Supplemental Digital Content is available in the text
Keywords: direct-acting antivirals, fatigue, work productivity
Abstract
Patient-reported outcomes (PROs) such as quality of life and work productivity are important for measuring patient's experience. We assessed PROs during and after treatment of hepatitis C virus (HCV) patients.
Data were obtained from a phase 3 open label study of sofosbuvir and ribavirin (SOF + RBV) with and without interferon (IFN). Patients completed 4 PRO assessment instruments (SF-36, Functional Assessment of Chronic Illness Therapy—Fatigue, Chronic Liver Disease Questionnaire— HCV, Work Productivity and Activity—Specific Health Problem) before, during, and after treatment.
A total of 533 patients with chronic HCV were enrolled; 28.9% treatment-naïve, 23.1% cirrhotic, 219 received IFN + SOF + RBV and 314 received IFN-free SOF + RBV. At baseline, there were no differences in PROs between the IFN-free and IFN-containing treatment arms (all P > 0.05). During treatment, patients receiving IFN + SOF + RBV had a substantial impairment in their PROs (up to −24.4% by treatment week 12, up to −8.3% at week 4 post-treatment). The PRO decrements seen in the SOF + RBV arm were smaller in magnitude (up to −7.1% by treatment week 12), and all returned to baseline or improved by post-treatment week 4. By 12 weeks after treatment cessation, patients who achieved sustained viral response-12 showed some improvement of PRO scores regardless of the regimen (up to +7.1%, P < 0.0001) or previous treatment experience. In multivariate analysis, the use of IFN was independently associated with lower PROs.
IFN-based regimens have a profoundly negative impact to PROs. By contrast, the impact of RBV on these PROs is relatively modest. Achieving HCV cure is associated with improvement of most of the PRO scores.
1. Introduction
The new direct-acting antiviral agents (DAAs) for treatment of hepatitis C virus (HCV) have revolutionized HCV treatment with high sustained viral response (SVR) rates and superior patient-reported outcomes (PROs).[1–12] Although new interferon (IFN)-free and ribavirin (RBV)-free regimens are currently being developed, both IFN and RBV remain a part of certain regimens.[1–4]
Previous studies have shown that treatment-naïve HCV patients treated with RBV and IFN experience a significant PRO impairment.[13,14] In particular, the use of IFN causes substantial side effects, including debilitating ones such as severe depression, which, in turn, affect patients’ ability to sustain treatment long enough to obtain a cure whether through medical discontinuation or through patients’ nonadherence. The use of RBV also has been shown to decrease PROs during treatment. However, the PRO data in patients who are retreated after having experienced another course of treatment have not been reported. Therefore, the aim of this study was to evaluate PROs during treatment with and without the use of IFN in patients who participated in a prior study of an IFN-containing or an IFN-free DAA-based regimen and has not achieved SVR.
2. Methods
Data were obtained from a phase 3 multicenter open label study investigating the use sofosbuvir (SOF); the study protocol GS-US-334-0109, ClinicalTrials.gov identifier NCT01625338. The target population was patients with all genotypes chronic HCV infection who participated in another study of an SOF-based regimen and did not achieve SVR after receiving active treatment or a placebo; in this study, patients who received a placebo were considered to be treatment-naive. The original intent of this trial was to provide the best opportunity to achieve SVR to participants of prior clinical trials who had experienced a treatment failure or happened to be assigned to a placebo arm. For this study, patients were assigned to receive either IFN + SOF + RBV for 12 weeks or SOF + RBV for 12 or 24 weeks; no randomization or blinding was used. Exclusion criteria were coinfection with HBV or human immunodeficiency virus, pregnancy, history of clinical hepatic decompensation, or the use of immunosuppressants, or other substances as specified in the study report.
We used the medical history collected at screening for the study participants to identify patients with a history of psychiatric disorders, sleep disorders, fatigue or asthenia, and type 2 diabetes or hyperglycemia. Adverse events related to treatment, as confirmed by the study investigators, were grouped into 9 disorder types depending on the organ system involved: blood and lymphatic, gastrointestinal, musculoskeletal and connective tissue, nervous, psychiatric, skin and subcutaneous tissue, fatigue, flu-like symptoms, and other disorders, as described previously.[11,12]
2.1. Assessment of PROs
In this study, PROs were collected as secondary endpoints using standard instruments.[15–19] All instruments were self-administered by patients prior to initiation of any study-related activities at baseline (day 1) visit, treatment week 12, and at post-treatment weeks 4, 12, and 24 while blinded to their most recent HCV RNA levels.
