Abstract
For vancomycin therapy of severe infections, the Infectious Diseases Society of America recommends high vancomycin trough levels, whose potential for inducing nephrotoxicity is controversial. We evaluated the incidence and risk factors of acute kidney injury (AKI) in critically ill patients given continuous intravenous vancomycin with target serum vancomycin levels of 20 to 30 mg/L.
We retrospectively studied 107 continuous intravenous vancomycin treatments of ≥48 hours’ duration with at least 2 serum vancomycin levels ≥20 mg/L in critically ill patients. Nephrotoxicity was defined according to the Kidney Disease Improving Global Outcomes Clinical Practice Guideline for AKI (ie, serum creatinine elevation by ≥26.5 μmoL/L or to ≥1.5 times baseline). Risk factors for AKI were identified by univariate and multivariate analyses.
AKI developed in 31 (29%) courses. Higher serum vancomycin levels were associated with AKI (P < 0.01). Factors independently associated with AKI were highest serum vancomycin ≥40 mg/L (odds ratio [OR], 3.75; 95% confidence interval [CI], 1.40–10.37; P < 0.01), higher cumulative number of organ failures (OR, 2.63 95%CI, 1.42–5.31; P < 0.01), and cirrhosis of the liver (OR, 5.58; 95%CI, 1.08–31.59; P = 0.04).
In this study, 29% of critically ill patients had AKI develop during continuous intravenous vancomycin therapy targeting serum levels of 20 to 30 mg/L. Serum vancomycin level ≥40 mg/L was independently associated with AKI.
Keywords: acute kidney injury, continuous infusion, intensive care, nephrotoxicity, vancomycin
1. Introduction
Vancomycin has been traditionally administered as brief intravenous infusions in a dosage of 1 g/12 hours to achieve trough vancomycin levels of 5 to 10 mg/L.[1,2] This schedule has been estimated to induce nephrotoxicity in 5% of patients.[1] Guidelines issued by the Infectious Diseases Society of America in 2006 recommend increasing the vancomycin dosage in patients with severe methicillin-resistant Staphylococcus aureus infections to achieve trough levels of 15 to 20 mg/L.[2–4] This recommendation is a response to reports of clinical treatment failure related to the limited vancomycin susceptibility of some methicillin-resistant Staphylococcus aureus strains. In patients with severe infections requiring intensive care unit (ICU) admission, an alternative vancomycin schedule is continuous intravenous administration in a dosage of 30 mg/kg/day after a loading dose of 15 to 30 mg/kg to achieve a serum plateau of 20 to 30 mg/L depending on the site of the infection.[5–7]
Following implementation of the latest Infectious Diseases Society of America recommendation to target higher serum vancomycin levels, several groups in the US reported an increase in the frequency of nephrotoxicity, to 12% to 43%.[8–12] In a recent meta-analysis, trough vancomycin level ≥15 mg/L was an independent risk factor for nephrotoxicity.[13] Most of the studies included in this meta-analysis were done in patients with limited illness severity who rarely required ICU admission and who received vancomycin on an intermittent basis. Furthermore, nephrotoxicity was defined using the traditional criterion of serum creatinine elevation by ≥44 μmol/L or ≥50% versus baseline.[14] The sensitivity of diagnostic criteria for acute renal failure was recently improved via the development of the acute kidney injury (AKI) concept, reported by the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI.[15] This concept facilitates the early detection, standardized definition, and stratification of renal function impairments.[15–18]
The objectives of this retrospective study were to determine the frequency and to identify the risk factors of AKI associated with continuous intravenous vancomycin therapy targeting high serum vancomycin levels of 20 to 30 mg/L in patients admitted to the ICU.
2. Methods
2.1. Study design and patient population
We performed a single-center, observational, retrospective study in our 18-bed medical-surgical adult ICU between November 2006 and January 2010. Complete case ascertainment was achieved by searching the hospital pharmacy database for delivery of vancomycin to ICU patients. Inclusion criteria were age >18 years, continuous intravenous vancomycin therapy for at least 48 hours, and at least 2 serum vancomycin levels ≥20 mg/L. Patients with probable or documented infection were eligible. Exclusion criteria were prophylactic vancomycin therapy, vancomycin therapy for less than 48 hours, one or no serum vancomycin level ≥20 mg/L, concomitant oral vancomycin administration, chronic dialysis, and pregnancy.
2.2. Treatment schedule and monitoring definitions
Vancomycin was routinely administered by continuous intravenous infusion via an electrical pump connected to a dedicated central venous catheter. Injectable powdered vancomycin (Sandoz, Holzkirchen, Germany) containing 1 g vancomycin base per vial was reconstituted with isotonic saline to obtain a final concentration of 20 mg/mL. A loading dose of 15 mg/kg injected over 60 minutes was followed by a continuous pump infusion of 30 mg/kg/day. Doses were computed using the most recent available body weight.
