczak, 2000). Nox isoforms Nox1, Nox2, and Nox4 are expressed in the lung, where Nox1 and Nox2 primarily produce superoxide and Nox4 primarily produces H2O2 (Mittal et al., 2007; Polikandriotis et al., 2006). Additionally, TGFβ1 up-regulates Nox4 expression (D. I. Brown & Griendling, 2009). Increased Nox1, Nox2, and Nox4 in ethanol-exposed mouse embryos (Dong, Sulik, & Chen, 2010), and ethanol-fed rat and mouse lungs (Polikandriotis et al., 2006; Wagner, Yeligar, Brown, & Hart, 2012), were found to be key sources of ROS production. AMs from ethanol-fed mice showed that increases in the expression of Nox1, Nox2, Nox4 (Yeligar, Harris, Hart, & Brown, 2012), and TGFβ1 (S. D. Brown & Brown, 2012) enhanced oxidative stress and impaired AM phagocytosis. These studies demonstrated that alcohol- induced expression of Noxes and TGFβ1 are also associated with AM oxidative stress and phagocytic dysfunction.
Collectively, these studies demonstrate that chronic alcohol ingestion induces an oxidized microenvironment within the lung that subsequently induces AM derangements, as manifested in an impaired capacity to phagocytose and clear bacteria from the alveolar space. Alcohol stimulates oxidative stress through multiple, and potentially interactive, mechanisms including oxidation of the GSH/GSSG redox status, decreased intracellular zinc, attenuated Nrf2, diminished GM-CSFRβ, depleted PPARγ, enhanced Noxes, and increased TGFβ1. Therefore, strategies to reverse any of these mechanisms for alcohol-induced exaggerated oxidative stress in the AM may improve lung immune function and susceptibility to developing respiratory infections in patients with a history of AUDs.
Unraveling the alcohol-pneumococcal pneumonia relationship: clues from translational research
Worldwide, individuals with AUDs are disproportionately affected by Streptococcus pneumoniae, the most common etiologic agent in bacterial pneumonia. Among patients hospitalized with pneumococcal infections, up to 20–30% will meet criteria for an AUD (de Roux et al., 2006; Garcia-Vidal et al., 2010; Plevneshi et al., 2009; van der Poll & Opal, 2009). Further, pneumonia in the setting of AUDs is often associated with extra-pulmonary spread of disease, including bacteremia, sepsis, and septic shock (Garcia-Vidal et al., 2010; Gentile, Sparo, Mercapide, & Luna, 2003; Plevneshi et al., 2009; Shariatzadeh, Huang, Tyrrell, Johnson, & Marrie, 2005). Both pneumonia and sepsis are major risk factors for the development of ARDS; therefore, in patients with AUDs who develop pneumococcal pneumonia, respiratory failure (de Roux et al., 2006) and the development of ARDS (Moss, Bucher, Moore, Moore, & Parsons, 1996; Moss et al., 2003) are unfortunately common in US intensive-care units. In ARDS patients who have AUDs, clinical outcomes are frequently poorer (Clark et al., 2013; Moss et al., 1996; O’Brien et al., 2007), including increased mortality and requirement for persistent hospitalization. Notably, anti-pneumococcal vaccines in individuals with AUDs are problematic in that this population is less likely to receive preventive medical services (Merrick et al., 2008), and that an efficacious response to pneumococcal vaccination is less assured (Benin et al., 2003; Luján et al., 2013). Therefore, understanding the pathophysiologic mechanisms underlying increased severity of illness and poorer outcomes in the setting of pneumococcal pneumonia among those with AUDs represents a major clinical need.
AUDs have been associated with both pulmonary and systemic oxidative stress (M. Y. Yeh, Burnham, Moss, & Brown, 2007) that may contribute to poorer outcomes in the setting of pulmonary infections, including pneumococcal pneumonia. Specifically, oxidative stress has been associated with a myriad of deleterious effects on pulmonary immune system function, including poorer apoptotic cell clearance (Moon, Lee, Park, Chong, & Kang, 2010), induction of autophagy (Malaviya, Laskin, & Laskin, 2014), abnormalities in ciliary morphology and function (Sunil et al., 2013), and impaired AM phagocytosis (Mehta et al., 2013). GSH is the quantitatively most abundant pulmonary antioxidant, and abnormalities in GSH homeostasis, favoring oxidation, have been consistently observed in the alveolar space in the setting of AUDs (Burnham, Brown, Halls, & Moss, 2003; M. Y. Yeh et al., 2007). Chronic alcohol exposure’s relationship to oxidative stress in the systemic circulation (Burnham et al., 2012; Yang et al., 2010), liver (C. H. Chen, Pan, Chen, & Huang, 2011; Leung & Nieto, 2013; Sid, Verrax, & Calderon, 2013), gut (Abdelmegeed et al., 2013; Keshavarzian et al., 2009; S. M. Tang, Gabelaia, Gauthier, & Brown, 2009), and brain (Herrera et al., 2003), both in animals and humans, has also been noted. Importantly, pulmonary GSH homeostasis remains abnormal even after abstinence from alcohol (Burnham et al., 2003, 2012).
