Abstract
Little is known on the relationship between herpes zoster and Parkinson's disease in older people. This study aimed to explore whether herpes zoster could be associated with Parkinson's disease in older people in Taiwan.
We conducted a retrospective cohort study using the claim data of the Taiwan National Health Insurance Program. There were 10,296 subjects aged 65 years and older with newly diagnosed herpes zoster as the herpes zoster group and 39,405 randomly selected subjects aged 65 years and older without a diagnosis of herpes zoster as the nonherpes zoster group from 1998 to 2010. Both groups were followed up until subjects received a diagnosis of Parkinson's disease. This follow-up design would explore whether subjects with herpes zoster were at an increased risk of Parkinson's disease. Relative risks were estimated by adjusted hazard ratio (HR) and 95% confidence interval (CI) using the multivariable Cox proportional hazards regression model.
The incidence of Parkinson's disease was higher in the herpes zoster group than that in the nonherpes zoster group (4.86 vs 4.00 per 1000 person-years, 95% CI 1.14, 1.29). After adjustment for confounding factors, the multivariable Cox proportional hazards regression model revealed that the adjusted HR of Parkinson's disease was 1.17 for the herpes zoster group (95% CI 1.10, 1.25), compared with the nonherpes zoster group.
Older people with herpes zoster confer a slightly increased hazard of developing Parkinson's disease when compared to those without herpes zoster. We think that herpes zoster correlates with increased risk of Parkinson's disease in older people. When older people with herpes zoster seek help, clinicians should pay more attention to the development of the cardinal symptoms of Parkinson's disease.
Keywords: herpes zoster, nonmotor, older people, Parkinson's disease
1. Introduction
Parkinson's disease is one of the most common neurodegenerative diseases in older people. As well established, the clinical diagnosis of Parkinson's disease is always based on patients presenting with the traditional motor symptoms including bradykinesia, rigidity, tremor, and postural instability.[1,2] In addition to motor symptoms, recently nonmotor symptoms have been regarded as cardinal components of Parkinson's disease. These nonmotor symptoms may include olfactory dysfunction, sleep disorder, constipation, depression, irritable bowel syndrome, hearing loss, cataract, and others. Nonmotor symptoms may occur during the course of the disease and also may predate the development of motor symptoms.[3–9] Yet, herpes zoster remains unmentioned.
Herpes zoster always results from reactivation of latent varicella-zoster virus as a result of the decline of human cell-mediated immunity.[10,11] Increasing age has been known as a risk factor for herpes zoster.[10,11] Herpes zoster not only could cause functional decline, but also could increase death risk in older people.[12,13] In a mouse model of Parkinson's disease, impairment of the central dopaminergic pathways can result in changes of some immune functions.[14] Increasing evidence has indicated that neuroinflammation and immunological changes play the significant roles in contributing to neuron death in Parkinson's disease, in which microglia activation and other immune cells at sites of neuronal injury is detected.[15–17]
In the light of aforementioned review, we make a theoretical hypothesis that there could be a link between herpes zoster and Parkinson's disease based on inflammation and immunological changes involved in both conditions. If herpes zoster is associated with Parkinson's disease in older people, clinicians may consider the possibility of Parkinson's disease when patients with herpes zoster concomitantly or gradually manifest with motor and/or nonmotor symptoms. This topic is not data mining because a clinical study by Ragozzino et al[18] has ever proposed such a potential link. However, no association could be detected between herpes zoster and Parkinson's disease. The authors explained that the limited power of the study could hide such an association.[18] Therefore, using the database of the Taiwan National Health Insurance Program, we conducted a retrospective cohort study to understand this hypothesis.
2. Methods
2.1. Design and data source
Taiwan is an independent country with more than 23 million people. We conducted a retrospective cohort study using data retrieved from claim information of the Taiwan National Health Insurance Program. This insurance program implemented in March 1995 and has covered nearly 99% of 23 million people living in Taiwan.[19] shortly speaking, the database contained information on patient encrypted identification number, sex, birth date, utilization of medical services, prescription International Classification of Diseases 9th Revision Clinical Modification drugs, and disease classification codes, and so on. Diseases were coded according to the (ICD-9 code), 2001 edition. The details of the program have been well written in previous studies.[20–24] The study was approved by the Institutional Review Board of China Medical University and Hospital in Taiwan (CMUH-104-REC2-115).