A Short Form-36 (SF-36) questionnaire is a generic instrument, which has been extensively validated in a variety of populations. It is used to calculate 8 health-related quality of life (HRQL) domains: Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health. The 2 summary scores, which are designed to be linear combinations of these 8 domains, summarize physical and mental health. The 2011 US population norms were used for normalization.[15]
The Functional Assessment of Chronic Illness Therapy—Fatigue (FACIT-F) is another widely used HRQL instrument with a fatigue-specific component. It includes a core which consists of the Physical, Emotional, Social, and Functional Well-Being domains and the Fatigue Scale (FS).[16] All domains add up to the total FACIT-F score.
The Chronic Liver Disease Questionnaire—HCV (CLDQ-HCV) is a disease-specific instrument that targets HRQL impairment in patients with chronic HCV infection. It includes 4 HRQL domains: Activity/Energy, Emotional, Worry, and Systemic, which are averaged to the total CLDQ-HCV score.[17]
The Work Productivity and Activity—Specific Health Problem (WPAI:SHP) is a different PRO instrument, which is widely used to quantify impairment in patients’ work productivity and daily activities that, as patient believes, is a consequence of a specific health problem (HCV infection, for the purpose of this study). It has 2 domains. The work productivity impairment domain is a sum of impairment in work productivity due to missed work hours (absenteeism) and due to decreased productivity while working (presenteeism); this domain is assessed in employed patients only. The other domain is activity impairment domain, which is impairment in daily activities other than work; it is assessed regardless of employment.[18] Unlike other instruments, all domains of WPAI:SHP are supposed to be inversely related to health status; that is, a greater impairment indicates worse health, and vice versa.
We also calculated SF-6D health utility scores, which are preference-based measures for health necessary for calculation of quality-adjusted years of life in economic analyses; an SF-36 instrument and a nonparametric Bayesian model were used as previously described.[19]
2.2. Statistical analysis
The treatment regimens were grouped into IFN-free (SOF + RBV) and IFN-containing (IFN + SOF + RBV) regardless of the duration. For all patients, all individual PRO domains, summary PROs, and health utility scores were calculated at each study time point and were further used for calculation of changes (decrements or improvements) in PROs and utilities from patients’ own baseline levels, thus, making patients their own controls. For the purpose of comparison between multiple PROs domains, these domains were transformed from their original scales to a universal 0 to 100 scale with greater scores representing better well-being.
All demographic and clinical parameters, as well as PROs and changes in those, were summarized as mean ± standard deviation or frequency (percentage) in the treatment arms separately, and were compared between the study arms using Wilcoxon rank sum nonparametric test (continuous parameters) or Pearson chi-squared test (categorical parameters). The decrements/improvements in PROs at the study time points from patients’ own baseline were tested for significance using Wilcoxon sign-rank test for matched pairs; a P value of 0.05 was used as a threshold for significance. In a separate round of analysis, independent predictors of summary PROs and SF-6D utility scores were assessed at the study time points separately using multiple linear regressions with stepwise selection of predictors; only predictors with P < 0.05 were left in the final regression models. In the regression analyses, demographic and clinical variables together with the treatment regimen were tested as potential predictors. All analyses were run in SAS 9.3 (SAS Institute, Cary, NC).
The study was separately approved by each site's Institutional Review Board. Participants signed an informed consent before being enrolled in the trial.
3. Results
There were 533 patients in this study (Table 1). Of those, 219 patients received 12 weeks of IFN + SOF + RBV, 114 patients received 12 weeks of SOF + RBV, and 200 patients received 24 weeks of SOF + RBV.
Table 1.
Baseline demographics and clinical presentation of the study cohort.

The demographic and clinical parameters of patients receiving IFN-containing versus IFN-free treatment are listed in Table 1. Patients receiving an IFN-containing regimen were older, more likely African-American, less cirrhotic and treatment-naïve, had predominantly HCV genotype 1, and reported having less depression or mood disorders at screening (all P < 0.05, Table 1). On the other hand, patients assigned to an IFN-free SOF + RBV regimen were predominantly HCV genotype 3 or 2 (Table 1).