Monitoring involved samples of the plateau vancomycin levels. Samples were collected once daily starting on the 2nd day of vancomycin therapy. Blood was drawn into standard dry tubes, which were kept at 4 °C, for less than 4 hours. An automated fluorescence polarization assay was performed using the Cobas Integra 800 analyzer (Roche Diagnostics, Basel, Switzerland). Patients with below-target vancomycin levels below 20 mg/L received an additional bolus and/or a higher daily dosage. Vancomycin levels above 30 mg/L were managed by interrupting the infusion and/or decreasing the daily dosage. No specific instruction was given to physicians for adjusting the vancomycin dosage to renal function.
For each course, we computed the dosage during the 1st 24 hours (D1 dosage) as the sum of the loading dose and continuously administered dose; as well as the overall mean daily dosage over the entire course, including the initial loading dose. Vancomycin therapy duration was recorded as the number of days with vancomycin therapy. We recorded the peak serum vancomycin level during the ICU stay.
2.3. Definition of nephrotoxicity
Renal function was monitored by repeatedly measuring the serum creatinine level and calculating the estimated glomerular filtration rate (using a glomerular filtration rate estimating equation derived from the serum creatinine value). We recorded the serum creatinine levels at the following time points: baseline (ie, just before vancomycin initiation), day of vancomycin discontinuation, and 3 and 7 days after vancomycin discontinuation. We also recorded the peak serum creatinine level between vancomycin initiation and 72 hours after vancomycin discontinuation, and we computed the serum creatinine increase from baseline to this peak (expressed as the absolute value and percentage).
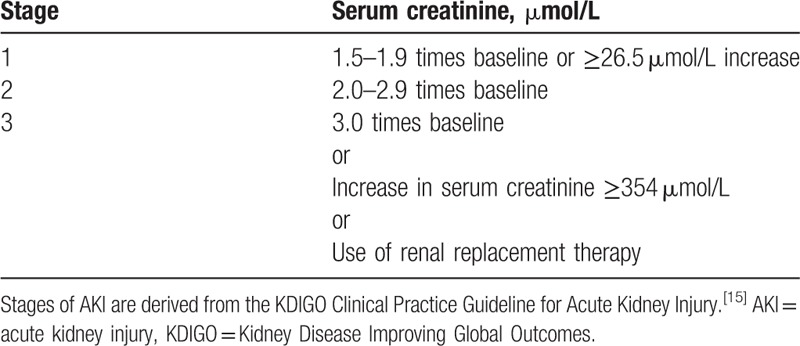
We defined nephrotoxicity as AKI, using criteria derived from the KDIGO Clinical Practice Guideline for AKI (serum creatinine increase by ≥26.5 μmol/L or to ≥1.5 times baseline).[15] AKI severity was categorized as shown in Table 1. The initiation of conventional dialysis before, during, or after vancomycin therapy was recorded.
Table 1.
Stages of acute kidney injury (AKI).
2.4. Study variables
We recorded age, gender, body weight, and body mass index. Comorbidities were classified as follows: cardiovascular (heart failure, coronary artery disease, and hypertension), diabetes, respiratory (chronic obstructive pulmonary disease and chronic respiratory failure), chronic renal dysfunction, cirrhosis of the liver, chronic alcohol abuse, solid malignancy, hematological malignancy, and immunosuppression (acquired immune deficiency syndrome, corticosteroid therapy, anticancer chemotherapy, and other immunosuppressants).
The Simplified Acute Physiology Score (SAPS) version II and Acute Physiology and Chronic Health Evaluation version II severity scores were recorded, as well as the reason for ICU admission (medical, emergent surgical, or scheduled surgical), hospital stay length, and vital status at ICU discharge. Organ failure was categorized as follows: respiratory (mechanical ventilation and/or PaO2/FiO2 < 200), hemodynamic (mean arterial pressure <65 mm Hg and/or vasoactive or inotropic drug therapy), hematological (platelet count ≤80,000/mm), hepatic (serum bilirubin ≥35 μmol/L), and neurological (Glasgow Coma Scale score ≤6 without sedation). The peak serum lactic acid value was recorded. For continuous variables, we recorded the highest value found during vancomycin therapy or within 72 hours after vancomycin discontinuation.
Exposures to other nephrotoxic agents within 72 hours before vancomycin therapy, during vancomycin therapy, and within 72 hours after vancomycin discontinuation were recorded. These agents were defined as intravenous iodinated contrast agents, aminoglycosides, loop diuretics, other diuretics (thiazides, aldosterone antagonists), angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists (Sartans), and nonsteroidal antiinflammatory drugs. For each exposure, the dose and duration were recorded.