2.2. Criteria and definition
The herpes zoster group consisted of subjects aged 65 years and older with newly diagnosed herpes zoster (ICD-9 code 053) from 1998 to 2010. The date of diagnosing herpes zoster was defined as the index date. The nonherpes zoster group consisted of randomly selected subjects aged 65 years and older who had never been clinically diagnosed with herpes zoster during the study period from 1998 to 2010 (herpes zoster group: nonherpes zoster group = 1:4). Both groups were matched with sex, age (every 5 years), comorbidities, and index year of diagnosing herpes zoster. Both groups were followed up until subjects received a new diagnosis of Parkinson's disease (ICD-9 code 332.0) or until December 31, 2011.
To diminish the biased analysis, subjects who had a previous diagnosis of Parkinson's disease or secondary Parkinsonism (ICD-9 code 332.1) before the index date were excluded from the study.
2.3. Comorbidities assessment
Comorbidities potentially related to Parkinson's disease were included as follows: alcohol-related diseases (ICD-9 codes 291, 303, 305.00, 305.01, 305.02, 305.03, 571.0-571.3, 790.3, and V11.3), cardiovascular diseases (ICD-9 codes 410–414, 428, 430–438, and 440–448), chronic kidney diseases (ICD-9 codes 585–586 and 588.8–588.9), chronic obstructive pulmonary diseases (ICD-9 codes 491, 492, 493, and 496), dementia (ICD-9 codes 290.0, 290.1, 290.2, 290.3, 290.4, 294.1, and 331.0), depression (ICD-9 codes 296.2, 296.3, 300.4, and 311), diabetes mellitus (ICD-9 code 250), head injury (ICD-9 codes 850-854 and 959.01), hyperlipidemia (ICD-9 codes 272.0, 272.1, 272.2, 272.3, and 272.4), and hypertension (ICD-9 codes 401–405). All comorbidities were diagnosed before the index date.
Some subjects could be mistakenly diagnosed, mistakenly coded by accident, or mistakenly coded by similar clinical features but without confirmed diagnosis. To ensure the validity of diagnosis, only subjects having at least 3 consensus same diagnoses in the ambulatory care and/or hospitalization records during the study period could be included. Principal diagnosis and secondary diagnosis were applied equally. Therefore, herpes zoster, Parkinson's disease, and comorbidities were documented for 3 or more records in the ambulatory care and/or hospitalization.
2.4. Statistical analysis
The differences of sex, age, and comorbidities were compared between the herpes zoster group and the nonherpes zoster group using the chi-square test for categorical variables and the t-test for continuous variables. The incidence of Parkinson's disease was estimated as the event number of Parkinson's disease found during the follow-up period, divided by the total follow-up person-years for each group. At first, all variables were included in the univariable Cox proportional hazards regression model. Those found to be significant in the