Abstract
Introduction:
Gastric carcinoma with lymphoid stroma is an unusual type of gastric tumor associated with a better prognosis than typical gastric carcinomas. The hallmark of this cancer is a prominent lymphoid infiltration of the stroma that represents an intense host lymphocytic response. The programmed death 1–programmed death-ligand 1 (PD-1/PD-L1) axis has recently emerged as a master immune checkpoint that controls antitumor immune responses against many neoplasms.
Patient's concerns case study and outcome:
We report the case of a male patient with gastric carcinoma with lymphoid stroma with a large mass infiltrating the gastric wall without nodal metastasis. He is alive without disease 10 months after surgery. We focused the study on factors that potentially modulate the prognosis. In this setting we demonstrate, for the first time in this type of tumor, by immunohistochemistry a strong PD-L1 expression in neoplastic cell and the presence of PD-1 positive infiltrating lymphocytes.
Conclusion:
The applied approach may contribute to the knowledge about host reaction in such tumor and it may also be used for tumor precise identification on the endoscopic biopsy time before excision surgery.
Keywords: case report, gastric cancer, immunohistochemistry, lymphoid stroma, PD-1/PD-L1 axis
1. Introduction
Gastric carcinoma with lymphoid stroma (GCL), also known as lymphoepithelioma-like carcinoma of the stomach, is an unusual type of gastric cancer (GC) that was first described by Watanabe et al in 1976[1] and account for about 4% of gastric carcinomas. This neoplasia arises frequently in the proximal stomach and is more common in males. More than 80% of cases are associated with infection with Epstein–Barr virus (EBV) while the remaining cases show microsatellite instability.[2] The histological hallmark of this cancer is a prominent lymphoid infiltration of the stroma with lymphocytic aggression of the neoplastic epithelial structures. This intense host lymphocytic response is associated with a better prognosis than that reported for patients with typical gastric carcinomas, suggesting an immune antitumor reaction.[1] In this regard, despite the complexity of cancer immunology, growing evidences suggest that the coinhibitory receptors, such as cytotoxic T lymphocyte-associated antigen-4 and programmed death 1 (PD-1), play a crucial role in cancer immune-editing, especially in the equilibrium and escape stages of the immune response.[3] Human programmed death-ligand 1 (PD-L1), as a dominant ligand, plays a central role in antigen-specific T cell response mediating PD-1-dependent immune suppression. The PD-1/PD-L1 axis has recently emerged as a master immune checkpoint that controls antitumor immune responses against many neoplasms.[4] A meta-analysis addressed to the expression of PD-L1 measured by immunohistochemistry in solid tumors, reported the relationship between positive PD-L1 reaction and worse prognosis in different tumor types.[5] However, the correlation between PD-L1 expression and clinical behavior in solid tumor is still in controversial. Numerous agents targeting PD-L1/PD-1 check-point are in clinical development.[6] Here, we present a case of GCL with review of the literature and a study addressed to the assessment of both tumor PD-L1 expression and lymphocytic population phenotype in order to evaluate the role of the microenvironment in this unusual type of tumor.
2. Case report
We report the case of a 76 years old man, with past medical history of hepatitis B virus-related liver cirrhosis, hypertension, ischemic heart disease (treated with percutaneous transluminal coronary angioplasty), and carotid atherosclerosis. He also received a pulmonary lobectomy for tuberculosis infection 20 years before.
The patient presented epigastric pain, weight loss (about 10 kg in 6 months), and lack of appetite. No nausea, vomiting, hematemesis were reported. He underwent endoscopic evaluation at Campus Bio Medico Hospital and a gastric lesion of about 6 cm in diameter was identified on the lesser curvature (Fig. 1). Multiple biopsies examination revealed a gastric carcinoma. He was referred to the surgical unit for further evaluation. On physical examination, he had mild pallor with ill-defined painless swelling in his gastric region. There was hepatomegaly. Laboratory tests were normal; tumor markers were negative. The computed tomography scan of the patient's abdomen showed a thickening of the stomach lining at the lesser curvature; imaging was negative for lymph nodes enlargement, peritoneal carcinomatosis, or liver metastases. On the basis of these findings, considering the patient's age and general condition, a gastrectomy was performed with lymph nodes dissection and a Roux-en-Y reconstruction. A nasogastric tube was placed for enteral nutrition. The surgical specimen was formalin fixed and submitted for histology.