2.5. Statistical analysis
Quantitative parameters were described as median (interquartile range) and qualitative parameters as number (percentage). We compared categorical variables using Fisher exact tests and continuous variables using Wilcoxon rank-sum tests. To identify associations between patient characteristics and AKI occurrence, we used a logistic regression model. Odds ratios are reported with their 95% confidence intervals. The final multivariate model was built using a backward stepwise procedure based on Akaike criterion. Continuous variables were checked for log-linearity, and when found to be nonlog-linear, they were categorized. Factors included in the multivariate regression model were selected as clinically relevant among variables yielding P values smaller than 0.20 by univariate analysis. Variables included in the model were as follows: number of organ failures (excluding AKI), highest vasoactive drug infusion rate, number of concomitant nephrotoxic agents, cirrhosis of the liver, and peak serum vancomycin ≥40 mg/L. Given their low numbers, missing values of covariates were handled by median imputation. Le Cessie-Van-Houwelingen goodness-of-fit tests were applied to final models. All tests were 2-sided, and P values < 0.05 were considered significant. Analyses were performed using R statistical software version 2.14.0 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org. Accessed June 29, 2016).
2.6. Ethical considerations
Our local ethics committee (Comité de Protection des Personnes of Paris, Ile de France VI) approved this study (#220114) and waived the need for informed consent. For each patient, the study data were collected in a standardized case-report form. Patients were identified only by a study inclusion alphanumeric combination.
3. Results
We identified 120 courses of intravenous vancomycin administered continuously using an electrical pump during the study period. Among them, 13 were excluded, for the following reasons: duration < 48 hours (n = 6), less than 2 serum vancomycin levels ≥20 mg/L (n = 2), concomitant oral vancomycin (n = 1), chronic dialysis (n = 2), and incomplete or unavailable medical records (n = 2) (Fig. 1). The remaining 107 vancomycin courses were studied. They were administered to 102 patients, of whom 5 received vancomycin courses during 2 different stays in our ICU.
Figure 1.
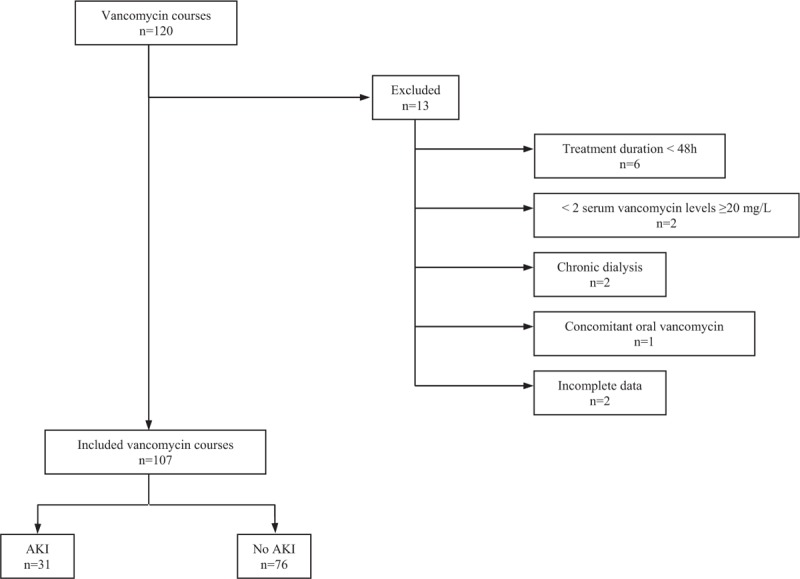
Flowchart of the study. Acute kidney injury (AKI) defined as serum creatinine elevation by ≥26.5 μmol/L or to ≥1.5 times baseline.
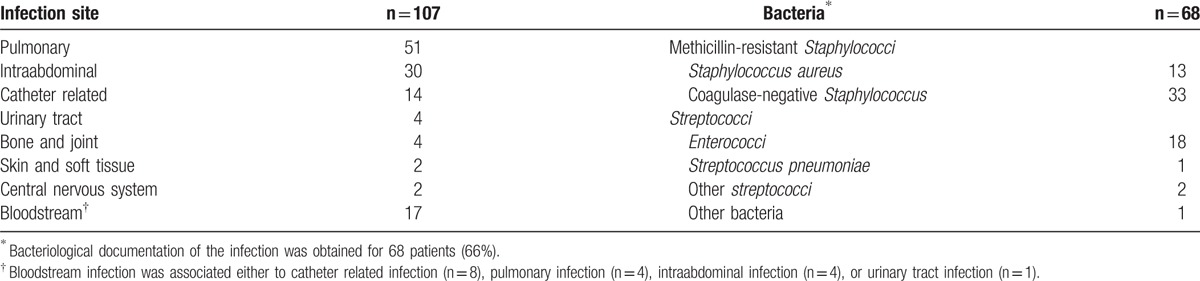
Table 2 reports the main patient characteristics (demographics, comorbidities, and ICU management), characteristics of vancomycin therapy, and concomitant use of nephrotoxic agents. Bacteriological documentation of the infection was obtained for 68 (66%) patients. The selection of vancomycin was probabilistic in 43 (36%) courses. Febrile bone marrow aplasia was the reason for vancomycin initiation in 8 (7%) patients. Table 3 reports the reasons for vancomycin treatment, bacteriological data, and infection sites.
Table 2.
Variables associated with AKI.
