Abstract
Rationale:
Chronic liver disease is a major cause of morbidity and mortality in patients with HIV. However, autoimmune hepatitis (AIH) in patients with HIV has rarely been reported. Our aim was to evaluate a cohort of patients with HIV and AIH and identify clinical presentations and outcomes.
Patient Concerns:
Management of autoimmune hepatitis in context of human immunodeficiency virus, long-term outcomes, and safety in setting of underlying immunocompromised state.
Diagnoses:
Autoimmune Hepatitis, Human Immunodeficiency Virus, Hepatotoxicity, Liver Injury, Liver Transplantation.
Interventions:
We retrospectively reviewed the charts of patients with HIV and AIH based on histological, serologic, biochemical demographic, and clinical data.
Outcomes:
Five patients were identified with autoimmune hepatitis; 4 of 5 were women, and all were African or African-American. The age at the time of AIH diagnosis was 46.6 ± 13.4 years. All patients acquired HIV sexually and all had CD4 counts >250 cells/uL (456–1011 cells/uL) and undetectable HIV viral loads at the time of AIH diagnosis. One patient presented with acute liver failure necessitating liver transplantation and developed AIH posttransplantation. At the time of diagnosis, the AST were 350 ± 448 U/L, ALT 247 ± 190 U/L, bilirubin 7 ± 12 mg/dL, and alkaline phosphatase 126 ± 53 U/L. All patients had histologic evidence of AIH on liver biopsies. Patients were successfully treated with prednisone and azathioprine, without a decrease in CD4 <250 cells/uL, infectious complications or significant side effects.
Lessons:
AIH occurs in patients with well-controlled HIV. In our patient cohort, immunosuppressive therapy with prednisone and azathioprine was safe and effective in inducing remission, without significant complications or development of opportunistic infections.
Keywords: autoimmune hepatitis, hepatotoxicity, human immunodeficiency virus, liver injury, liver transplantation
1. Introduction
Autoimmune hepatitis (AIH) is a progressive, chronic liver disease that has a varying clinical presentation and course. The diagnosis of autoimmune hepatitis is made based on criteria including hypergammaglobulinemia, positive serologic tests including antinuclear and anti-smooth muscle antibodies, and a characteristic hepatic histological appearance, namely interface hepatitis, plasmacytic infiltrate, and regenerative liver-cell rosettes as outlined by American and European practice guidelines.[1–3] Other etiologies of liver disease such as alpha-1-antitrpsysin deficiency, Wilson disease, hemochromatosis, viral hepatitis, drug-induced liver injury, and alcoholic/nonalcoholic liver disease also warrant exclusion. The disease typically affects women and those with coexisting autoimmune conditions, but it occurs in many of different populations. While it is common to have complications of liver disease including chronic hepatitis B or C, drug hepatotoxicity, opportunistic infections, or neoplasms in patients with HIV, few cases of AIH occurring in HIV-infected individuals have previously been reported, and AIH is rarely suspected. Despite its rare incidence, the finding of AIH in HIV-infected patients has important therapeutic and prognostic implications. In the few cases that have previously been reported, the majority of patients benefited in receiving treatment for AIH with standard therapy of prednisone with or without azathioprine or budesonide in the setting of HIV.[4–6] This is in contrast to a case described by German et al where treatment with highly active anti-retroviral therapy (HAART) led to improvement in the course of AIH.[7] In addition, no correlations have been made between CD4 counts or other markers in predicting the presence or severity of AIH, mainly due to limitations from studying small group of patients. Furthermore, no assessment of safety or disease progression has been made in this cohort of HIV seropositive patients treated for AIH.
Our aim was to evaluate trends and patterns in a cohort of patients with HIV and AIH at our institution and to identify presentations and outcomes in this complex population of patients.
2. Methods
We retrospectively reviewed the charts of patients with HIV and AIH identified within a clinical cohort in the outpatient clinic setting. The diagnosis of AIH was made based on the American Association of the Study of Liver Diseases practice guidelines.[2] Following IRB approval, consent was obtained from all patients to access their medical record. Charts were retrospectively reviewed for demographic, clinical, laboratory, and treatment data; including response to therapy based on biochemical response and status of HIV infection. Means and standard deviations for continuous variables and frequencies for categorical values were obtained.