The precise impact of alcohol-associated oxidative stress on pulmonary innate immunity remains to be determined in humans, but may represent one modifiable factor that could positively influence outcomes in AUD patients with pneumococcal pneumonia. As noted above, AMs are responsible for initiating and regulating the pulmonary immune response in the setting of infection. When stimulated by pathogen-associated molecular peptides (PAMPs), AMs secrete a variety of pro-inflammatory chemokines and cytokines, including IL-6, IL-1β, and tumor necrosis factor (TNF)-α. Although these mediators play an important role in generating an immune response against invading pathogens (D. Tang, Kang, Coyne, Zeh, & Lotze, 2012), they have also been implicated in the pathogenesis of lung injury and ARDS (Meduri et al., 1995; Park et al., 2001). Since bacterial pneumonia is a common risk factor for the development of ARDS (Matthay, Ware, & Zimmerman, 2012), the marked lung inflammation, followed by progressive epithelial and endothelial damage, and subsequent neutrophil influx to the alveolar space (Williams & Chambers, 2014), are also thought to be related, in part, to PAMP activation of AMs (Aggarwal, King, & D’Alessio, 2014; Herold, Mayer, & Lohmeyer, 2011; Rosseau et al., 2000; Steinberg et al., 1994). Earlier investigations have supported an association between AUDs with diminished AM activation (Omidvari, Casey, Nelson, Olariu, & Shellito, 1998). However, in experiments with human BAL cells that are comprised of ~90% AMs, it was recently reported that AUDs appear to be associated with an activated, pro-inflammatory BAL cell phenotype with an exaggerated response to LPS (Gaydos et al., 2016; O’Halloran et al., 2016). Moreover, data indicated that the BAL cells’ response may be partially driven by oxidative stress (Gaydos et al., 2016). Other pre-clinical investigations performed in models of chronic alcohol exposure further support a pro-inflammatory monocyte response to LPS stimulation in vitro (Mandrekar, Bala, Catalano, Kodys, & Szabo, 2009), as well as augmented BAL neutrophils in response to LPS exposure in vivo (Boé et al., 2010). Therefore, AMs may be activated in the setting of AUDs, and upon exposure to pathogens, AMs display an over-exuberant, pro-inflammatory response that contributes to the increased morbidity in pneumonia and the predisposition to develop ARDS.
Preliminary experiments in a small number of healthy subjects with AUDs and smoking-matched controls (n = 10 in each group) were performed to determine if exposure of BAL cells (~90% AMs) to heat-killed S. pneumoniae protein (ATCC #55143, strain JY2008) at doses ranging from 0–10 μg over a 42-h time period would elicit a pro-inflammatory response, analogous to what was previously observed with LPS (Gaydos et al., 2016). BAL cell elaboration of IFNγ, IL-6, and TNFα were increased among AUD subjects with the 10-μg S. pneumoniae protein dose after 18 h in culture (p < 0.05). Addition of 10-mM NAC concurrent with S. pneumoniae protein diminished, but did not normalize AM pro-inflammatory cytokine secretion at 18 hours’ culture time. In separate experiments, when NAC was added after BAL cells had been in culture with S. pneumoniae protein for 18 h, IL-6, TNFα, and IFNγ secretions were less than in BAL cells cultured with S. pneumoniae protein, but without NAC, after 42 h in culture.
These data suggest that when BAL cells (primarily AMs) from individuals with AUDs are stimulated by pathogens, pro-inflammatory cytokine production is more robust. The over-exuberant response by AMs may have implications for the severity of illness among individuals with pulmonary infections. Modulation of AM oxidative stress represents a potential mechanism to restore appropriate immune cell function in the setting of pneumococcal infection.
Conclusion
Patients with AUDs have enhanced susceptibility to lung injury and respiratory infections, which increase economic costs, morbidity, and mortality. There are several potential mechanisms by which alcohol causes lung injury and impairs lung immunity. Alcohol intoxication, combined with burn injuries, activates the gut-liver axis and drives pulmonary inflammation, promoting morbidity and mortality. Other mechanisms that activate the gut-liver axis may have similar results when superimposed on alcohol intoxication. During alcohol-induced lung immune dysfunction, the upper airway is the first checkpoint to fail in the clearance of respiratory pathogens due to differential post-translational modifications of novel proteins that control cilia function. Proteomic approaches are needed to identify novel alcohol targets and post-translational modifications in airway cilia for therapeutic interventions. When inhaled pathogens are not cleared in the upper airway, they enter the alveolar space, where they are phagocytized and cleared by AMs. With chronic alcohol ingestion, oxidative stress pathways in the AM are stimulated, thereby impairing AM immune capacity and pathogen clearance. AUDs increase both the predisposition and illness severity of pneumococcal pneumonia infections, which may be due to the pro-inflammatory and oxidative stress response of AMs.
