Supplemental Digital Content is available in the text
Keywords: cholesterol, high-sensitivity C-reactive protein, polyunsaturated fatty acids
Abstract
Growing evidence suggests that the effects of diet on cardiovascular disease (CVD) occur through mechanisms involving subclinical inflammation. We assessed whether reported dietary fatty acid intake correlates with a serum high-sensitivity C-reactive protein (hs-CRP) concentration in a population-based sample of US men and women.
In this cross-sectional analysis, participants were selected from the US National Health and Nutrition Examination Survey (NHANES) and restricted to those with available data on dietary intake, biochemical and anthropometric measurements from 2001 to 2010. All statistical analyses accounted for the survey design and sample weights by using SPSS Complex Samples v22.0 (IBM Corp, Armonk, NY).
Of the 17,689 participants analyzed, 8607 (48.3%) were men. The mean age was 45.8 years in the overall sample, 44.9 years in men, and 46.5 years in women (P = 0.047). The age-, race-, and sex-adjusted mean dietary intakes of total polyunsaturated fatty acids (PUFAs), PUFAs 18:2 (octadecadienoic), and PUFAs 18:3 (octadecatrienoic) monotonically decreased across hs-CRP quartiles (P < 0.001), whereas dietary cholesterol increased across hs-CRP quartiles (P < 0.001)
This study provides further evidence of an association between fatty acid intake and subclinical inflammation markers. hs-CRP concentrations are likely modulated by dietary fatty acid intake. However, the causality of this association needs to be demonstrated in clinical trials.
1. Introduction
Subclinical chronic inflammation is known to play an important role in the development of atherosclerotic cardiovascular disease (CVD).[1] Serum concentrations of high-sensitivity C-reactive protein (hs-CRP) and proinflammatory cytokines, including interleukin 6 (IL-6), are associated with an increased risk of CVD.[1,2] There have been several reports of associations between dietary factors and the level of serum CRP and other inflammatory biomarkers.[1,3] Dietary guidelines recommend the consumption of n-3 and n-6 polyunsaturated fatty acids (PUFAs), in preference, saturated and trans-fatty acids; however, it has been reported that a high intake of n-6 PUFAs may increase subclinical inflammation.[4,5] The relationship between hs-CRP and dietary n-6 fatty acids (n-6 FAs) remains controversial.[6–8]
A recent systematic review and meta-analysis found that short-term marine-derived omega-3 supplementation decreases systemic inflammatory biomarkers, including hs-CRP, IL-6, and tumor necrosis factor-α (TNF-α) in different populations.[9] However, another systematic review provided weak support for omega-3 fatty acid supplementation in reducing chronic inflammation, reporting no convincing evidence supporting that a low intake of specific omega-3 fatty acids is associated with increased inflammation.[10] International dietary guidelines provide varying recommendations on the amounts and types of fatty acids for decreasing inflammatory markers and improving cardiovascular health.[11–13] This lack of consensus may reflect the lack of conclusive scientific evidence regarding the effects of dietary fatty acids intake on levels of hs-CRP.[11–13]
To our knowledge, no previous study has comprehensively examined the association of dietary fatty acids intake with hs-CRP level in the US population. We aimed to explore the association between reported dietary intake of fatty acids and serum hs-CRP concentrations in the NHANES population sample.
2. Methods
2.1. Population
The National Health and Nutrition Examination Survey (NHANES) is an ongoing, repeated set of cross-sectional surveys conducted by the National Center for Health Statistics (NCHS). NHANES uses a multistage probabilistic sampling strategy that oversamples certain segments of the population, including African-Americans, Mexican-Americans, and those of lower socioeconomic status. Approximately, 5000 subjects are recruited into NHANES each year, and the data are publicly available in 2-year cycles. Demographic, dietary, and behavioral information are gathered through in-home questionnaires, whereas anthropometric and biomarker data are collected by trained staff using mobile examination units. The NCHS Research Ethics Review Board approved the underlying protocol, and written informed consent was obtained from all subjects. The interview consists of questions on sociodemographic characteristics and previously diagnosed medical conditions. More detailed information on the NHANES survey design and questionnaires is reported elsewhere.[14]
The present study is based on analysis of data for two, 2-year NHANES survey cycles between 2001 and 2010 using data from the day 1 dietary recall. Overall response rates for these years ranged from 73% to 84% for interviews, and from 70% to 80% for examinations.[15,16] We identified 17,689 eligible participants aged 18 years or older for the analyses.