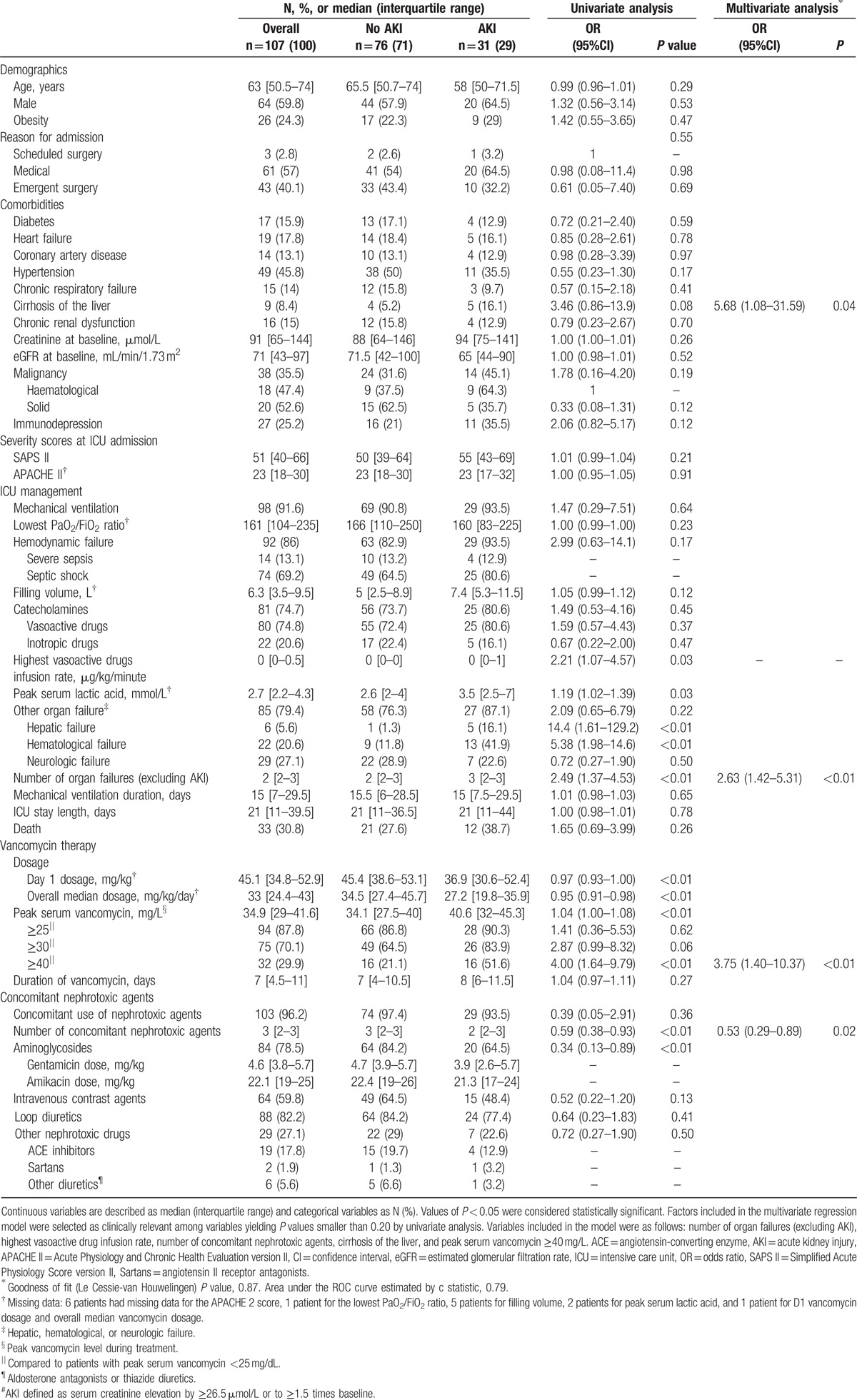
Table 3.
Bacteriological data and infection sites.
AKI developed during 31 (29%) courses. AKI severity was stage 1 in 17 (54.8%) patients, stage 2 in 4 (12.9%) patients, and stage 3 in 10 (32.3%) patients. Baseline serum creatinine values were not significantly different between patients with and without AKI. Of the 7 patients who required de novo renal replacement therapy (RRT), only 1 survived with a return to the previous level of kidney function. Of the 31 courses with AKI, 17 (54.8%) were followed by recovery of normal renal function; 2 (6.5%) required continuation of RRT started before vancomycin initiation; and 12 (38.7%) were in patients who died with persistent renal dysfunction, including 6 (6/31, 19.4%) after receiving de novo RRT.
By univariate analysis (Table 2), the clinical variables associated with AKI were peak serum lactic acid level, cirrhosis of the liver, and higher cumulative number of organ failures (excluding AKI and including hepatic failure and hematological failure). Higher peak serum vancomycin levels were significantly associated with AKI (Figs. 2 and 3). Lower values of D1 dosage and overall dosage were also associated with AKI (Table 2). Aminoglycoside exposure and total number of concomitant nephrotoxic agents were more common in the group without AKI (Table 2).