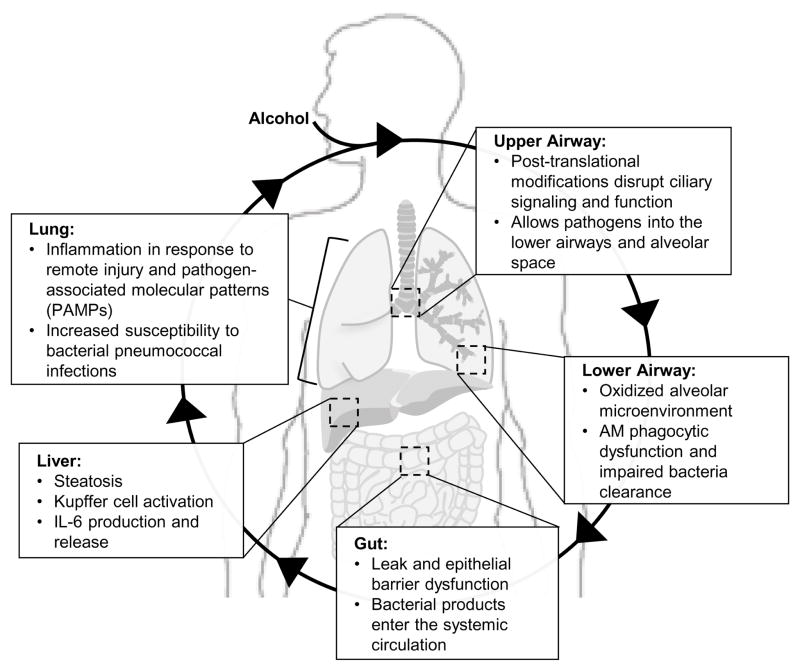
These combined studies suggest that alcohol-induced gut leakiness and liver macrophage activation may drive cytokine expression, resulting in systemic oxidative stress and lung injury. This lung injury starts with the desensitization of the ciliated airway epithelium, causing impaired clearance of inhaled pathogens from the upper airway. Altered activation of alcoholic alveolar macrophages in the lower airway not only impairs bacterial phagocytosis and clearance, but may also induce the release of more cytokines into the circulation. Kupffer cells, resident liver macrophages, demonstrate up-regulation of pro-inflammatory transcription factors and pathways, including hypoxia inducible factor-1 alpha and activator protein-1 (Yeligar, Machida, & Kalra, 2010). Circulating cytokines may further perpetuate systemic oxidative stress that results from alcohol-use disorders. This cycle of systemic oxidative stress and the effects it may have on the gut-liver-lung axis have been summarized in Fig. 1.
Fig. 1. Hypothetical schema summarizing alcohol-induced systemic oxidative stress.
During prolonged alcohol use, the ciliated airway epithelium of the upper airway undergoes post-translational modifications that disrupt ciliary signaling and function, allowing inhaled pathogens into the lower airways and alveolar space. The oxidized alveolar microenvironment in the lower airway alters alveolar macrophage activation, impairing phagocytosis and bacterial clearance. Alcohol-induced gut leak and epithelial barrier dysfunction in the gut cause bacterial products to enter the systemic circulation, leading to subsequent liver steatosis, Kupffer cell activation, and interleukin-6 (IL-6) production and release. Circulating bacterial products and chemokines exacerbate the lung inflammatory response to remote injury and pathogen-associated molecular patterns (PAMPs), increasing susceptibility to bacterial pneumococcal infections.
Although macrophages are derived from monocytes, the ability of AMs to be recruited into the alveolar space suggests that they represent a population of phagocytes present in this microenvironment that are very different from the resident macrophages of other tissues. During alcohol use, the AM response to remote injury and systemic oxidative stress renders the host susceptible to infection due to an over-exuberant, pro-inflammatory response that perpetuates even further systemic oxidative stress. However, AMs exhibit an immunosuppressed phenotype. In fact, some studies suggest that alcohol compromises host defenses against bacterial pneumonia, such as K. pneumoniae, by suppressing the recruitment and bactericidal activity of polymorphonuclear leukocytes into the lung (Nelson et al., 1991) and attenuating lung expression of CXC chemokines bearing the Glu-Leu-Arg motif, which attracts T cells (Happel et al., 2007). There is high plasticity in AM activation that influences how they clear microbes and respond to pathogens. The molecular mechanisms by which AM activation affects their response, and their plasticity in range of responses to pathogens and clearance of microbes, warrant further study. The commonly accepted distinguishing factor between AM activation states centers around their ability to perform phagocytosis. In AMs isolated from human subjects with alcohol-use disorders, phagocytosis is only impaired by ~50% (Mehta et al., 2013; Yeligar et al., 2015), suggesting a heterogeneous AM population where some cells can phagocytize and clear bacteria from the alveolar space and others cannot.
Additionally, current tests that are commonly used to identify individuals with alcohol-use disorders and to categorize severity of consumption, including the Short Michigan Alcohol Screening Test and the Alcohol Use Disorders Identification Test (AUDIT), do not factor in other clinical variables that may affect the response of AMs to various stimuli, such as age, sex, race/ethnicity, or environmental effects of cigarette smoking, that are common among unhealthy alcohol consumers. Therefore, clinical investigations should carefully characterize these potential confounders to most accurately study the effect of alcohol on outcome variables since these confounders have the potential to contribute to the heterogeneity of human AM populations and phenotype plasticity. Further study in suitably powered populations is warranted to delineate and characterize heterogeneous AM populations and their phenotypes in individuals with alcohol-use disorders.