Details on NHANES Laboratory/Medical Technologists Procedures and Anthropometry Procedures are described elsewhere.[17,18] Moreover, complete laboratory procedures for collection, storage, calibration, and quality control of blood samples for determination of hs-CRP concentrations are available elsewhere (http://www.cdc.gov/NCHS/data/nhanes/nhanes_09_10/CRP_F_met.pdf. [accessed August 19, 2013]). In this study National Cholesterol Education Program's Adult Treatment Panel III report (NCEP/ATPIII) have been used to describe metabolic syndrome (MetS).[19] If a subject has at least 3 of the following 5 criteria, then he was classified as having MetS: waist circumference ≥102 cm in men or ≥88 cm in women; triglycerides ≥150 mg/dL; high-density lipid (HDL) cholesterol <40 mg/dL in men or <50 mg/dL in women; systolic blood pressure ≥130 or diastolic blood pressure ≥85 mmHg; fasting blood glucose ≥100 mg/dL.
For assessment of the diet 24-h recall was applied by a skilled assessor throughout the mobile examination center (MEC) as described previously.[20,21] In this study, we have used the data on fatty acids intake such as total daily fat intake, total saturated fatty acid intake, total monounsaturated fatty acid (MUFA) intake, total PUFA intake, cholesterol intake, saturated fatty acids (SFA) 4:0 (butanoic), SFA 6:0 (hexanoic), SFA 8:0 (octanoic), SFA 10:0 (decanoic), SFA 12:0 (dodecanoic), SFA 14:0 (tetradecanoic), SFA 16:0 (hexadecanoic), SFA 18:0 (octadecanoic), MUFA 16:1 (hexadecenoic), MUFA 18:1 (octadecenoic), MUFA 20:1 (eicosenoic), MUFA 22:1 (docosenoic), PUFA 18:2 (octadecadienoic), PUFA 18:3 (octadecatrienoic), PUFA 18:4 (octadecatetraenoic), PUFA 20:4 (eicosatetraenoic), PUFA 20:5 (eicosapentaenoic), PUFA 22:5 (docosapentaenoic), PUFA 22:6 (docosahexaenoic).
2.2. Statistical analysis
Analyses were conducted according to the guidelines set by the Centers for Disease Control and Prevention for analysis of the NHANES dataset, accounting for the masked variance and using their suggested weighting methodology.[22] Continuous and categorical demographic variables were compared across quartiles of hs-CRP using analysis of variance (ANOVA) and χ2 tests, respectively. Age-, sex-, race-, body mass index (BMI)- and energy-adjusted mean intakes of nutrients were compared across quartiles of serum hs-CRP using analysis of co-variance (ANCOVA). Comparison of dietary intakes across quartile of Serum hs-CRP scores was conducted using ANCOVA with Bonferroni correction. All tests were 2-sided, and P <0.05 was the level of significance unless otherwise stated. Results were analyzed using SPSS complex sample module version 22.0 (IBM Corp, Armonk, NY). Sample weights were applied to account for unequal probabilities of selection, nonresponse bias, and oversampling.