Figure 2.
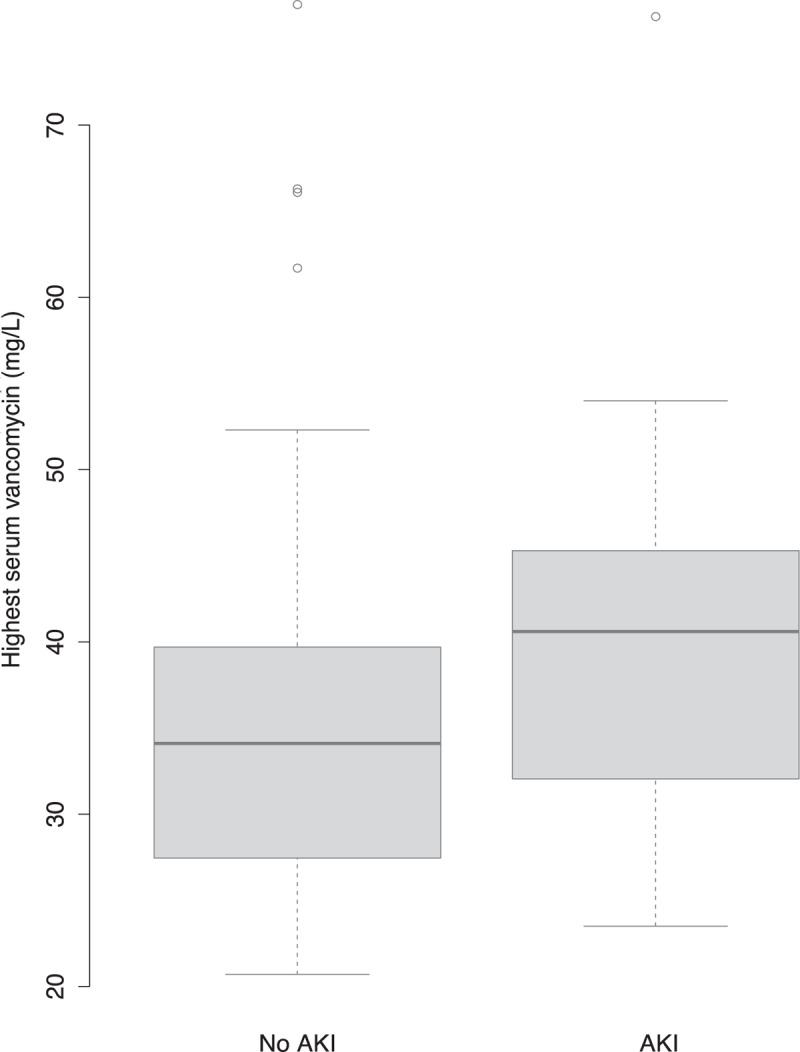
Acute kidney injury (AKI) according to peak serum vancomycin level. The difference between the 2 groups was statistically significant (P < 0.01). AKI defined as serum creatinine elevation by ≥26.5 μmol/L or to ≥1.5 times baseline.
Figure 3.
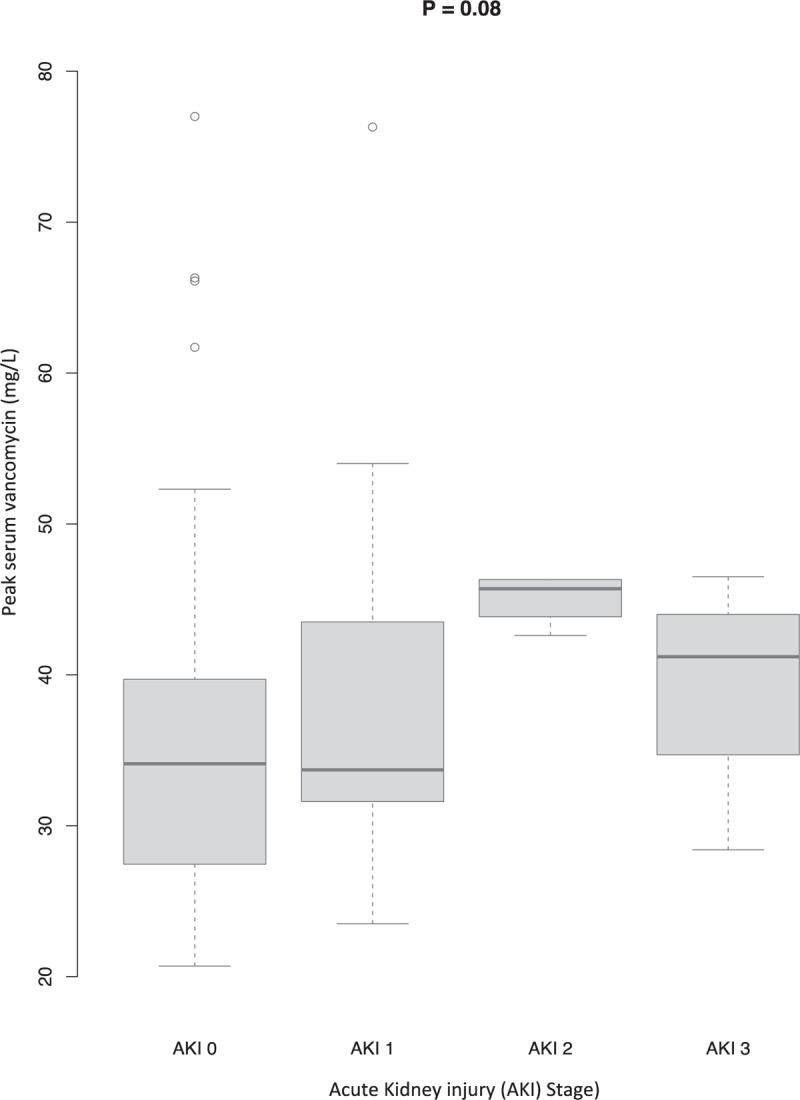
Boxplot of peak serum vancomycin (mg/L) according to stages of acute kidney injury (AKI). A boxplot is shown for each of the four each AKI stages. The X axis shows the 4 AKI stages (0–3) and the Y axis the peak serum vancomycin level (mg/L). The shaded box indicates the middle 50% of the data; the lower and upper ends of this box are the lowest and highest quartiles, respectively. The solid black horizontal line through each shaded box indicates the median value. The circles above the vertical solid black lines are individual outliers. The P value is for the overall comparison of peak serum vancomycin levels for each AKI stage. The AKI stages are defined in the Kidney Disease Improving Global Outcomes Clinical Practice Guideline,[15] reported in Table 1.
By multivariate analysis (Table 2), factors independently associated with AKI were peak serum vancomycin ≥40 mg/L, higher cumulative number of organ failures (excluding AKI), and cirrhosis of the liver. The number of concomitant nephrotoxic agents was associated with a lower risk of AKI.
4. Discussion
In this retrospective cohort of 107 patients with severe acute illnesses requiring ICU admission, the frequency of AKI during high-dose continuous intravenous vancomycin therapy targeting plateau concentrations of 20 to 30 mg/L was 29%. Factors independently associated with AKI were peak serum vancomycin ≥40 mg/L, higher cumulative number of organ failures (excluding AKI), and cirrhosis of the liver. The number of concomitant nephrotoxic agents was associated with a lower risk of AKI.