Figure 1.
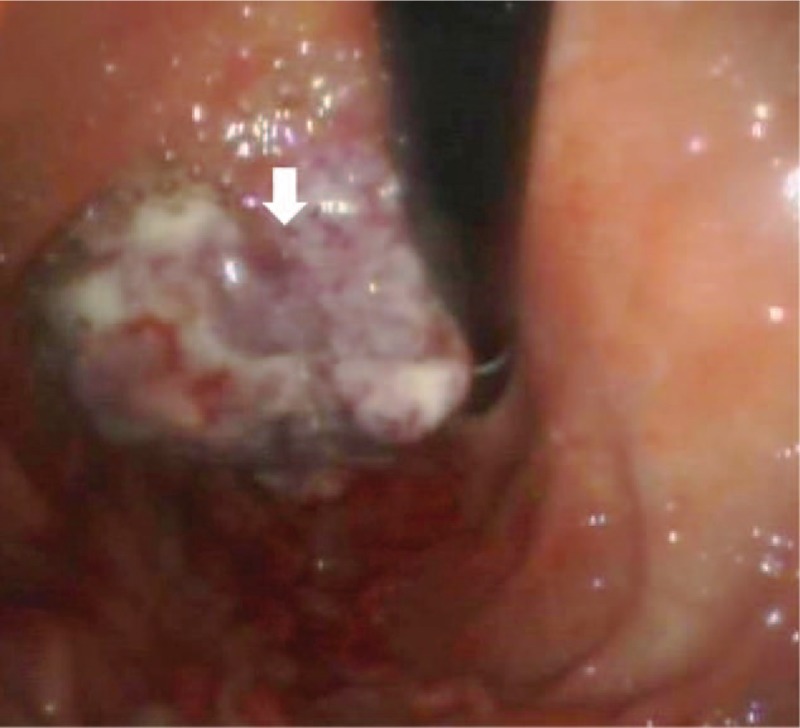
Endoscopic appearance of the gastric lesion. Note the polypoid endophytic shape with extensive ulceration of the mucosal surface (arrow).
Gross examination of the resected gastric specimen revealed the presence of a mass measuring about 6.5 cm in the largest diameter, partially ulcerated on the mucosal surface, located on the lesser curvature, and extending from the fundus to the esophageal margin of resection. Formalin fixed, paraffin-embedded slices of the resected surgical sample, performed in agreement with international guidelines[7] were submitted for histological examination. Microscopically, the neoplastic mass corresponded to a gastric carcinoma growing in solid nests and sheets of malignant epithelial elements and it was characterized by a prominent lymphoid infiltrate. Tumor cells were arranged in microalveolar and thin trabecular pattern surrounded by an abundant lymphocytic stroma showing a lympho-epithelial paradoxical lesion with small lymphocytes that invade and lyse neoplastic epithelia (Fig. 2A). Neoplastic epithelial cells were atypical, medium size, and oval or polygonal in shape, with poorly defined cell borders, vesicular to clear nuclei, prominent nucleoli, and abundant light eosinophilic cytoplasm. None of the 13 dissected lymph nodes revealed tumor metastasis. The case was reported G3, pT3, and pN0 according to the 7th edition of the Union for International Cancer Control guidelines.[8]
Figure 2.

Microscopic features of the tumor: (A) hematoxylin/eosin staining showed heavy lymphocytic infiltration and lymphocytic exocytosis on the neoplastic cells. (B) EBER nuclear reactivity (black-purple) is evident in neoplastic cells. (C) PD-L1 immunohistochemistry shows membranous staining in neoplastic epithelia. (D) PD-1 immunohistochemistry evidences positive staining in infiltrating lymphocytes. EBER = Epstein–Barr encoded small RNA, PD-1 = programmed death 1, PD-L1 = programmed death-ligand 1.
Immunohistochemical characterization was achieved with automatized Omnis System (Dako, Carpinteria, CA) in peroxidase method; it was performed using the following monoclonal antibodies: CD56 (123C3, Dako), CD3 (policlonal, Dako), CD20 (L26, Dako), and pan CK (MNF116, Dako). Proliferation fraction was evaluated by Ki67 (MIB1, Dako). Immunohistochemistry for PD-1 (NAT105, Dako) and PD-L1 (PD-L1/CD274, BioSB, Santa Barbara, CA) was performed on seriate paraffin sections after the diagnosis was established. In situ hybridization for EBV latent infection was performed using Epstein–Barr encoded small RNA (EBER) probes (Dako) on paraffin sections.