univariable model were further included in the multivariable Cox proportional hazards regression model to estimate the hazard ratio (HR) and 95% confidence interval (CI) of Parkinson's disease associated with herpes zoster and comorbidities. The proportional hazard model assumption was examined by using a test of scaled Schoenfeld residuals. In the model evaluating the Parkinson's disease risk throughout overall follow-up period, results of the test revealed a significant relationship between Schoenfeld residuals for herpes zoster and follow-up time, suggesting that the proportionality assumption was violated (P value = 0.001). In the subsequent analyses, we stratified the follow-up period to deal with the violation of proportional hazard assumption. The statistical significance level was set at 2-sided probability value of <0.05. All analyses were used by SAS software version 9.2 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Baseline characteristics of the study population
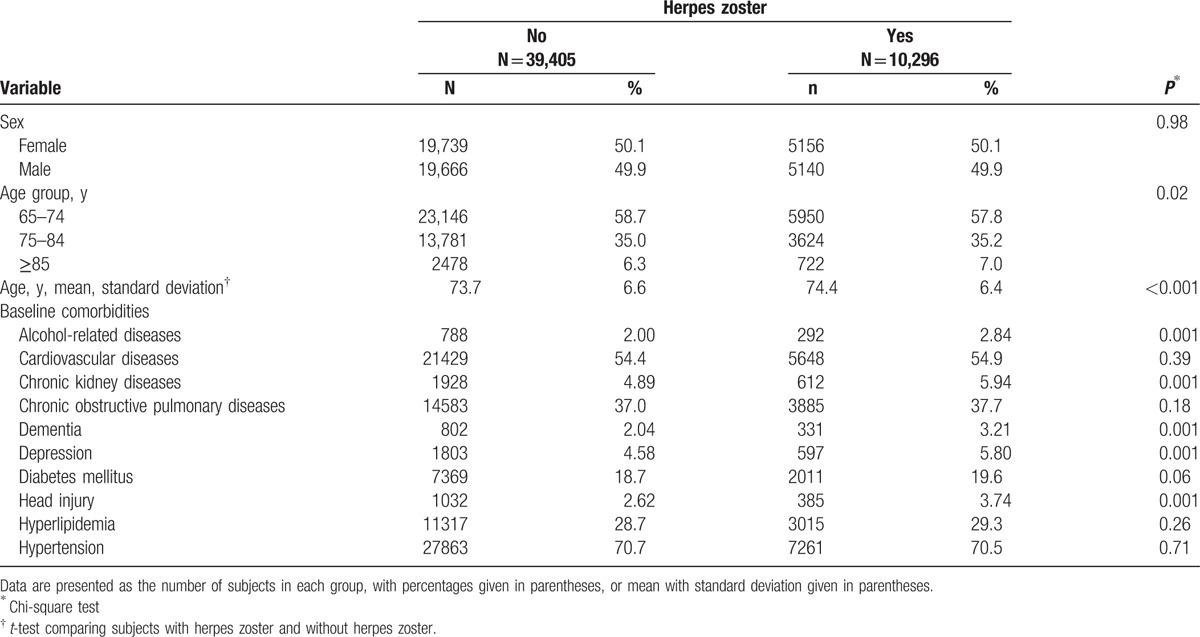
Table 1 reveals the baseline information of the study population. There were 10,296 subjects in the herpes zoster group and 39,405 subjects in the nonherpes zoster group, with a similar distribution of sex. The mean ages (standard deviation) were 74.4 (6.4) years in the herpes zoster group and 73.7 (6.6) years in the nonherpes zoster group (t-test, P < 0.001). The herpes zoster group was more likely to have a history of alcohol-related diseases, chronic kidney diseases, dementia, depression, and head injury (chi-square test, P = 0.001).
Table 1.
Baseline characteristics between the herpes zoster group and the nonherpes zoster group.
3.2. Incidence density of Parkinson's disease stratified by sex, age, and follow-up period
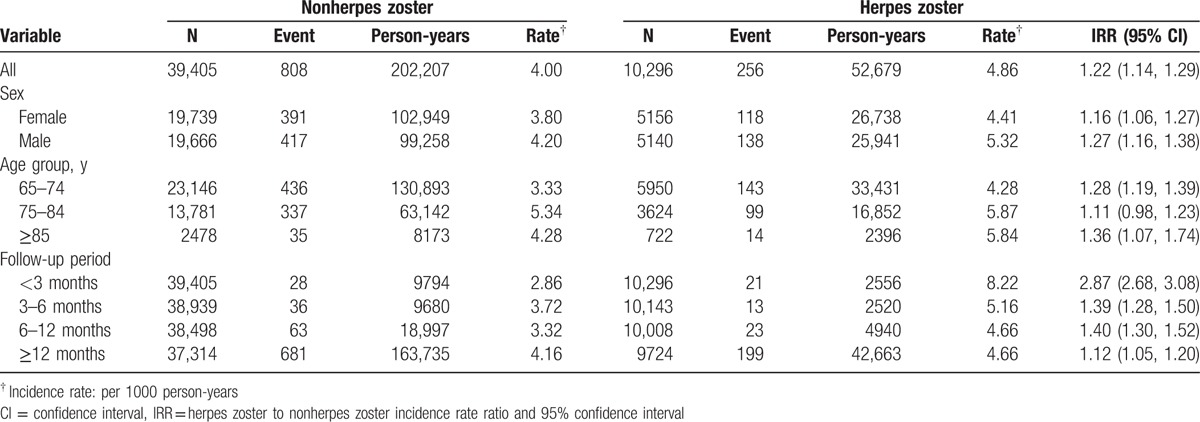
At the end of the study period, there were 256 events of Parkinson's disease and 52,679 person-years in the herpes zoster group. There were 808 events of Parkinson's disease and 202,207 person-years in the nonherpes zoster group. Table 2 reveals that a higher incidence of Parkinson's disease in the herpes zoster group than that in the nonherpes zoster group (4.86 vs 4.00 per 1000 person-years, incidence rate ratio 1.22, 95% CI 1.14, 1.29). The incidence rates of Parkinson's disease, as stratified by sex, age, and follow-up period, were all higher in the herpes zoster group than those in the nonherpes zoster group. The incidence rate of Parkinson's disease decreased with the follow-up period in the herpes zoster group, with the highest in the first 3 months (8.22 per 1000 person-years). The incidence rate ratio of Parkinson's disease was higher in the first 3 months (incidence rate ratio 2.87, 95% CI 2.68, 3.08). The risk of Parkinson's disease persisted for 12 months and longer after diagnosing herpes zoster (incidence rate ratio 1.12, 95% CI 1.05, 1.20).