3. Results
Five patients were identified to have AIH and HIV, 4 were women and 1 was male, and all were African or African-American. The age at the time of AIH diagnosis after the diagnosis of HIV was 46.6 ± 13.4 years. All patients had CD4 counts >250 cells/uL (456–1011 cells/uL) and nondetectable HIV viral loads at the time of HIV diagnosis. The mode of HIV transmission was sexual in all patients. Table 1 includes laboratory data at the time of AIH diagnosis. All patients underwent liver biopsies with varying degrees of histologic evidence of AIH on their pathology. Note that Case 2 had borderline biochemical markers (antinuclear antibody negative, smooth-muscle antibody 1:20), but the histologic findings were highly suggestive of AIH, characterized by extensive plasma cell infiltrate. Figure 1 shows representative histology from 3 of the 4 patients. Note that 1 patient underwent biopsy at another institution and his slides were not available for review at the time of drafting of this manuscript, though our pathologists reviewed his slides previously.
Table 1.
Clinical findings at the time of AIH diagnosis.
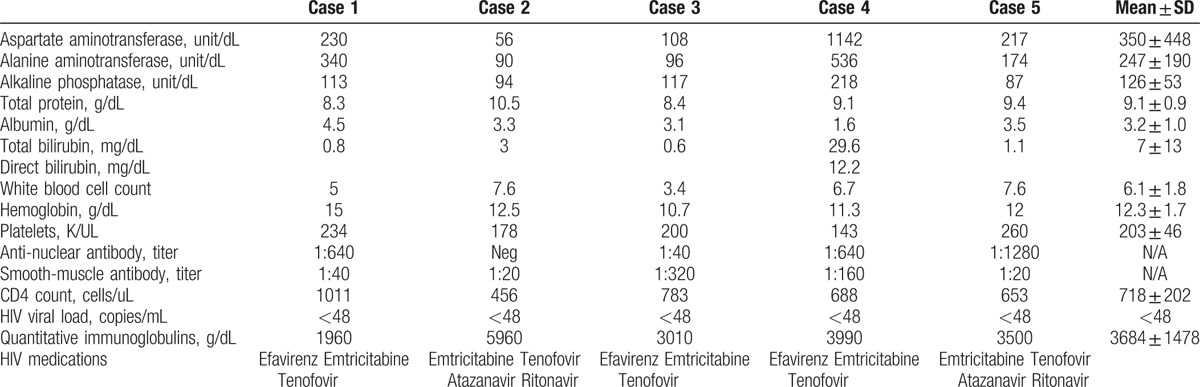
Figure 1.
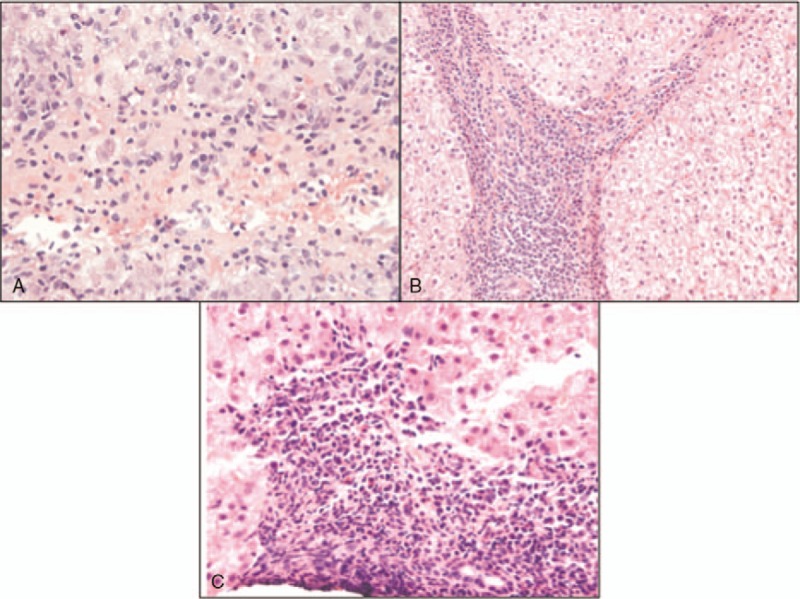
A, High power view showing plasma cells. B, High power view showing sheets of plasma cells and cirrhotic bands. C, High power view of portal area showing plasma cells.