At immunohistochemistry, infiltrating lymphocytes consisted primarily of T cells that are CD3+, CD56− and showed PD-1 positivity in more than 50% of the cellularity (Fig. 2D). PD-1 expression was observed neither in tumor nor stroma cells. Lymphocytes with B phenotype (CD20+) were less than 10% of lymphoid infiltrate. Neoplastic cells resulted positive for pan CK and over 80% of them showed membrane positivity for PD-L1, with variable intensity among the cancer population, ranging from marked to weak with zonal distribution (Fig. 2C). PD-L1 expression was observed in tumor but not in nonneoplastic gastric epithelium. Proliferation fraction evaluated by Ki67 immunoreactivity was 65%. More than 90% of malignant cells showed positive nuclear reaction for EBV (Fig. 2B). EBER positivity was also present in normal mucosa near the tumor.
No complications were recorded during clinical course. The patient was initially fed with enteral nutrition and then oral which was gradually increased. Discharge was in 15th postoperative day. Ten months after the operation the patient stays well and free for recurrent disease.
Written informed consent was obtained from the patient for publication of this case report in the setting of a protocol for the study of biological material in oncology approved by the Ethical Committee (Prot. 30.08 ComEt CBM).
3. Discussion
Gastric carcinoma with lymphoid stroma is an uncommon type of GC and the interest for these lesions is pointed to the prognosis that for these patients is reported better than that for patients with typical cancer, independently of the tumor dimension or infiltrative stage. In this setting, GCL may represent a model of antitumor immune reaction in the host. Our case showed cellular and architectural morphology similar to the previous reported for the histological diagnosis of these tumors. We focused the study on PD-L1 expression in cancer cells and on the stromal lymphocytes within the tumor mass, in order to investigate their phenotype and the possible role of the PD-1/PD-L1 checkpoint in the host immune response to tumor cells.
The PD-L1/PD-1 pathways can protect tumors from cytotoxic T cells, ultimately inhibiting the antitumor immune response in 2 ways: deactivating cytotoxic T cells in the tumor microenvironment; preventing priming and activation of new T cells in the lymph nodes and subsequent recruitment to the tumor. The mechanism is hypothesized to act by tumoral expression of PD-L1 that binds to T cell receptor PD-1, deactivating cytotoxic T cells. Once deactivated, T cells remain inhibited in the tumor microenvironment. This complex mechanism observed in vivo and in vitro[9] was evidenced on tissues by immunohistochemistry. Recent meta-analysis showed that overexpression of PD-L1 in solid tumor tissues, as measured by IHC, is associated with worse prognosis in different tumor types, which suggests that the development of strategies against the PD-L1/PD-1 axis would be a promising therapeutic approach for solid tumors.[5] The strong evidence of the PD-L1/PD-1 pathway presence in a case of GCL, which is consider a tumor with a relatively good prognosis when compared with other type of gastric carcinomas, raises the thought that this checkpoint is not able to determine the immuno-escape in the tumor environment alone. Recently, PD-L1 expression in GC of Western patients was reported to correlate significantly with overall and tumor specific survival as well as distinct clinic-pathological patient characteristics; the authors showed, in a large series of gastric adenocarcinoma not including cases of GCL, that patients with PD-L1 positive tumor cells had a significant better overall (P = 0.028) and tumor specific survival (P = 0.018).[10] The better prognosis of GCL has been related to the fact that the patient's inflammatory response may prevent the spread of tumor through the gastric wall and to the lymph nodes or remote organs; the role of PD-L1 expression however has not been previously analyzed in this subtype of CG. Our results could suggest that the presence of intense PD-L1 expression on epithelial neoplastic cells may represent a way of cancer cells to inhibit the host defense reaction. Other mechanisms however should be able to restore the PD-1 lymphocytes activity and act in the maintenance of the host immune-defense. The reported case showed negative nodes despite a G3 and pT3 tumor; moreover, the patient is well without evidence of recurrent disease 10 months after surgery. The presence of diffuse EBV latent infection within cancer cells, a frequent finding in GCL, may contribute to the stimulatory action on the immune system as in the lymphoepithelioma-like carcinoma of the nasopharynx, bearing the same EBV association. The role of EBV in oncogenesis differs according to the host cell type and the immune status of the host. EBV-associated lymphoepithelioma-like carcinoma belongs to the latency I neoplasms, in which latent gene products are restricted to EBV nuclear antigen I, EBER, latent membrane protein 2A, and BamHI-A rightward transcripts while viral proteins, EBNA2 and LMP1, are not expressed.