Table 2.
Incidence density of Parkinson's disease associated with herpes zoster stratified by sex, age, and follow-up period.
3.3. Hazard ratio of Parkinson's disease associated with herpes zoster and comorbidities
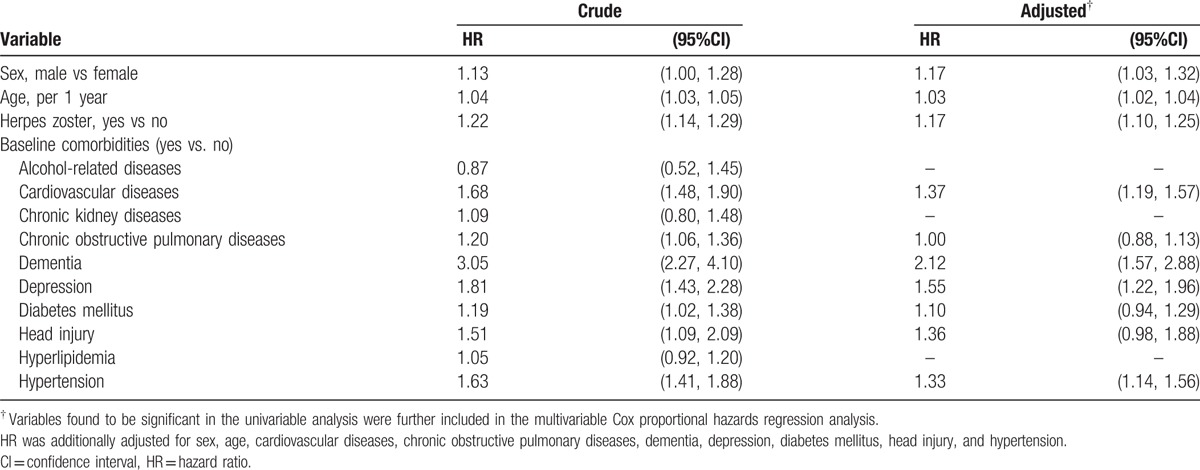
Table 3 reveals the HR of Parkinson's disease associated with herpes zoster and comorbidities. After adjustment for confounding factors, the multivariable Cox proportional hazards regression model revealed that the adjusted HR of Parkinson's disease was 1.17 for the herpes zoster group (95% CI 1.10, 1.25), compared with the nonherpes zoster group. In addition, male (adjusted HR 1.17, 95% 1.03, 1.32), age (per 1 year, adjusted HR 1.03, 95% 1.02, 1.04), cardiovascular diseases (adjusted HR 1.37, 95% 1.19, 1.57), dementia (adjusted HR 2.12, 95% 1.57, 2.88), depression (adjusted HR 1.55, 95% 1.22, 1.96), and hypertension (adjusted HR 1.33, 95% CI 1.14, 1.56) were factors significantly associated with Parkinson's disease.
Table 3.
Cox model measured hazard ratio and 95% confidence interval of Parkinson's disease associated with herpes zoster and comorbidities.