During treatment, significantly more patients in the IFN + SOF + RBV arm experienced at least 1 treatment-related side effect when compared to the SOF + RBV arm: 89.0% versus 69.7% (P < 0.0001). The most frequently reported side effects were fatigue (52.0% vs 30.2%, P < 0.0001), depression and other psychiatric issues (45.2% vs 30.6%, P = 0.0006), and joint or muscle soreness (39.3% vs 13.1%, P < 0.0001) (Table 2). The overall rate of SVR was higher in the IFN + SOF + RBV arm: 82.6% versus 75.5% in SOF + RBV (P = 0.048). That included SVR-12 of 79.85% in HCV genotype 1 patients treated with IFN + SOF + RBV, and 90.5% in IFN + RBV + SOF arm versus 69.8% in SOF + RBV arm in HCV genotype 3 patients (P = 0.0003).
Table 2.
Treatment-related adverse events.
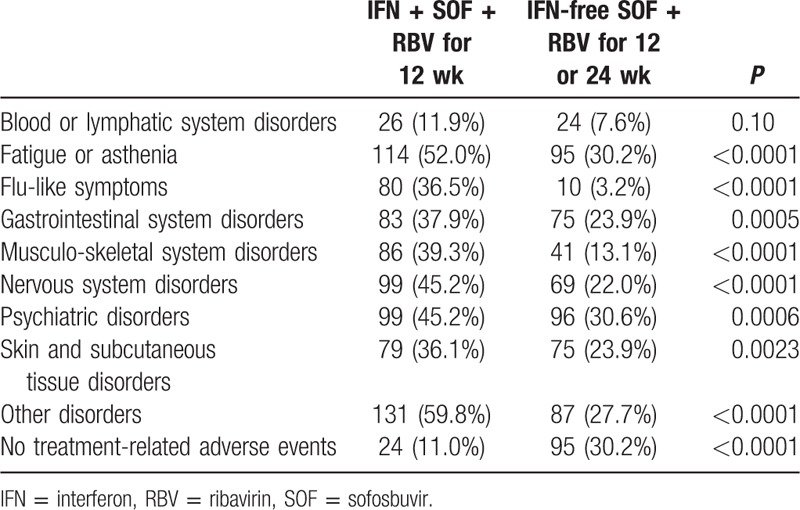
3.1. PROs during treatment with and without interferon
At baseline, despite some difference in demographic and clinical presentation, there were no difference in PROs between the 2 treatment arms (all P > 0.05) (Supplementary Table 1).
After 4 weeks of treatment with IFN + SOF + RBV, the PRO scores were found to be significantly lower than patients’ own baseline scores (Supplementary Fig. 1). The negative impact of treatment was found to be most apparent in the domains of patients’ Work Productivity (the greatest average decrease of 18.1 on a universal 0–100 scale), Role Physical of SF-36 (average −16.0), Activity/Energy of CLDQ-HCV (−15.9), Activity of WPAI:SHP (−15.5), Physical Well-Being of FACIT-F (−15.3), and FS of FACIT-F (−13.8) (all P < 0.0001). The average decrement across 26 studied PROs at that time point was −9.7 points.
These significant decreases in scores continued throughout treatment with IFN + RBV + SOF (Fig. 1A). Specifically, at 12 weeks, the decrements were up to −24.4 points from baseline; the largest decrements were again observed in Work Productivity and a number of physical functioning-related domains including Role Physical of SF-36, Activity of WPAI:SHP, Physical Well-Being and FS of FACIT-F, and Activity/Energy of CLDQ-HCV (all P < 0.0001). The average decrement across 26 PROs was −12.8 points, and the only 2 PRO domains in which no statistically significant decrement was found were the Worry domain of CLDQ-HCV and the Emotional Well-Being domain of FACIT-F (Fig. 1A).
Figure 1.