3. Results
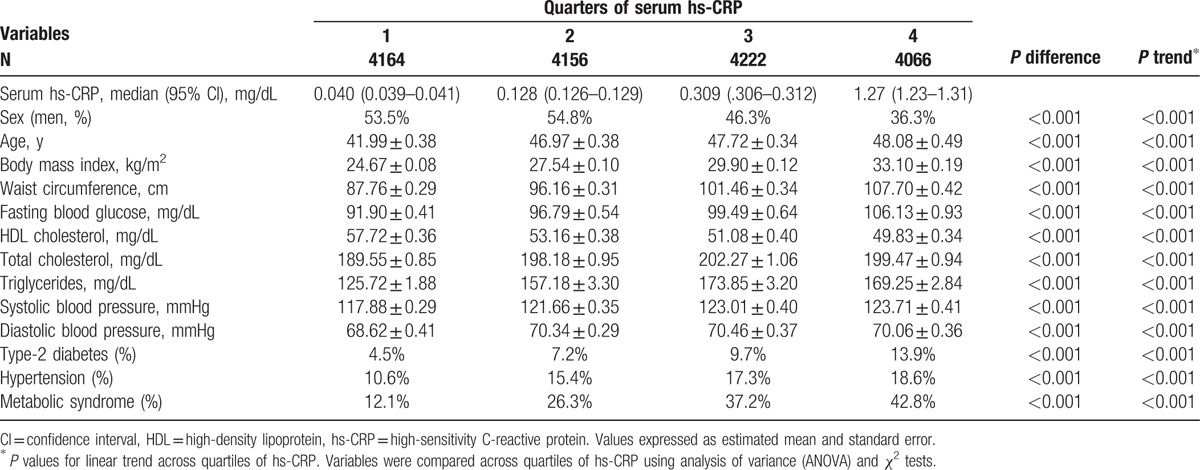
The weighted distributions of study population characteristics are shown in Table 1. Of the 17,689 eligible participants, 48.3% (n = 8607) were men. The mean age was 45.8 years overall, 44.9 years in men and 46.5 in women (P = 0.047). The distribution of the clinical, biochemical, and anthropometrical characteristics across quarters of serum hs-CRP is shown in Table 2, with significant differences (all P < 0.001) in a linear manner (all P < 0.001 for linear trends). This reflects monotonically increasing trend (decreasing for high-density lipoprotein cholesterol) across increasing quarters of hs-CRP level for a range of measures including BMI, waist circumference, and triglycerides. The prevalence of diabetes, hypertension, and MetS increased across quarters of hs-CRP.
Table 1.
Sample size and weighted characteristics of NHANES 2001–2010 adult participants.
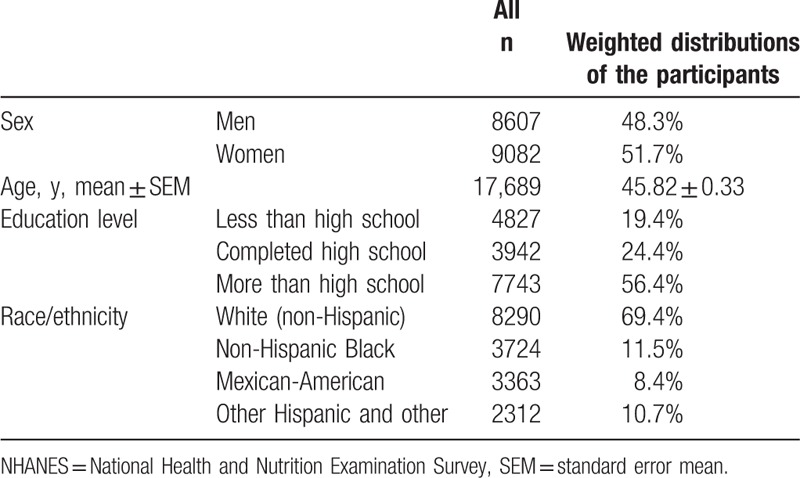
Table 2.
Clinical and biochemical measures across quartiles of hs-CRP.