Four patients were treated with prednisone and azathioprine, with improvement in the serum liver chemistry tests, and without a decrease in the CD4 to below 250 cells/uL, infectious complications or significant side effects. Table 2 includes laboratory data 1 month after initiation of therapy in the 4 patients started on treatment. Complete biochemical remission was achieved in 2 patients within 40 days of initiating treatment. A third patient achieved biochemical remission after 2 years of therapy (alanine transaminase (ALT) at 1 month was 48 U/L), and the fourth patient had partial biochemical response but was noted to be intermittently compliant with treatment and was subsequently lost to follow-up a year after initiation of therapy. She was well and denied being on steroid therapy at the last follow-up which was approximately 2 years after initiation of treatment, but it is unclear what her aminotransferases were at that time. Note that 3 of the 4 patients (not including the one lost to follow-up) had CD4 counts >250 and undetectable viral loads at 1 year follow-up. No adverse events were noted related to their HIV.
Table 2.
Laboratory values 1 mo after treatment.
The fifth patient had a clinical presentation of acute liver failure. She presented to the emergency room with anorexia, weakness, confusion, and jaundice, with markedly elevated liver chemistry tests including an aspartate transaminase (AST) 1416 U/L, ALT 650 U/L, total bilirubin 21 mg/dL, direct bilirubin 16 mg/dL, alkaline phosphatase 269 U/L, and international normalized ratio (INR) of 2.1. Her CD4 count was 430 cells/uL and VL was undetectable (<49 copies/mL). She had been on HAART since the time of her diagnosis 8 years prior to admission but had recently been changed to therapy with efavirenz, emtricitabine and tenofovir 2 weeks prior to presentation. The patient was noted to have a positive ANA (1:40–1:180), positive antismooth muscle antibody (1:20), and negative AMA. Further evaluation for other etiologies of liver failure including viral hepatitis and metabolic disorders (hemochromatosis, Wilson disease, alpha-1-anti-trypsin deficiency) was negative. She denied acetaminophen ingestion and her level was negative. A liver biopsy at that time was notable for acute hepatitis with bridging necrosis. Few plasma cells were seen. The etiology of liver failure remained uncertain. Diagnoses of AIH and drug toxicity related to efavirenz were entertained. The patient continued to clinically deteriorate and her biochemical profile did not improve despite holding her medications, so a diagnosis of AIH was favored, but was not confirmed histologically. A brief trial of steroids (3 days) was attempted, but discontinued as the patient became septic. The patient subsequently underwent transplantation within 1 month of presentation. She did well in the immediate perioperative period. Long-term follow-up revealed development of AIH 4 years following transplantation, as confirmed by liver biopsy (Fig. 2). She was subsequently treated with augmentation of her immunosuppression and did well, without infectious or HIV-related complications.
Figure 2.
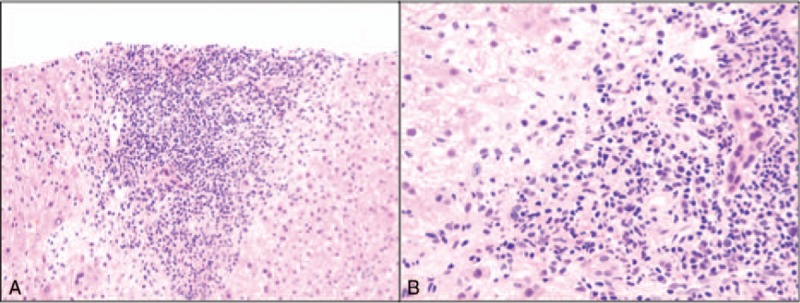
A, Low power view showing plasma cells in portal area. B, High power view showing plasma cells.