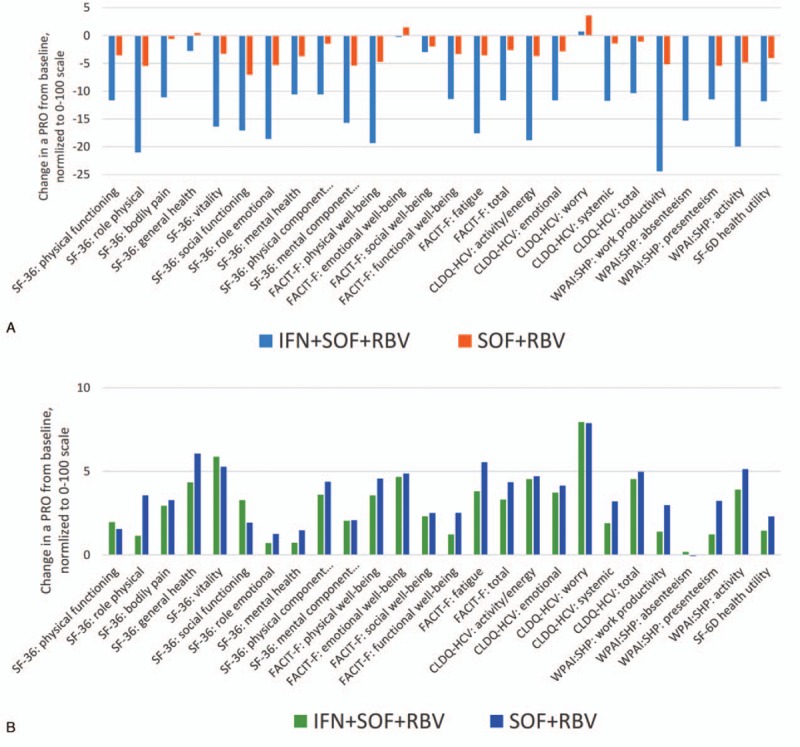
Average changes in PROs from baseline to (A) treatment week 12 (all P < 0.05 between regimens except for FACIT-F: emotional well-being and FACIT-F social well-being), and (B) post-treatment week 12 (in patients with SVR-12 only; all P > 0.05 between regimens). All PROs were transformed to a universal 0 to 100 scale. FACIT-F = Functional Assessment of Chronic Illness Therapy—Fatigue, PRO = patient-reported outcome, SVR = sustained viral response.
The PRO scores in the IFN + RBV + SOF arm remained low until 4 weeks post-treatment, predominantly in the same areas of impaired Work Productivity (average −8.3), Role Physical (−5.3), Role Emotional (−7.9), Activity (−6.2), and Social Functioning (−5.7) (all P < 0.002; the average across PROs −3.3) (Supplementary Fig. 2). Despite this, by post-treatment week 12 in patients with SVR, no PRO remained lower than the baseline level, and 14 out of 26 PRO domains improved relative to baseline (by up to +8.0, and by +4.2 on average; all P < 0.05) (Fig. 1B). Additionally, by week 24 after treatment discontinuation, all but 4 average PRO scores (Physical Functioning, Bodily Pain, Social Functioning of SF-36, and Absenteeism of WPAI:SHP) were significantly above their baseline levels (all P < 0.05).
In contrast to the IFN-containing regimen, the on-treatment PRO decrements in patients receiving an SOF + RBV-based treatment were smaller in magnitude (Fig. 2). In particular, the average PRO decrement by treatment week 4 was −2.0, with the greatest decrements observed in Social Functioning of SF-36 (−4.4), Physical Well-Being of FACIT-F (−4.0), Role Emotional of SF-36 (−3.8), and Presenteeism of WPAI:SHP (−3.5) (all P < 0.005) (Supplementary Fig. 1). Similarly, the decrements observed by treatment week 12 were also substantially smaller in comparison to those seen in the IFN-containing arm: up to −7.1 points in Social Functioning of SF-36 and −2.9 points across all studied PROs (Fig. 1A).
Figure 2.