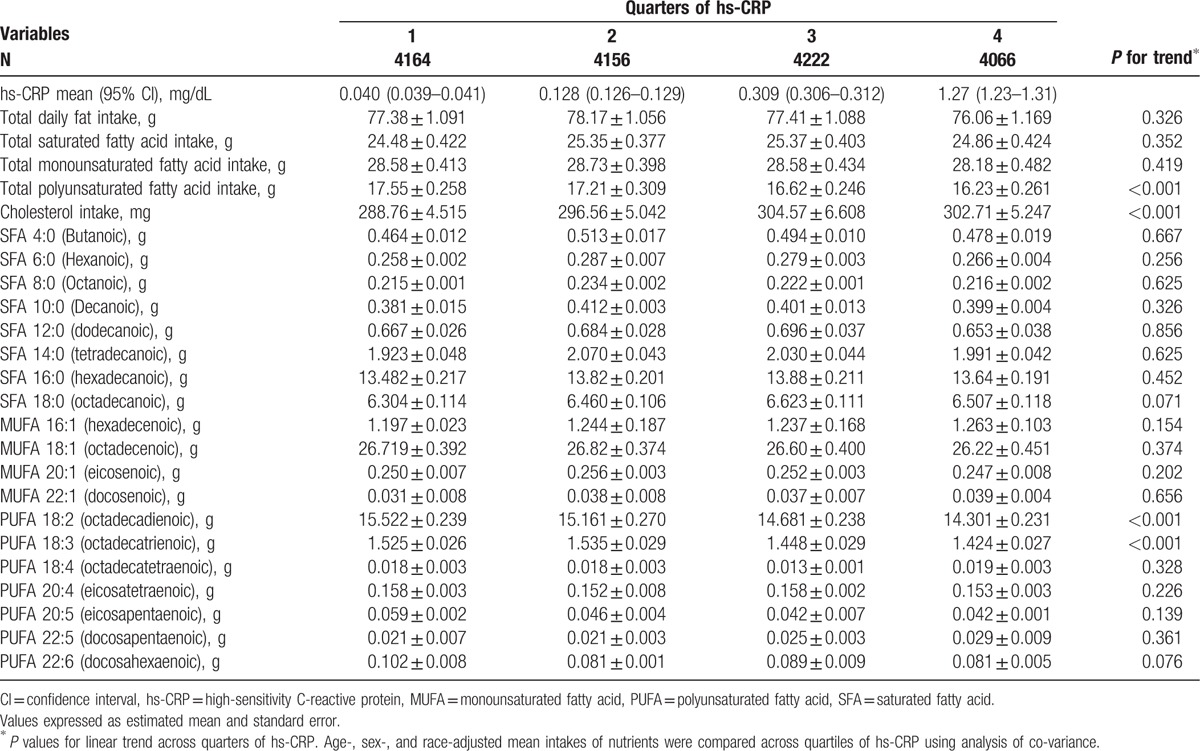
The association of fatty acid intake with serum hs-CRP is summarized in Table 3. Mean dietary intakes of total PUFA, PUFA 18:2 (octadecadienoic), and PUFA 18:3 (octadecatrienoic) monotonically decreased across hs-CRP quarters (P < 0.001), and dietary cholesterol increases across hs-CRP quartiles (P < 0.001), whereas intake of total fat, MUFA, and SFA was not correlated with serum hs-CRP levels (Table 3).
Table 3.
Age-, sex-, and race-adjusted mean nutrient intakes across quartiles of hs-CRP levels.
In models adjusted for BMI, age, race, and sex, we found that total daily fat intake, total MUFA intake, total PUFA intake, MUFA 18:1 (octadecenoic), PUFA 18:2 (octadecadienoic), and PUFA 18:3 (octadecatrienoic) monotonically decreased across hs-CRP quarters (all P < 0.001), whereas dietary total SFA, SFA 4:0 (Butanoic), SFA 6:0 (Hexanoic), SFA 8:0 (octanoic), SFA 10:0 (decanoic), and SFA 14:0 (tetradecanoic) increased across hs-CRP quarters (all P < 0.001). In models adjusted for age, race, sex, BMI, and energy, we found that total PUFA intake, PUFA 18:2 (octadecadienoic), and PUFA 18:3 (octadecatrienoic) monotonically decreased across hs-CRP quarters (all P < 0.001), whereas total SFA intake, SFA 4:0 (butanoic), SFA 6:0 (hexanoic), SFA 8:0 (octanoic), SFA 10:0 (decanoic), SFA 14:0 (tetradecanoic), and SFA 18:0 (Octadecanoic) increased across hs-CRP quarters (all P < 0.001, Supplemental Table 1).