Our work is a pragmatic study in a large cohort of ICU patients who had multiple comorbidities and organ failures. The high SAPS II and Acute Physiology and Chronic Health Evaluation version II scores reflect the severity of the acute illnesses in our patients. In contrast to others, we did not exclude patients with preexisting acute or chronic renal dysfunction, iodinated contrast agent exposure, or neutropenia.[9–12,19] Our results highlight the strong associations linking organ failures and comorbidities to AKI development in ICU patients: cirrhosis of the liver, high serum lactic acid level (reflecting circulatory failure), and cumulative number of organ failures (excluding AKI) including hepatic failure and hematological failure were associated with a higher risk of AKI by univariate analysis. By multivariate analysis, a higher cumulative number of organ failures and cirrhosis of the liver remained independent risk factors for AKI. Our results confirm previous findings.[15,20] The KDIGO Clinical Practice Guideline for AKI states that critical illness, circulatory shock, and chronic diseases including liver disease are common causes of AKI.[15] More recently, the multinational Acute Kidney Injury–Epidemiologic Prospective Investigation study reported specific data on the epidemiology of AKI in ICU patients.[20] In this cohort, the frequency of AKI was nearly 60%, and illness severity (ie, higher SAPS III score) was also an independent risk factor of AKI. Similarly, shock, organ failures including liver failure, and comorbidities including cirrhosis were associated with a higher frequency of AKI.[20] These results are consistent with ours and emphasize the very high risk of AKI in ICU patients: in this specific population, assessing the risk of AKI is a priority, as identifying high-risk patients may help to avoid additional iatrogenicis. Nephrotoxic treatments such as high-dose vancomycin must be prescribed with caution and monitored closely by daily serum vancomycin assays.