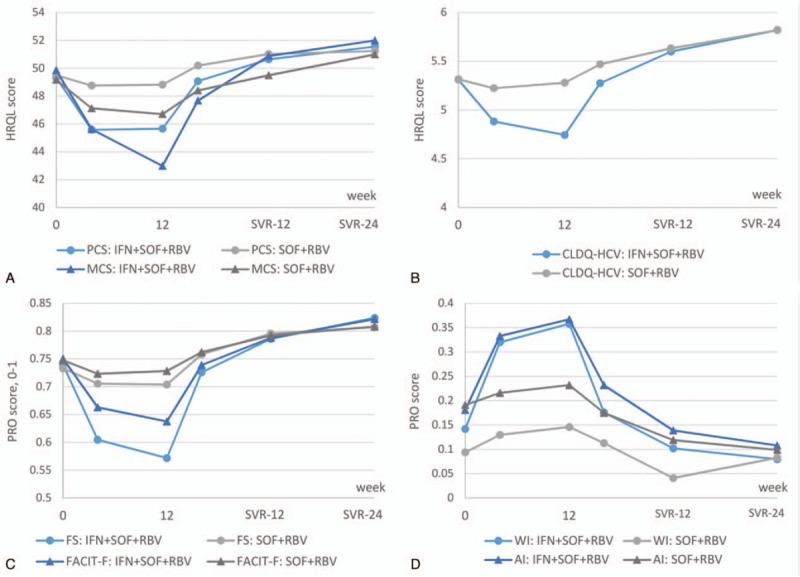
Summary PROs during and after treatment with IFN + RBV + SOF and IFN-free RBV + SOF. AI = activity impairment, FS = Fatigue Scale, IFN = interferon, MCS = Mental Summary Score, PCS = Physical Summary Score, PRO = patient-reported outcome, RBV = ribavirin, SOF = sofosbuvir, WI = work productivity impairment. All P < 0.05 between SOF+RBV and IFN+SOF+RBV treatment arms at treatment weeks 4 and 12.
Furthermore, in patients who received an IFN-free SOF + RBV regimen, all PROs returned to baseline levels or started to improve by post-treatment week 4 (improvements up to +4.1 in the Worry domain of CLDQ-HCV, average improvement across 7 improved PROs +2.1; all P < 0.05); no residual PRO decrement relative to baseline was observed at that time point (Supplementary Fig. 2). By 12 weeks after treatment cessation, in patients with SVR-12, all but 6 PRO scores significantly improved in patients treated with SOF + RBV by up to +7.1 points (+4.2 on average; all P < 0.03); note that these improvements are now similar to those observed in the IFN-containing arm (all P > 0.05) (Fig. 1B), and a similar observation was made at post-treatment week 24 as well.
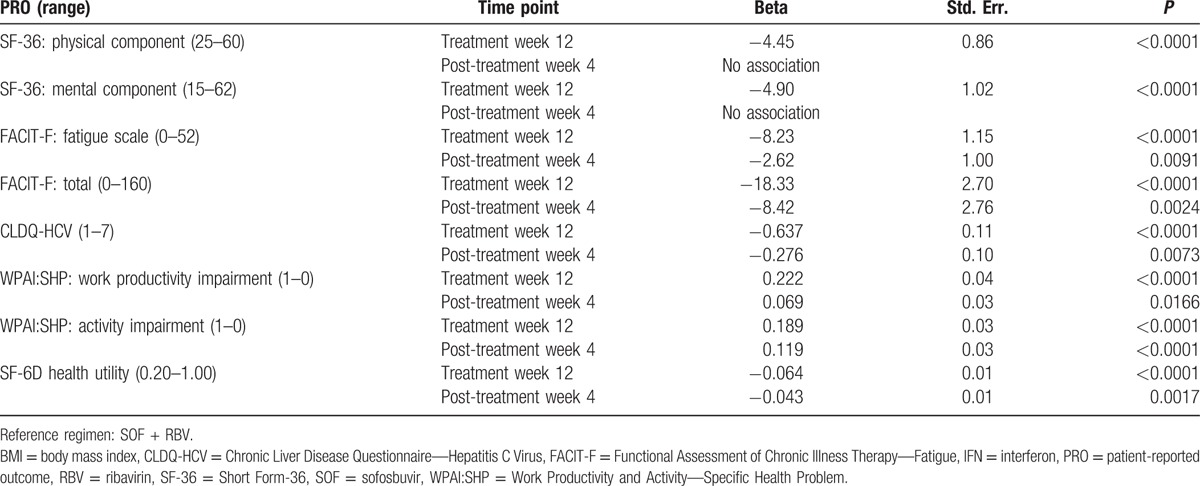
In multivariate analysis, after adjustment for demographic and clinical predictors of PROs, which were similar to those reported previously[8–12,20–23] and included site location, history of psychiatric disorders, fatigue, cirrhosis, the use of IFN in addition to SOF + RBV was independently associated with a greater impairment in PROs during and soon after treatment discontinuation (Table 3). In particular, the magnitude of association (beta) ranged from −8.1 to −22.2 at treatment week 12 (all P < 0.0001) and from −4.6 to −11.9 at post-treatment week 4 (all P < 0.02). By post-treatment week 12, the Physical Component Summary of SF-36, the CLDQ-HCV score, and Work Productivity Impairment score were still associated with treatment received: from −4.5 to −5.6 points for the use of IFN in comparison to the reference SOF + RBV regimen (all P < 0.05).