4. Discussion
This study investigated the association between dietary fatty acid intake and serum hs-CRP concentrations in a representative sample of US adults. The main findings were the association of increasing serum hs-CRP levels with increasing cholesterol intake and decreasing PUFA intake, suggesting a relationship between fatty acid intake and subclinical inflammation in this population.
Consistent with our findings, there are reports in the literature to suggest that inflammatory markers such as hs-CRP increase quickly after consumption of an excess amount of dietary lipids, while nutritional cholesterol itself is closely linked to inflammation markers through particular transcriptional regulators and may contribute to increasing the inflammatory component of atherogenesis.[23–25] We also recently reported an inverse relationship between cholesterol intake and hs-CRP in adult Iranians without a history of CVD.[26] Murakami et al[27] stated no significant association between SFA intake and raised hs-CRP, and they ascribed their results to the low baseline degree of raised hs-CRP level in their population (Japanese women). In this regard, a study in an elderly subjects could not detect a significant association between concentration of saturated myristic, palmitic or stearic acids, measured in serum cholesteryl esters, and hs-CRP level.[28] In line with these previous results, an augmented SFA intake was not significantly related with changes in hs-CRP in an Italian subjects. They stated that, in dysmetabolic subjects, the role of dietary factors including PUFA is associated with enhanced postprandial inflammatory factors and lipids profile.[29,30] Moreover, it has been proposed that the Mediterranean diet has a converse correlation with inflammatory factors such as hs-CRP level.[20,31]
Studies have reported that n-3 FAs work both directly by substituting arachidonic acid as an eicosanoid substrate and stopping arachidonic acid metabolism, and indirectly by changing the expression of inflammatory genes via influences on transcription factor activation.[6,32] Additionally, it has been suggested that both n-3 PUFAs and n-6 PUFAs halt the activities of δ-6 desaturase, δ-5 desaturase, and cyclooxygenase, all of which have a role in fatty acid control that affects pro- and anti-inflammatory mediators. Therefore, high intake of both n-3 PUFAs and n-6 PUFAs could lessen inflammation.[5,33] Additional proposed mechanism is that PUFAs can change the action of transcription factors, such as peroxisome proliferator-activated receptors (PPARs) and nuclear factor κB. PPARs via stopping signaling molecules can impact the initiation of nuclear factor κB, and hence obstructs the construction production of pro-inflammatory cytokines.[5,34]
Some inconsistent findings have been reported in different type of studies, with some suggesting no significant differences in subjects with a MUFA-rich diet.[35–37] In line with our study, recently Muke et al,[38] in a prospective study of 4707 individuals, found that higher intakes of PUFAs (mainly n-6 PUFAs) were correlated with lower levels of hs-CRP, which might reflect reduced chronic systemic inflammation. Julia et al[39] hypothesized that the inverse relation found between total n-3 PUFAs and hs-CRP was mostly driven by long-chain n-3 PUFAs.
The present study has some limitations. Its cross-sectional nature does not allow inferences about causality. Also, the use of a single 24-hour dietary recall may not fully capture the usual dietary behaviors. However, this concern is mitigated by the large sample size, increasing the probability of inclusion of diverse dietary behaviors. Moreover, we did not control for chronic diseases that might elevate hs-CRP.
As fatty acid intake has been a topic of interest in relation to CVD risk, understanding the effects of SFA, MUFA, and PUFA on subclinical inflammation could yield useful clinical insights and therapeutic potential. Our findings provide further evidence on the association between fatty acid intake and subclinical inflammation as reflected in hs-CRP levels. This raises the possibility that hs-CRP concentrations could be improved by changes in dietary fatty acid intake. However, these need to be formally tested in well-designed trials to comprehensively understand the impact of dietary fatty acids on subclinical inflammation.