Focused proteomic approaches are needed to identify alcohol-driven post-translational modifications of key functional proteins that regulate airway host defenses. Such approaches will have broad applications in the identification of novel protein targets for alcohol in the lungs and other organs affected by alcohol exposure. These studies may well lead to the development of useful biomarkers of alcohol exposure and therapeutic approaches to correct impaired lung host defenses. Additionally, novel therapeutic strategies to abrogate alcohol-induced AM oxidative stress, such as treatments with NAC or GSH, could potentially restore pathogen clearance by AMs. Therapies that target oxidative stress as a means to normalize the immune response have potentially particular benefit in the AUD setting; N-acetylcysteine (NAC), a thiol precursor of GSH, represents one potential therapeutic option. NAC has been demonstrated to replenish intracellular GSH and to directly scavenge ROS at target cells. Small trials (n < 30 subjects) of NAC in patients with ARDS have demonstrated that intravenous NAC was efficacious in increasing the number of organ failure-free days (days a patient was both alive and without organ failure) (Bernard et al., 1997; Soltan-Sharifi et al., 2007), and had an excellent safety profile in the critically ill. Understanding the value of NAC to diminish pulmonary oxidative stress in the setting of chronic alcohol exposure, and its impact on AM activation, would help to determine whether antioxidant therapy might be useful as an adjunct for patients with pneumococcal pneumonia to ameliorate outcomes. AMs isolated from ethanol-fed rats also showed enhanced oxidation of the GSH/GSSG redox potential and impaired in vitro fluorescent S. aureus internalization that could be reversed with GSH treatments (L. A. Brown, Ping, Harris, & Gauthier, 2007). Further explorations into the molecular mechanisms of alcohol-mediated respiratory derangements are necessary for the discovery of novel therapeutic interventions to mitigate alcohol-induced lung injury and immune dysfunction.
Highlights.
Mechanisms by which alcohol causes lung injury and immune dysfunction are reviewed.
Gut-liver-lung axis regulates lung inflammation after intoxication and burn injury.
Alcohol mediates ciliated airway dysfunction via post-translational modifications.
Alcohol induces alveolar macrophage oxidative stress and phagocytic dysfunction.
Translational research shows the link between alcohol and pneumococcal pneumonia.
Acknowledgments
The section titled, “Alcohol intoxication exacerbates pulmonary response to remote injury: gut-liver-lung axis regulates pulmonary inflammation after intoxication and burn injury”, was supported by NIH grants R01 GM115257 (EJK), R01 AA012034 (EJK), R21 AA023193 (EJK), T32 AA013527 (EJK), F30 AA022856 (MMC), and the Dr. Ralph and Marian C. Falk Medical Research Trust (EJK). The section titled, “Alcohol-mediated ciliated airway dysfunction”, was supported by the NIH grants R01 AA008769 (JHS), F32 AA019859 (Samantha M. Simet), and F30 AA024676 (Michael E. Price). The section titled, “Alcohol-induced alveolar macrophage oxidative stress and dysfunction”, was supported by the Emory Alcohol & Lung Biology Center P50 AA135757 (LAB), AHA SDG 13SDG13930003 (SMY), K99 AA021803 (SMY), and R00 AA021803 (SMY). The section titled, “Unraveling the alcohol-pneumococcal pneumonia relationship: clues from translational research”, was supported by the R24 AA019661 (ELB) and the University of Colorado Department of Medicine Team Science Award (ELB). We would also like to extend our thanks to Brandy E. Wade, PhD for her contributions to the illustration depicted in Fig. 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABA. National Burn Repository: 2012 Report. Chicago, IL: American Burn Association; 2012. [Google Scholar]
- Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, et al. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radical Biology & Medicine. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual Review of Immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. American Journal of Physiology Lung Cellular and Molecular Physiology. 2014;306:L709–725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami S, Redl H, Yao YM, Schlag G. Involvement of bacteria/endotoxin translocation in the development of multiple organ failure. Current Topics in Microbiology and Immunology. 1996;216:239–258. doi: 10.1007/978-3-642-80186-0_11. [DOI] [PubMed] [Google Scholar]
- Baron P, Traber LD, Traber DL, Nguyen T, Hollyoak M, Heggers JP, et al. Gut failure and translocation following burn and sepsis. The Journal of Surgical Research. 1994;57:197–204. doi: 10.1006/jsre.1994.1131. [DOI] [PubMed] [Google Scholar]
- Benin AL, O’Brien KL, Watt JP, Reid R, Zell ER, Katz S, et al. Effectiveness of the 23-valent polysaccharide vaccine against invasive pneumococcal disease in Navajo adults. The Journal of Infectious Diseases. 2003;188:81–89. doi: 10.1086/375782. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- Bird MD, Morgan MO, Ramirez L, Yong S, Kovacs EJ. Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice. Journal of Burn Care & Research. 2010;31:652–660. doi: 10.1097/BCR.0b013e3181e4c58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MD, Zahs A, Deburghgraeve C, Ramirez L, Choudhry MA, Kovacs EJ. Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcoholism: Clinical and Experimental Research. 2010;34:1733–1741. doi: 10.1111/j.1530-0277.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boé DM, Richens TR, Horstmann SA, Burnham EL, Janssen WJ, Henson PM, et al. Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response. Alcoholism: Clinical and Experimental Research. 2010;34:1723–1732. doi: 10.1111/j.1530-0277.2010.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventive Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Brezel BS, Kassenbrock JM, Stein JM. Burns in substance abusers and in neurologically and mentally impaired patients. The Journal of Burn Care & Rehabilitation. 1988;9:169–171. doi: 10.1097/00004630-198803000-00009. [DOI] [PubMed] [Google Scholar]
- Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radical Biology & Medicine. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. American Journal of Physiology Lung Cellular and Molecular Physiology. 2007;292:L824–832. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- Brown SD, Brown LA. Ethanol (EtOH)-induced TGF-β1 and reactive oxygen species production are necessary for EtOH-induced alveolar macrophage dysfunction and induction of alternative activation. Alcoholism: Clinical and Experimental Research. 2012;36:1952–1962. doi: 10.1111/j.1530-0277.2012.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham EL, Brown LA, Halls L, Moss M. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcoholism: Clinical and Experimental Research. 2003;27:1167–1172. doi: 10.1097/01.ALC.0000075821.34270.98. [DOI] [PubMed] [Google Scholar]
- Burnham EL, McCord JM, Bose S, Brown LA, House R, Moss M, et al. Protandim does not influence alveolar epithelial permeability or intrapulmonary oxidative stress in human subjects with alcohol use disorders. American Journal of Physiology Lung Cellular and Molecular Physiology. 2012;302:L688–699. doi: 10.1152/ajplung.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Pan CH, Chen CC, Huang MC. Increased oxidative DNA damage in patients with alcohol dependence and its correlation with alcohol withdrawal severity. Alcoholism: Clinical and Experimental Research. 2011;35:338–344. doi: 10.1111/j.1530-0277.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- Chen MM, Bird MD, Zahs A, Deburghgraeve C, Posnik B, Davis CS, et al. Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol. 2013;47:223–229. doi: 10.1016/j.alcohol.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, O’Halloran EB, Ippolito JA, Choudhry MA, Kovacs EJ. Alcohol potentiates postburn remote organ damage through shifts in fluid compartments mediated by bradykinin. Shock. 2015;43:80–84. doi: 10.1097/SHK.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, O’Halloran EB, Shults JA, Kovacs EJ. Kupffer Cell p38 Mitogen-Activated Protein Kinase Signaling Drives Postburn Hepatic Damage and Pulmonary Inflammation When Alcohol Intoxication Precedes Burn Injury. Critical Care Medicine. 2016 doi: 10.1097/CCM.0000000000001817. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, Palmer JL, Ippolito JA, Curtis BJ, Choudhry MA, Kovacs EJ. Intoxication by intraperitoneal injection or oral gavage equally potentiates postburn organ damage and inflammation. Mediators of Inflammation. 2013;2013:971481. doi: 10.1155/2013/971481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, Zahs A, Brown MM, Ramirez L, Turner JR, Choudhry MA, et al. An alteration of the gut-liver axis drives pulmonary inflammation after intoxication and burn injury in mice. American Journal of Physiology Gastrointestinal and Liver Physiology. 2014;307:G711–718. doi: 10.1152/ajpgi.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. American Journal of Physiology Gastrointestinal and Liver Physiology. 2002;282:G937–947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Williams A, Feemster LM, Bradley KA, Macht M, Moss M, et al. Alcohol screening scores and 90-day outcomes in patients with acute lung injury. Critical Care Medicine. 2013;41:1518–1525. doi: 10.1097/CCM.0b013e318287f1bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantoni A, Duffner LA, De Maria N, Fontanilla CV, Messingham KA, Van Thiel DH, et al. Dose-dependent effect of ethanol on hepatic oxidative stress and interleukin-6 production after burn injury in the mouse. Alcoholism: Clinical and Experimental Research. 2000;24:1443–1448. [PubMed] [Google Scholar]
- Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, et al. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutrition, Metabolism, and Cardiovascular Diseases. 2012;22:471–476. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Curtis BJ, Hlavin S, Brubaker AL, Kovacs EJ, Radek KA. Episodic binge ethanol exposure impairs murine macrophage infiltration and delays wound closure by promoting defects in early innate immune responses. Alcoholism: Clinical and Experimental Research. 2014;38:1347–1355. doi: 10.1111/acer.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CS, Esposito TJ, Palladino-Davis AG, Rychlik K, Schermer CR, Gamelli RL, et al. Implications of alcohol intoxication at the time of burn and smoke inhalation injury: an epidemiologic and clinical analysis. Journal of Burn Care & Research. 2013;34:120–126. doi: 10.1097/BCR.0b013e3182644c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, et al. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129:1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Archives of Surgery. 1990;125:403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- Deitch EA. Role of the gut lymphatic system in multiple organ failure. Current Opinion in Critical Care. 2001;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Maejima K, Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. The Journal of Trauma. 1985;25:385–392. doi: 10.1097/00005373-198505000-00002. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcoholism: Clinical and Experimental Research. 2006;30:76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Dong J, Sulik KK, Chen SY. The role of NOX enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos. Toxicology Letters. 2010;193:94–100. doi: 10.1016/j.toxlet.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost AC, Burleson DG, Cioffi WG, Jr, Jordan BS, Mason AD, Jr, Pruitt BA., Jr Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. The Journal of Trauma. 1993;35:335–339. doi: 10.1097/00005373-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Bradford B, Rivera C, Kono H, Brenner DA, et al. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Vidal C, Ardanuy C, Tubau F, Viasus D, Dorca J, Liñares J, et al. Pneumococcal pneumonia presenting with septic shock: host- and pathogen-related factors and outcomes. Thorax. 2010;65:77–81. doi: 10.1136/thx.2009.123612. [DOI] [PubMed] [Google Scholar]