Table 3.
Independent association of the use of an IFN-containing treatment regimen with PROs during and soon after treatment cessation (adjusted for age, gender, location, BMI, history of psychiatric disorders, fatigue, and treatment history).
3.2. Subgroup analysis: PROs in treatment-experienced patients treated with IFN
In a separate round of analysis, we tested the hypothesis that the effect of IFN on PROs may be less pronounced in treatment-experienced patients who have already been treated with IFN. For that purpose, we used the subgroup of treatment-experienced patients currently treated with IFN + SOF + RBV, and compared baseline PROs and treatment-emergent changes in PROs between those who had received an IFN-containing regimen before and those who had received an IFN-free regimen with or without RBV.
As shown in Supplementary Table 2, most of the baseline PROs in patients with history of IFN treatment were significantly higher when compared to baseline PROs in patients who were being retreated after an IFN-free regimen. These PROs included 4 domains of SF-36 (Role Physical, General Health, Social Functioning, and Role Emotional), all but one domains of FACIT-F, all domains of CLDQ-HCV, Activity Impairment of WPAI:SHP, and the SF-6D health utility score (P < 0.05); nearly all of these PROs remained higher during treatment with IFN in this study. However, no difference in treatment-emergent changes in PROs with reference to patients’ own baseline levels was found between patients treated with IFN after having failed an IFN-free versus IFN-containing regimen neither in univariate nor in multivariate analysis (all P > 0.05).
4. Discussion
The purpose of this study was to investigate the impact of alternative hepatitis C treatment regimens on PROs. In this study, we have found that patients who were treated with a regimen containing IFN experienced a substantial decrease in their PRO scores during and even shortly after treatment. According to the multivariate analysis, the use of IFN was also found to be an independent predictor of substantial PRO impairment, and that association was observed up to 12 weeks after treatment discontinuation. Although PROs were also impacted by IFN-free regimens that contained RBV, the magnitude of such impact is much smaller. These data are consistent with previously published PRO data.[2–10,21–23]
Across both IFN-free and IFN-containing treatment arms, PRO domains that were most affected by the treatment were the domains primarily associated with daily functioning and physical activity. Thus, it is imperative for healthcare practitioners to assist patients in dealing with these areas by identifying strategies patients can pursue to stay active and social.
A number of previous studies of the use of IFN for treatment of chronic HCV infection have shown the negative effect of IFN on PROs.[13,14] In this study, we have shown that the impact of IFN-containing and IFN-free RBV-containing regimens on PROs of patients who are retreated is similar to those who have never been treated or have been treated with and IFN-free regimen only. In fact, experience with previous IFN-based treatment does not seem to predispose patients for better or worse experience during another course of treatment. Although HCV patients who were treatment-experienced with IFN had higher baseline PRO scores, most of these were likely due to previously applied strict treatment eligibility criteria.
A major limitation of this study is related to the original clinical trial design, which lacks both randomization and blinding. We, however, believe that the bias caused by this could be adequately accounted for by multivariate analysis.
In conclusion, treatment-experienced patients who were retreated with an IFN-containing regimen experienced significantly more impairment of their PROs in comparison to patients who were treated with an IFN-free regimen regardless of duration. However, it is important to note that, albeit accompanied by an unfavorable patients’ well-being profile, the IFN-containing regimen was still associated with a higher chance of SVR, especially in patients with HCV genotype 3. These 2 outcomes represent a trade-off that patients who have failed a DAA-based regimen may face, although the use of IFN, hopefully, will be revisited once new DAAs come to market and provide this cohort of patients with more treatment options.