Supplementary Material
Footnotes
Abbreviations: AMPM = automated multiple-pass method, ANCOVA = analysis of co-variance, ANOVA = analysis of variance, BMI = body mass index, CVD = cardiovascular disease, HDL = high-density lipid, hs-CRP = high-sensitivity C-reactive protein, IL-6 = interleukin 6, MEC = mobile examination center, MetS = metabolic syndrome, MUFA = monounsaturated fatty acids, n-6 fAs = N-6 fatty acids, NCEP/ATPIII = National Cholesterol Education Program's Adult Treatment Panel III report, NCHS = National Center for Health Statistics, NHANES = National Health and Nutrition Examination Survey, PPARs = peroxisome proliferator-activated receptors, PUFAs = polyunsaturated fatty acids, SFA = saturated fatty acid, US = United States.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
MM was supported by a TWAS studentship of the Chinese Academy of Sciences.
References
- [1].Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr 2015;6:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis 2014;24:929–39. [DOI] [PubMed] [Google Scholar]
- [3].Lim S, Jang HC, Lee HK, et al. The relationship between body fat and C-reactive protein in middle-aged Korean population. Atherosclerosis 2006;184:171–7. [DOI] [PubMed] [Google Scholar]
- [4].Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119:902–7. [DOI] [PubMed] [Google Scholar]
- [5].Muka T, Kiefte-de Jong JC, Hofman A, et al. Polyunsaturated fatty acids and serum C-reactive protein: The Rotterdam Study. Am J Epidemiol 2015;181:846–56. [DOI] [PubMed] [Google Scholar]
- [6].El-Saed A, Masaki K, Okamura T, et al. The associations of C-reactive protein with serum levels of polyunsaturated fatty acids and trans fatty acids among middle-aged men from three populations. J Nutr Health Aging 2016;20:16–21. [DOI] [PubMed] [Google Scholar]
- [7].Schmitz G, Ecker J. The opposing effects of n−3 and n−6 fatty acids. Prog Lipid Res 2008;47:147–55. [DOI] [PubMed] [Google Scholar]
- [8].Yoneyama S, Miura K, Sasaki S, et al. Dietary intake of fatty acids and serum C-reactive protein in Japanese. J Epidemiol 2007;17:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li K, Huang T, Zheng J, et al. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor (: a meta-analysis. PloS One 2014;9:e88103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Atlantis E, Cochrane B. The association of dietary intake and supplementation of specific polyunsaturated fatty acids with inflammation and functional capacity in chronic obstructive pulmonary disease: a systematic review. Int J Evid Based Healthc 2016;14:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Di Angelantonio E, Chowdhury R, Forouhi NG, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk. In response. Ann Intern Med 2014;161:458. [DOI] [PubMed] [Google Scholar]
- [12].Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011;46:209–28. [DOI] [PubMed] [Google Scholar]
- [13].Hammad S, Pu S, Jones PJ. Current evidence supporting the link between dietary fatty acids and cardiovascular disease. Lipids 2016;51:507–17. [DOI] [PubMed] [Google Scholar]
- [14].Kalk WJ, Joffe BI. The metabolic syndrome, insulin resistance, and its surrogates in African and white subjects with type 2 diabetes in South Africa. Metab Syndr Relat Disord [Research Support, Non-US Gov’t] 2008;6:247–55. [DOI] [PubMed] [Google Scholar]
- [15].Alebiosu CO, Odusan BO. Metabolic syndrome in subjects with type-2 diabetes mellitus. J Natl Med Assoc 2004;96:817–21. [PMC free article] [PubMed] [Google Scholar]
- [16].Yoon SS, Dillon CF, Illoh K, et al. Trends in the prevalence of coronary heart disease in the U.S.: National Health and Nutrition Examination Survey, 2001-2012. Am J Prev Med 2016;51:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Konnov MV, Dobordzhinidze LM, Deev AD, et al. [Waist circumference below metabolic syndrome harmonizing criteria is associated with increased cardiovascular risk]. Kardiologiia 2010;50:23–7. [PubMed] [Google Scholar]