Available data on the nephrotoxicity associated with high vancomycin dosages are scant and somewhat conflicting. The overall frequency of nephrotoxicity in a meta-analysis of studies published between 2003 and 2007 was 14.6%.[21] A 2013 review reported considerable variation in the incidence of nephrotoxicity across studies (from 5% to 43%).[13] Most studies identified trough vancomycin level ≥15 mg/L and treatment duration ≥7 days as the most common risk factors.[13,21,22] In 188 ICU patients, initial trough vancomycin level ≥15 mg/L was associated with higher frequencies of nephrotoxicity ranging from 15% to 25%.[19] More recently, Hanrahan et al[23] reported nephrotoxicity in 20% of 1430 critically ill patients; higher serum vancomycin concentrations and longer treatment duration were independently associated with higher odds of nephrotoxicity. Furthermore, continuous infusion was associated with a lower frequency of nephrotoxicity compared with intermittent infusion.[23]
Vancomycin nephrotoxicity was usually defined in previous studies as creatinine elevation ≥44 μmol/L or ≥50% of the baseline value.[8–12,19] Instead, we used the AKI concept described in the KDIGO Clinical Practice Guideline for AKI.[15] This concept combines the definitions issued by the international Acute Kidney Injury Network[16,17] and the RIFLE classification developed by the Acute Dialysis Quality Initiative group.[24] Only 3 other previous studies used the AKI concept to define nephrotoxicity.[20,21,24] Changes in the definition of nephrotoxicity over time may lead to spurious variations in incidence. The AKI concept has been reported to improve the early detection of nephrotoxicity and the management of patients treated with vancomycin.[18] A single previous study evaluated the nephrotoxicity of continuous intravenous vancomycin in ICU patients using AKI criteria.[25] Of 129 retrospectively evaluated patients, 38 (29.5%) experienced AKI, in keeping with our findings. AKI developed in only 8% of patients whose serum vancomycin levels remained within the 15 to 25 mg/L range and was associated with longer vancomycin exposures (14.9 vs 9.2 days in patients without AKI).[25] Treatment duration was not associated with AKI in our study. This apparent discrepancy may be related to the overall shorter treatment duration in our study (median, 7 days).[4–12]
Several characteristics of our study are worth noting. The continuous vancomycin delivery and high target serum level are original features. In most of the available studies, vancomycin was given as twice-daily injections, with dosage adjustments based on trough serum vancomycin levels. Many studies support high-dose continuous vancomycin administration to produce a serum plateau of 20 to 30 mg/L in ICU patients.[5–7,26–28] In previous studies, the independent variable was either the mean of all serum vancomycin levels available for each patient[9,10,12] or the serum vancomycin levels during the 1st few treatment days.[8,11,19,29] We looked at individual assays to identify the peak serum level in each patient. This parameter is useful in clinical practice to adjust the dosage to the target. An unexpected finding from our study is the lower frequency of AKI in patients given concomitant nephrotoxic therapy including aminoglycosides. A possible explanation is selection bias due to the retrospective study design, with preferential aminoglycoside use in patients at lower risk of AKI. In contrast to early studies,[30,31] recent work found little evidence of heightened toxicity with concomitant aminoglycoside and vancomycin therapy. However, concomitant aminoglycoside therapy was an independent risk factor for nephrotoxicity in a multicenter retrospective study of patients with pneumonia.[19]
There are several limitations to our study. First, the observational single-center design and small sample size may have failed to detect confounding by unmeasured factors (such as age, severity scores, and emergency admission) and may limit the general applicability of our findings. Given the small number of patients with AKI stages 2 and 3 (n = 14), a statistical analysis of AKI determinants in the patient subgroup with severe impairment was not feasible. Second, the oliguria criterion in the KDIGO Clinical Practice Guideline for AKI was not used in our study.[15] ICU patients frequently exhibit multifactorial functional or organic renal dysfunction with preserved urine output. In addition, urine output is often noninterpretable because of the concomitant use of loop diuretics (82% of our patients). We therefore elected not to consider oliguria. Third, the retrospective design is an important limitation to our work. Only prospective randomized studies could show whether vancomycin exposure is a cause of AKI. Conceivably, the higher serum vancomycin levels in the group with AKI may be ascribable to decreased drug clearance due to renal dysfunction, since vancomycin is excreted almost entirely by the kidneys.[9,10,13,22] Vancomycin dosages differed significantly between the groups with and without AKI in our study. Given our finding that baseline renal function was similar in the group with and without AKI, the significantly lower overall median vancomycin dosage in the AKI group is probably ascribable to dosage reduction in response to high serum vancomycin levels.
5. Conclusions
AKI developed in 29% of high-dose continuous intravenous vancomycin treatments targeting serum levels of 20 to 30 mg/L in ICU patients with severe acute illnesses. Peak serum vancomycin level ≥40 mg/L, higher cumulative number of organ failures, and cirrhosis of the liver were independently associated with AKI. A higher number of concomitant nephrotoxic agents were associated with a lower risk of AKI. In ICU patients exhibiting multiple risk factors for AKI and receiving vancomycin with high serum level targets, assessing the risk of AKI is crucial to avoid additional injury, and close monitoring by daily serum vancomycin assays is in order. Prospective randomized studies are needed to determine the serum vancomycin levels above which ICU patients are at risk for AKI and to determine whether vancomycin exposure is a cause of AKI.
Acknowledgments
The authors thank A Wolfe, MD (Issy-les-Moulineaux, France), for helping to prepare the article. The authors also thank the Centre Hospitalier de Versailles for editorial assistance.
Footnotes
Abbreviations: AKI = acute kidney injury, AKIN = Acute Kidney Injury Network, ICU = intensive care unit, KDIGO = Kidney Disease Improving Global Outcomes, RRT = renal replacement therapy, SAPS = Simplified Acute Physiology Score.