[11] The in situ hybridization finding (strong nuclear hybridization signal in tumor cells) in our case is consistent with the latency I type. GCL generally has a better prognosis than other forms of EBV-associated gastric carcinomas, suggesting the EBV infection alone does not serve as an independent prognostic factor. On the other hand, EBV-positive GCs are classified as a definite set of GC with related molecular alterations and are known to be associated with PD-L1 overexpression[12] although this information was not specifically assessed for GCL subtype. The main challenge in histological diagnosis of these tumors resides in the difficult to preoperatively recognize the lymphoid pattern within small bioptic specimens, so the correct definition of this histotype is usually achieved on the final surgical sample. Since these patients are known to have a better prognosis, it is of relevance for clinicians and pathologists to obtain an accurate diagnosis at the time of endoscopic evaluation. Localization of EBV in malignant cells by EBER in situ hybridization remains the gold standard for assigning the EBV-positive class of cancers.[13] Our results suggest that when the GCL subtype is suspected on the biopsy specimens, both PD-L1 and EBV evaluation may support the diagnosis before surgical time. These observations require a validation on a large number of GCL cases.
In conclusion, we report a case of EBV-associated GCL with a study of the tumor microenvironment that showed significant PD-L1/PD-1 pathway expression. This uncommon type of GC has generally a better prognosis than other forms of gastric carcinomas. The advantage in clinical behavior has been related to the presence of intense lymphoid infiltrate. Our results are of interest because merge the reported better prognosis for both GCL subtype and PD-L1 expression in GC and confirm the association between EBV infection and PD-L1 membranous expression; moreover, this report is the first PD-L1 characterization of GCL subtype of GC. This case confirms that the relationship between PD-L1/PD-1 pathway and prognosis of solid tumor is a critical point for therapy and follow-up in oncological patients.
Footnotes
Abbreviations: EBER = Epstein–Barr encoded small RNA, EBV = Epstein–Barr virus, GC = gastric cancer, GCL = gastric carcinoma with lymphoid stroma, PD-1 = programmed death 1, PD-L1 = programmed death-ligand 1.
The authors have no conflicts of interest to disclose.
References
- [1].Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma: its morphologic characteristic and prognostic correlations. Cancer 1976;38:232–43. [DOI] [PubMed] [Google Scholar]
- [2].Bittar Z, Fend F, Quintanilla-Martinez L. Lymphoepithelioma-like carcinoma of the stomach: a case report and review of the literature. Diagn Pathol 2013;4:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases – elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guo Y, Wang AY. Novel immune check-point regulators in tolerance maintenance. Front Immunol 2015;18:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu P, Wu D, Li L, et al. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One 2015;10:e0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Page DB, Postow MA, Callahan MK, et al. Immune modulation in cancer with antibodies. Annu Rev Med 2014;65:185–202. [DOI] [PubMed] [Google Scholar]
- [7].Robert ME, Lamps L, Lauwers GY. Association of directors of anatomic and surgical pathology; recommendations for the reporting of gastric carcinoma. Hum Pathol 2008;39:9–14. [DOI] [PubMed] [Google Scholar]
- [8].Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual (7th ed). New York, NY: Springer; 2010. [Google Scholar]
- [9].Chen J, Jiang CC, Jin L, et al. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 2016;27:409–16. [DOI] [PubMed] [Google Scholar]
- [10].Böger C, Behrens HM, Mathiak M, et al. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016;7:24269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fukayama M, Ushiku T. Epstein-Barr virus-associated gastric carcinoma. Pathol Res Pract 2011;207:529–37. [DOI] [PubMed] [Google Scholar]
- [12].Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gulley ML, Tang W. Laboratory assays for Epstein–Barr virus-related disease. J Mol Diagn 2008;10:279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