Supplementary Material
Footnotes
Abbreviations: CLDQ-HCV = Chronic Liver Disease Questionnaire—Hepatitis C Virus, DAA = direct-acting antiviral agent, FACIT-F = Functional Assessment of Chronic Illness Therapy—Fatigue, FS = Fatigue Scale, HCV = hepatitis C virus, HRQL = health-related quality of life, IFN = interferon, PRO = patient-reported outcome, RBV = ribavirin, SF-36 = Short Form-36, SOF = sofosbuvir, SVR = sustained viral response, WPAI:SHP = Work Productivity and Activity—Specific Health Problem.
ZMY is a consultant or advisory board of Abbvie, Intercept, Gilead Sciences, Salix, GSK, BMS, and Janssen.
The study was funded by Gilead Sciences.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878–87. [DOI] [PubMed] [Google Scholar]
- [2].Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370:1993–2001. [DOI] [PubMed] [Google Scholar]
- [3].Bourlière M, Bronowicki JP, de Ledinghen V, et al. Ledipasvir–sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis 2015;15:397–404. [DOI] [PubMed] [Google Scholar]
- [4].Gane EJ, Hyland RH, An D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology 2015;149:1454–61. [DOI] [PubMed] [Google Scholar]
- [5].Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889–98. [DOI] [PubMed] [Google Scholar]
- [6].Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483–93. [DOI] [PubMed] [Google Scholar]
- [7].Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879–88. [DOI] [PubMed] [Google Scholar]
- [8].Younossi ZM, Stepanova M, Henry L, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH-C). J Hepatol 2014;60:741–7. [DOI] [PubMed] [Google Scholar]
- [9].Younossi Z, Henry L. The impact of the new antiviral regimens on patient reported outcomes and health economics of patients with chronic hepatitis C. Dig Liver Dis 2014;46suppl 5:S186–96. [DOI] [PubMed] [Google Scholar]
- [10].Younossi ZM, Stepanova M, Henry L, et al. Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2014;12:1349–59. [DOI] [PubMed] [Google Scholar]
- [11].Younossi ZM, Stepanova M, Sulkowski M, et al. Sofosbuvir and ribavirin for treatment of chronic hepatitis C in patients coinfected with hepatitis C virus and HIV: the impact on patient-reported outcomes. J Infect Dis 2015;212:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Younossi ZM, Stepanova M, Marcellin P, et al. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: results from the ION-1, -2, and -3 clinical trials. Hepatology 2015;61:1798–808. [DOI] [PubMed] [Google Scholar]
- [13].Mchutchison JG, Ware JE, Bayliss MS, et al. The effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivity. J Hepatol 2001;34:140–7. [DOI] [PubMed] [Google Scholar]
- [14].Hassanein T, Cooksley G, Sulkowski M, et al. The impact of peginterferon alfa-2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis C. J Hepatol 2004;40:675–81. [DOI] [PubMed] [Google Scholar]
- [15].Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res 2001;10:405–13. [DOI] [PubMed] [Google Scholar]
- [16].Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res 1999;8:604. [Google Scholar]
- [17].Younossi ZM, Guyatt G, Kiwi M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999;45:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. [DOI] [PubMed] [Google Scholar]
- [19].Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271–92. [DOI] [PubMed] [Google Scholar]
- [20].Younossi ZM, Stepanova M, Afdhal N, et al. Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol 2015;63:337–45. [DOI] [PubMed] [Google Scholar]
- [21].Younossi ZM, Stepanova M, Sulkowski M, et al. Sofosbuvir and ledipasvir improve patient-reported outcomes in patients co-infected with hepatitis C and human immunodeficiency virus. J Viral Hepat 2016;23:857–65. [DOI] [PubMed] [Google Scholar]
- [22].Younossi ZM, Stepanova M, Sulkowski M, et al. Ribavirin-free regimen with sofosbuvir and velpatasvir is associated with high efficacy and improvement of patient-reported outcomes in patients with genotypes 2 and 3 chronic hepatitis C: results from astral-2 and -3 clinical trials. Clin Infect Dis 2016;63:1042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol 2016;111:808–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