4. Discussion
The presentation of AIH in a patient with HIV is a rare, but should be considered in patients with HIV who are not found to have other etiologies of elevated aminotransferases or liver injury. The presentation is varied, and can manifest as life-threatening acute liver failure necessitating liver transplantation, or mild aminotransferase elevations of unknown etiologies. Note that it can also present as de-novo AIH in a posttransplant patient in the context of immunosuppression. In our series of patients, 4 were women, and all were African or African-American. The 4 patients who did not present with acute liver failure underwent treatment without a change or deterioration in their HIV status, or increase in infectious complications. The patient who developed posttransplant AIH was also successfully treated following diagnosis with increase in immunosuppression. Although it is difficult to definitively determine, given the small size of our series, it appears that treatment with corticosteroids and immunomodulators is safe and effective despite the theoretical risks of worsening cell-mediated immunosuppression manifested as development of opportunistic infections or malignancies.
The pathogenesis of liver damage in AIH in patients with HIV may be mediated by autoreactive CD4 or CD8 T-cells, or by an antibody-dependent cell-mediated cytotoxic response directed against liver antigens.[8] Interestingly, initiation of HAART has also been implicated in de novo AIH, and has thought to “unmask” AIH during the immune reconstitution phase following initiation of HAART treatment, mediated by a loss of peripheral tolerance and autoreactivity.[6] Moreover, beyond serving as a catalyst, drugs can have a direct cytotoxic effect and lead to drug-induced AIH, which is a well-described clinical entity, though typically implicating minocycline or nitrofurantoin. In fact, the patient who presented with acute liver failure may have demonstrated this pathophysiology, with efavirenz serving as the catalyst in this clinical scenario. This is the prevailing theory, however, other cases have purported improvement of liver biochemistry tests following initiation of HAART, suggesting that the virus itself may somehow be the causative agent in certain instances.[7] The majority of reported cases in the literature support the former hypothesis, as most cases did not show improvement with HAART and required treatment with corticosteroids. The patients presented in this case also support this mechanistic theory.
Albeit a rare disease, de novo AIH should be considered in patients with HIV, particularly in those with well-controlled disease where no other clear etiologies can be identified. A liver biopsy, albeit not mandatory, is very useful for diagnosis, and typically shows findings consistent with AIH, but this is not always the case. A combination of serologic, biochemical, clinical, and histologic data is needed to arrive at the diagnosis. Prompt treatment should be initiated, particularly in cases of worsening liver injury and evolving liver failure. Monitoring for infectious complications should be undertaken, but the risk appears to be minimal in patients with well-controlled HIV.
Footnotes
Abbreviations: AIH = autoimmune hepatitis, ALT = alanine transaminase, AST = aspartate transaminase, HAART = highly active anti-retroviral therapy, HIV = human immunodeficiency virus, INR = international normalized ratio.
The authors have no conflicts of interest to disclose.
References
- [1].Krawitt EL. Autoimmune hepatitis. N Engl J Med 2006;354:54–66. [DOI] [PubMed] [Google Scholar]
- [2].Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology 2002;36:479–97. [DOI] [PubMed] [Google Scholar]
- [3].European Association for the Study of the L. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol 2015;63:971–1004. [DOI] [PubMed] [Google Scholar]
- [4].Puius YA, Dove LM, Brust DG, et al. Three cases of autoimmune hepatitis in HIV-infected patients. J Clin Gastroenterol 2008;42:425–9. [DOI] [PubMed] [Google Scholar]
- [5].Wan DW, Marks K, Yantiss RK, et al. Autoimmune hepatitis in the HIV-infected patient: a therapeutic dilemma. AIDS Patient Care STDS 2009;23:407–13. [DOI] [PubMed] [Google Scholar]
- [6].O’Leary JG, Zachary K, Misdraji J, et al. De novo autoimmune hepatitis during immune reconstitution in an HIV-infected patient receiving highly active antiretroviral therapy. Clin Infect Dis 2008;46:e12–14. [DOI] [PubMed] [Google Scholar]
- [7].German V, Vassiloyanakopoulos A, Sampaziotis D, et al. Autoimmune hepatitis in an HIV infected patient that responded to antiretroviral therapy. Scand J Infect Dis 2005;37:148–51. [DOI] [PubMed] [Google Scholar]
- [8].Diamantis I, Boumpas DT. Autoimmune hepatitis: evolving concepts. Autoimmun Rev 2004;3:207–14. [DOI] [PubMed] [Google Scholar]