- [18].Ogbera AO. Prevalence and gender distribution of the metabolic syndrome. Diabetol Metab Syndr 2010;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433–8. [DOI] [PubMed] [Google Scholar]
- [20].Ahluwalia N, Andreeva VA, Kesse-Guyot E, et al. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab 2013;39:99–110. [DOI] [PubMed] [Google Scholar]
- [21].Ahluwalia N, Dwyer J, Terry A, et al. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr 2016;7:121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Statistics. NCfH. ANALYTIC AND REPORTING GUIDELINES http://www.cdc.gov/nchs/data/nhanes/nhanes 03 04/nhanes analytic guidelines dec 2005.pdf. DOI: 10.5114/aoms.2017.64714. [Google Scholar]
- [23].Kleemann R, Kooistra T. HMG-CoA reductase inhibitors: effects on chronic subacute inflammation and onset of atherosclerosis induced by dietary cholesterol. Current Drug Targets Cardiovasc Hematol Disord 2005;5:441–53. [DOI] [PubMed] [Google Scholar]
- [24].Kleemann R, Verschuren L, van Erk MJ, et al. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol 2007;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tannock LR, O’Brien KD, Knopp RH, et al. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation 2005;111:3058–62. [DOI] [PubMed] [Google Scholar]
- [26].Mazidi M, AH-B, Zadeh SK, Azarpazhooh MR, et al. Dietary cholesterol, but not dietary fatty acid intake, varies with serum hs-CRP concentrations in individuals free of any history of cardiovascular disease. Eur J Clin Nutr 2016;1–4. [DOI] [PubMed] [Google Scholar]
- [27].Murakami K, Sasaki S, Takahashi Y, et al. Total n-3 polyunsaturated fatty acid intake is inversely associated with serum C-reactive protein in young Japanese women. Nutr Res 2008;28:309–14. [DOI] [PubMed] [Google Scholar]
- [28].Petersson H, Lind L, Hulthe J, et al. Relationships between serum fatty acid composition and multiple markers of inflammation and endothelial function in an elderly population. Atherosclerosis 2009;203:298–303. [DOI] [PubMed] [Google Scholar]
- [29].McKiernan F, Lokko P, Kuevi A, et al. Effects of peanut processing on body weight and fasting plasma lipids. Br J Nutr 2010;104:418–26. [DOI] [PubMed] [Google Scholar]
- [30].Moreira Alves RD, Boroni Moreira AP, Macedo VS, et al. High-oleic peanuts: new perspective to attenuate glucose homeostasis disruption and inflammation related obesity. Obesity (Silver Spring) 2014;22:1981–8. [DOI] [PubMed] [Google Scholar]
- [31].Smidowicz ARJ. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr 2015;6:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006;83(6 suppl):1505s–19s. [DOI] [PubMed] [Google Scholar]
- [33].Ringbom T, Huss U, Stenholm A, et al. Cox-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod 2001;64:745–9. [DOI] [PubMed] [Google Scholar]
- [34].Marx N, Sukhova GK, Collins T, et al. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 1999;99:3125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Harvey KA, Walker CL, Xu Z, et al. Oleic acid inhibits stearic acid-induced inhibition of cell growth and pro-inflammatory responses in human aortic endothelial cells. J Lipid Res 2010;51:3470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599–610. [DOI] [PubMed] [Google Scholar]
- [37].Barbour JA HP, Buckley JD, Bryan J, et al. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients 2015;7:7381–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Muka T K-dJJ, Hofman A, Dehghan A, et al. Polyunsaturated fatty acids and serum C-reactive protein: the Rotterdam study. Am J Epidemiol 2015;181:846–56. [DOI] [PubMed] [Google Scholar]
- [39].Julia C TM, Meunier N, Papet I, et al. Intakes of PUFAs were inversely associated with plasma C-reactive protein 12 years later in a middle-aged population with vitamin E intake as an effect modifier. J Nutr 2013;143:1760–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