Funding/support: The study was funded by the French public funding agency Délégation à la Recherche Clinique et à l’Innovation, Versailles, France.
Authorship: GL, SL, and VC designed the study, analyzed the data, and drafted the manuscript. JPB helped to coordinate the study and draft the manuscript. FB, CP, and SL participated in designing the study. DG and SL helped to draft the manuscript. GL, SL, and ME performed the statistical analysis. All authors revised and approved the manuscript.
Ethics committee approval: Our local ethics committee (Comité de Protection des Personnes, Paris, Ile de France VI) approved this study (#220114) and waived the need for informed consent in compliance with French law on retrospective studies of anonymized data.
The authors have no conflicts of interest to disclose.
References
- [1].Levine DP. Vancomycin: a history. Clin Infect Dis Off Publ Infect Dis Soc Am 2006;42suppl 1:S5–12. [DOI] [PubMed] [Google Scholar]
- [2].Rybak MJ, Lomaestro BM, Rotschafer JC. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis Off Publ Infect Dis Soc Am 2009;49:325–7. [DOI] [PubMed] [Google Scholar]
- [3].Kullar R, Davis SL, Levine DP, et al. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 2011;52:975–81. [DOI] [PubMed] [Google Scholar]
- [4].Liu C, Bayer A, Cosgrove SE. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52:e18–55. [DOI] [PubMed] [Google Scholar]
- [5].Bingen E, Mariani-Kurkdjian P, Nebbad B. Optimal vancomycin serum level in Staphylococcus aureus infections? Médecine Mal Infect 2006;36:439–42. [DOI] [PubMed] [Google Scholar]
- [6].James JK, Palmer SM, Levine DP, et al. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996;40:696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wysocki M, Delatour F, Faurisson F. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001;45:2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bosso JA, Nappi J, Rudisill C. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother 2011;55:5475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hidayat LK, Hsu DI, Quist R, et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006;166:2138–44. [DOI] [PubMed] [Google Scholar]
- [10].Jeffres MN, Isakow W, Doherty JA, et al. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther 2007;29:1107–15. [DOI] [PubMed] [Google Scholar]
- [11].Lodise TP, Patel N, Lomaestro BM, et al. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis Off Publ Infect Dis Soc Am 2009;49:507–14. [DOI] [PubMed] [Google Scholar]
- [12].Lodise TP, Lomaestro B, Graves J, et al. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008;52:1330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2013;57:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- [15].Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter 2012;2Suppl:1–38. [Google Scholar]
- [16].Bagshaw SM, George C, Bellomo R, et al. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008;23:1569–74. [DOI] [PubMed] [Google Scholar]
- [17].Mehta RL, Kellum JA, Shah SV. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Minejima E, Choi J, Beringer P, et al. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother 2011;55:3278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cano EL, Haque NZ, Welch VL. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther 2012;34:149–57. [DOI] [PubMed] [Google Scholar]
- [20].Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. [DOI] [PubMed] [Google Scholar]
- [21].Pritchard L, Baker C, Leggett J, et al. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med 2010;123:1143–9. [DOI] [PubMed] [Google Scholar]
- [22].Wong-Beringer A, Joo J, Tse E, et al. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents 2011;37:95–101. [DOI] [PubMed] [Google Scholar]
- [23].Hanrahan TP, Harlow G, Hutchinson J, et al. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med 2014;42:2527–36. [DOI] [PubMed] [Google Scholar]
- [24].Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care Lond Engl 2004;8:R204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Spapen HD, Doorn KJ van, Diltoer M. Retrospective evaluation of possible renal toxicity associated with continuous infusion of vancomycin in critically ill patients. Ann Intensive Care 2011;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jeurissen A, Sluyts I, Rutsaert R. A higher dose of vancomycin in continuous infusion is needed in critically ill patients. Int J Antimicrob Agents 2011;37:75–7. [DOI] [PubMed] [Google Scholar]
- [27].Roberts JA, Taccone FS, Udy AA, et al. Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob Agents Chemother 2011;55:2704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rello J, Sole-Violan J, Sa-Borges M. Pneumonia caused by oxacillin-resistant Staphylococcus aureus treated with glycopeptides. Crit Care Med 2005;33:1983–7. [DOI] [PubMed] [Google Scholar]
- [29].Chung J, Oh JM, Cho EM. Optimal dose of vancomycin for treating methicillin-resistant Staphylococcus aureus pneumonia in critically ill patients. Anaesth Intensive Care 2011;39:1030–7. [DOI] [PubMed] [Google Scholar]
- [30].Rybak MJ, Albrecht LM, Boike SC, et al. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother 1990;25:679–87. [DOI] [PubMed] [Google Scholar]
- [31].Wood MJ. Comparative safety of teicoplanin and vancomycin. J Chemother Florence Italy 2000;12suppl 5:21–5. [DOI] [PubMed] [Google Scholar]