- Gaydos J, McNally A, Guo R, Vandivier RW, Simonian PL, Burnham EL. Alcohol abuse and smoking alter inflammatory mediator production by pulmonary and systemic immune cells. American Journal of Physiology Lung Cellular and Molecular Physiology. 2016;310:L507–518. doi: 10.1152/ajplung.00242.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile JH, Sparo MD, Mercapide ME, Luna CM. Adult bacteremic pneumococcal pneumonia acquired in the community. A prospective study on 101 patients. Medicina (B Aires) 2003;63:9–14. [PubMed] [Google Scholar]
- George SC, Hlastala MP, Souders JE, Babb AL. Gas exchange in the airways. Journal of Aerosol Medicine. 1996;9:25–33. doi: 10.1089/jam.1996.9.25. [DOI] [PubMed] [Google Scholar]
- Grobmyer SR, Maniscalco SP, Purdue GF, Hunt JL. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. The Journal of Burn Care & Rehabilitation. 1996;17(6 Pt 1):532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, et al. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41:325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Frontiers in Immunology. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. The Journal of Clinical Investigation. 1998;101:761–768. doi: 10.1172/JCI1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43:474–484. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- Jong GM, Hsiue TR, Chen CR, Chang HY, Chen CW. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest. 1995;107:214–217. doi: 10.1378/chest.107.1.214. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Mitchell PO, Fernainy K, Roman J, Eaton DC, et al. GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. American Journal of Physiology Lung Cellular and Molecular Physiology. 2006;291:L1150–1158. doi: 10.1152/ajplung.00150.2006. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, et al. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. Journal of Immunology. 2005;175:6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. American Journal of Physiology Lung Cellular and Molecular Physiology. 2007;292:L813–823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. American Journal of Respiratory and Molecular Biology. 2009;41:207–216. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J, Murdoch EL, Deburghgraeve C, Ramirez L, Kovacs EJ. Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcγR-mediated phagocytosis. Cellular Immunology. 2012;274:61–71. doi: 10.1016/j.cellimm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh MJ, Clark C, Goto M, Kovacs EJ, Gamelli RL, Sayeed MM, et al. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31:290–296. doi: 10.1016/j.burns.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Kelley D, Lynch JB. Burns in alcohol and drug users result in longer treatment times with more complications. The Journal of Burn Care & Rehabilitation. 1992;13(2 Pt 1):218–220. doi: 10.1097/00004630-199203000-00008. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, et al. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. Journal of Hepatology. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. American Journal of Physiology Gastrointestinal and Liver Physiology. 2002;282:G6–15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. The Journal of Biological Chemistry. 2001;276:41930–41937. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- Konomi JV, Harris FL, Ping XD, Gauthier TW, Brown LA. Zinc insufficiency mediates ethanol-induced alveolar macrophage dysfunction in the pregnant female mouse. Alcohol and Alcoholism. 2015;50:30–38. doi: 10.1093/alcalc/agu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauing KL, Roper PM, Nauer RK, Callaci JJ. Acute alcohol exposure impairs fracture healing and deregulates β-catenin signaling in the fracture callus. Alcoholism: Clinical and Experimental Research. 2012;36:2095–2103. doi: 10.1111/j.1530-0277.2012.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. Journal of Hepatology. 2013;58:395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Li X, Akhtar S, Kovacs EJ, Gamelli RL, Choudhry MA. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. Journal of Burn Care & Research. 2011;32:489–497. doi: 10.1097/BCR.0b013e3182223c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Harris FL, Brown LA. Alcohol induced mitochondrial oxidative stress and alveolar macrophage dysfunction. BioMed Research International. 2014;2014:371593. doi: 10.1155/2014/371593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Yeligar SM, Brown LA. Chronic-alcohol-abuse-induced oxidative stress in the development of acute respiratory distress syndrome. TheScientificWorldJournal. 2012;2012:740308. doi: 10.1100/2012/740308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liffner G, Bak Z, Reske A, Sjöberg F. Inhalation injury assessed by score does not contribute to the development of acute respiratory distress syndrome in burn victims. Burns. 2005;31:263–268. doi: 10.1016/j.burns.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Luján M, Burgos J, Gallego M, Falcó V, Bermudo G, Planes A, et al. Effects of immunocompromise and comorbidities on pneumococcal serotypes causing invasive respiratory infection in adults: implications for vaccine strategies. Clinical Infectious Diseases. 2013;57:1722–1730. doi: 10.1093/cid/cit640. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Laskin JD, Laskin DL. Oxidative stress-induced autophagy: role in pulmonary toxicity. Toxicology and Applied Pharmacology. 2014;275:145–151. doi: 10.1016/j.taap.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. Journal of Immunology. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. The Journal of Clinical Investigation. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- Mehta AJ, Joshi PC, Fan X, Brown LA, Ritzenthaler JD, Roman J, et al. Zinc supplementation restores PU.1 and Nrf2 nuclear binding in alveolar macrophages and improves redox balance and bacterial clearance in the lungs of alcohol-fed rats. Alcoholism: Clinical and Experimental Research. 2011;35:1519–1528. doi: 10.1111/j.1530-0277.2011.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, Yeligar SM, Elon L, Brown LA, Guidot DM. Alcoholism causes alveolar macrophage zinc deficiency and immune dysfunction. American Journal of Respiratory and Critical Care Medicine. 2013;188:716–723. doi: 10.1164/rccm.201301-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick EL, Hodgkin D, Garnick DW, Horgan CM, Panas L, Ryan M, et al. Unhealthy drinking patterns and receipt of preventive medical services by older adults. Journal of General Internal Medicine. 2008;23:1741–1748. doi: 10.1007/s11606-008-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M, Roth M, König P, Hofmann S, Dony E, Goyal P, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circulation Research. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moon C, Lee YJ, Park HJ, Chong YH, Kang JL. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. American Journal of Respiratory and Critical Care Medicine. 2010;181:374–387. doi: 10.1164/rccm.200907-1061OC. [DOI] [PubMed] [Google Scholar]
- Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clinical Infectious Diseases. 2005;41(Suppl 7):S490–497. doi: 10.1086/432003. [DOI] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. American Journal of Respiratory and Critical Care Medicine. 2000;161(2 Pt 1):414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Critical Care Medicine. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- Murdoch EL, Brown HG, Gamelli RL, Kovacs EJ. Effects of ethanol on pulmonary inflammation in postburn intratracheal infection. Journal of Burn Care & Research. 2008;29:323–330. doi: 10.1097/BCR.0b013e3181667599. [DOI] [PubMed] [Google Scholar]
- Murdoch EL, Karavitis J, Deburghgraeve C, Ramirez L, Kovacs EJ. Prolonged chemokine expression and excessive neutrophil infiltration in the lungs of burn-injured mice exposed to ethanol and pulmonary infection. Shock. 2011;35:403–410. doi: 10.1097/SHK.0b013e31820217c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Summer W, Bagby G, Nakamura C, Stewart L, Lipscomb G, et al. Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. The Journal of Infectious Diseases. 1991;164:901–906. doi: 10.1093/infdis/164.5.901. [DOI] [PubMed] [Google Scholar]
- O’Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Critical Care Medicine. 2007;35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- O’Halloran EB, Curtis BJ, Afshar M, Chen MM, Kovacs EJ, Burnham EL. Alveolar macrophage inflammatory mediator expression is elevated in the setting of alcohol use disorders. Alcohol. 2016;50:43–50. doi: 10.1016/j.alcohol.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidvari K, Casey R, Nelson S, Olariu R, Shellito JE. Alveolar macrophage release of tumor necrosis factor-alpha in chronic alcoholics without liver disease. Alcoholism: Clinical and Experimental Research. 1998;22:567–572. doi: 10.1111/j.1530-0277.1998.tb04294.x. [DOI] [PubMed] [Google Scholar]
- Osler W. The principles and practice of medicine :designed for the use of practitioners and students of medicine. New York: D. Appleton and Company; 1892. [Google Scholar]
- Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, 2nd, Park DR, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2001;164(10 Pt 1):1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- Piotrowski WJ, Marczak J. Cellular sources of oxidants in the lung. International Journal of Occupational Medicine and Environmental Health. 2000;13:369–385. [PubMed] [Google Scholar]
- Plevneshi A, Svoboda T, Armstrong I, Tyrrell GJ, Miranda A, Green K, et al. Population-based surveillance for invasive pneumococcal disease in homeless adults in Toronto. PLoS One. 2009;4:e7255. doi: 10.1371/journal.pone.0007255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, et al. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. American Journal of Respiratory Cell and Molecular Biology. 2006;34:314–319. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ME, Pavlik JA, Sisson JH, Wyatt TA. Inhibition of protein phosphatase 1 reverses alcohol-induced ciliary dysfunction. American Journal of Physiology Lung Cellular and Molecular Physiology. 2015;308:L577–585. doi: 10.1152/ajplung.00336.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseau S, Hammerl P, Maus U, Walmrath HD, Schütte H, Grimminger F, et al. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. American Journal of Physiology Lung Cellular and Molecular Physiology. 2000;279:L25–35. doi: 10.1152/ajplung.2000.279.1.L25. [DOI] [PubMed] [Google Scholar]
- Rush B. An inquiry into the effects of ardent spirits upon the human body and mind: with an account of the means of preventing, and of the remedies for curing them. 4. Philadelphia: Printed for Thomas Dobson; Archibald Bartram, printer; 1808. [Google Scholar]
- Savola O, Niemelä O, Hillbom M. Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol and Alcoholism. 2005;40:269–273. doi: 10.1093/alcalc/agh159. [DOI] [PubMed] [Google Scholar]
- Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clinical and Molecular Hepatology. 2012;18:337–346. doi: 10.3350/cmh.2012.18.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariatzadeh MR, Huang JQ, Tyrrell GJ, Johnson MM, Marrie TJ. Bacteremic pneumococcal pneumonia: a prospective study in Edmonton and neighboring municipalities. Medicine (Baltimore) 2005;84:147–161. doi: 10.1097/01.md.0000164302.03972.d7. [DOI] [PubMed] [Google Scholar]
- Shults JA, Curtis BJ, Chen MM, O’Halloran EB, Ramirez L, Kovacs EJ. Impaired respiratory function and heightened pulmonary inflammation in episodic binge ethanol intoxication and burn injury. Alcohol. 2015;49:713–720. doi: 10.1016/j.alcohol.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sid B, Verrax J, Calderon PB. Role of oxidative stress in the pathogenesis of alcohol-induced liver disease. Free Radical Research. 2013;47:894–904. doi: 10.3109/10715762.2013.819428. [DOI] [PubMed] [Google Scholar]
- Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, et al. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. Journal of Burn Care & Research. 2008;29:784–789. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simet SM, Pavlik JA, Sisson JH. Dietary antioxidants prevent alcohol-induced ciliary dysfunction. Alcohol. 2013a;47:629–635. doi: 10.1016/j.alcohol.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simet SM, Pavlik JA, Sisson JH. Proteomic analysis of bovine axonemes exposed to acute alcohol: role of endothelial nitric oxide synthase and heat shock protein 90 in cilia stimulation. Alcoholism: Clinical and Experimental Research. 2013b;37:609–615. doi: 10.1111/acer.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH, Pavlik JA, Wyatt TA. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcoholism: Clinical and Experimental Research. 2009;33:610–616. doi: 10.1111/j.1530-0277.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Branas CC, Miller TR. Fatal nontraffic injuries involving alcohol: A metaanalysis. Annals of Emergency Medicine. 1999;33:659–668. [PubMed] [Google Scholar]
- Soltan-Sharifi MS, Mojtahedzadeh M, Najafi A, Reza Khajavi M, Reza Rouini M, Moradi M, et al. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Human & Experimental Toxicology. 2007;26:697–703. doi: 10.1177/0960327107083452. [DOI] [PubMed] [Google Scholar]
- Staitieh BS, Fan X, Neveu W, Guidot DM. Nrf2 regulates PU.1 expression and activity in the alveolar macrophage. American Journal of Physiology Lung Cellular and Molecular Physiology. 2015;308:L1086–1093. doi: 10.1152/ajplung.00355.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 1994;150:113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- Su GL, Goyert SM, Fan MH, Aminlari A, Gong KQ, Klein RD, et al. Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. American Journal of Physiology Gastrointestinal and Liver Physiology. 2002;283:G640–645. doi: 10.1152/ajpgi.00253.2001. [DOI] [PubMed] [Google Scholar]
- Sunil VR, Vayas KN, Massa CB, Gow AJ, Laskin JD, Laskin DL. Ozone-induced injury and oxidative stress in bronchiolar epithelium are associated with altered pulmonary mechanics. Toxicological Sciences. 2013;133:309–319. doi: 10.1093/toxsci/kft071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World Journal of Gastroenterology. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibian JH, O’Hara SP, Larusso NF. Primary sclerosing cholangitis: the gut-liver axis. Clinical Gastroenterology and Hepatology. 2012;10:819. doi: 10.1016/j.cgh.2012.01.024. author reply 819–820. [DOI] [PubMed] [Google Scholar]
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunological Reviews. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SM, Gabelaia L, Gauthier TW, Brown LA. N-acetylcysteine improves group B streptococcus clearance in a rat model of chronic ethanol ingestion. Alcoholism: Clinical and Experimental Research. 2009;33:1197–1201. doi: 10.1111/j.1530-0277.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. American Journal of Physiology Lung Cellular and Molecular Physiology. 2000;279:L1005–1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Tulloh BR, Collopy BT. Positive correlation between blood alcohol level and ISS in road trauma. Injury. 1994;25:539–543. doi: 10.1016/0020-1383(94)90097-3. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- Volta U, Caio G, Tovoli F, De Giorgio R. Gut-liver axis: an immune link between celiac disease and primary biliary cirrhosis. Expert Review of Gastroenterology & Hepatology. 2013;7:253–261. doi: 10.1586/egh.13.5. [DOI] [PubMed] [Google Scholar]
- Wagner MC, Yeligar SM, Brown LA, Hart CM. PPARγ ligands regulate NADPH oxidase, eNOS, and barrier function in the lung following chronic alcohol ingestion. Alcoholism: Clinical and Experimental Research. 2012;36:197–206. doi: 10.1111/j.1530-0277.2011.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AE, Chambers RC. The mercurial nature of neutrophils: still an enigma in ARDS? American Journal of Physiology Lung Cellular and Molecular Physiology. 2014;306:L217–230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E. Kupffer cell reactions in rat liver under various conditions as observed in the electron microscope. Journal of Ultrastructure Research. 1974;46:499–520. doi: 10.1016/s0022-5320(74)90070-7. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcoholism: Clinical and Experimental Research. 2004;28:998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Latchoumycandane C, McMullen MR, Pratt BT, Zhang R, Papouchado BG, et al. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. The Journal of Biological Chemistry. 2010;285:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FL, Lin WL, Shen HD, Fang RH. Changes in circulating levels of interleukin 6 in burned patients. Burns. 1999;25:131–136. doi: 10.1016/s0305-4179(98)00150-8. [DOI] [PubMed] [Google Scholar]
- Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. American Journal of Respiratory and Critical Care Medicine. 2007;176:270–276. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh MY, Burnham EL, Moss M, Brown LA. Non-invasive evaluation of pulmonary glutathione in the exhaled breath condensate of otherwise healthy alcoholics. Respiratory Medicine. 2008;102:248–255. doi: 10.1016/j.rmed.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM, Brown LA. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. Journal of Immunology. 2012;188:3648–3657. doi: 10.4049/jimmunol.1101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM, Brown LA. Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by downregulating NADPH oxidases. American Journal of Physiology Lung Cellular and Molecular Physiology. 2014;306:L429–441. doi: 10.1152/ajplung.00159.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Machida K, Kalra VK. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. The Journal of Biological Chemistry. 2010;285:35359–35373. doi: 10.1074/jbc.M110.138636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Mehta AJ, Harris FL, Brown LA, Hart CM. Peroxisome Proliferator-Activated Receptor γ Regulates Chronic Alcohol-Induced Alveolar Macrophage Dysfunction. American Journal of Respiratory Cell and Molecular Biology. 2015;55:35–46. doi: 10.1165/rcmb.2015-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs A, Bird MD, Ramirez L, Choudhry MA, Kovacs EJ. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock. 2013;39:373–379. doi: 10.1097/SHK.0b013e318289d6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]