4. Discussion
In this retrospective cohort study, we noticed that the incidence rate of Parkinson's disease in the herpes zoster group seems to be slightly higher than that in the nonherpes zoster group (4.86 vs 4.00 per 1000 person-years, Table 2). We also noticed that the incidence rate of Parkinson's disease was the highest during the first 3 months (8.22 per 1000 person-years, Table 2). After adjustment for confounding factors, we also noticed that patients with herpes zoster were associated with increased hazard of Parkinson's disease (adjusted HR 1.17, Table 3). Ragozzino et al[18] have ever proposed such a potential link, but no association could be detected between herpes zoster and Parkinson's disease. The authors explained that the limited power of the study could hide such an association. [18] To the best of our knowledge, this present study was the first cohort study using the population database to reveal such an association between herpes zoster and Parkinson's disease. To diminish the biased results, subjects who had a diagnosis of Parkinson's disease before the index date were excluded from the study. All study subjects were followed up until subjects received a new diagnosis of Parkinson's disease or until 2011. Therefore, we really examined the first reported diagnosis of Parkinson's disease for the study subjects. That is, patients with herpes zoster were included in the study before the confirmed diagnosis of Parkinson's disease. However, whether herpes zoster could be a nonmotor manifestation of Parkinson's disease in older people needs further research to confirm.
The pathogenetic basis between herpes zoster and Parkinson's disease cannot be explored in our observation. Although infection could be a risk factor for Parkinson's disease, such as herpes simplex,[25,26] no research has conclusively shown a link between herpes zoster and Parkinson's disease. Increasing evidence has indicated that neuroinflammation and immunological changes play the significant roles in contributing to neuron death in Parkinson's disease, in which microglia activation and other immune cells at sites of neuronal injury is detected.[15–17] During the natural course of Parkinson's disease, these cells can cause chronic inflammation and thereby lead to the progressive degeneration and death of dopaminergic neurons in the substantia nigra.[15–17] Finally, the resulting dopamine depletion reflects the pathological abnormalities and clinical manifestations of Parkinson's disease. In addition, the animal study has revealed that peripheral T lymphocytes and B lymphocytes could be decreased in rate model of Parkinson's disease.[27] Similarly, the human studies also have found that peripheral T lymphocytes and B lymphocytes could be decreased in patients with Parkinson's disease.[28–30] Therefore, we rationally hypothesize that peripheral T lymphocytes and B lymphocytes could be decreased during the natural course of Parkinson's disease. Due to the decline of cell-mediated immunity, herpes zoster might have a chance to develop later during the natural course of Parkinson's disease.
We noticed that patients with herpes zoster seemed to have more comorbidities, such as alcohol-related diseases, chronic kidney diseases, dementia, depression, and head injury (Table 1). Therefore, these patients could frequently seek medical care and consecutively they would have more chances to be diagnosed with Parkinson's disease. Clinically, not all patients acquiring herpes zoster would develop Parkinson's disease. It is unlikely that patients acquiring herpes zoster would be immediately diagnosed with Parkinson's disease. It needs to take time to make the right diagnosis. Based on neuroinflammation and immunological changes involved in both conditions mentioned on the above discussion, patients acquiring herpes zoster might concomitantly or gradually manifest with cardinal symptoms of Parkinson's disease during their follow-up period. Patients who concomitantly manifested with cardinal symptoms could be diagnosed with Parkinson's disease more quickly, maybe less than 3 months. Patients who gradually manifested with cardinal symptoms could be diagnosed with Parkinson's disease more later, maybe longer than 3 months. Therefore, these accompanied cardinal symptoms can alert clinicians about the possibility of Parkinson's disease. Therefore, we suggest that clinicians should take a thorough history on motor or nonmotor symptoms when patients presented with herpes zoster, particularly in the first 3 months after diagnosing herpes zoster. Thus, Parkinson's disease can be detected more earlier.
A number of limitations should be discussed. First, the lag period between the onset of cardinal symptoms of Parkinson's disease and the date of confirmed diagnosis of this disease really exists. Therefore, whether herpes zoster developed before or after the onset of cardinal symptoms of Parkinson's disease cannot be definitely determined in this observational study. Second, although the diagnoses of herpes zoster, Parkinson's disease, and comorbidities were based on ICD-9 codes, the diagnosis accuracy based on ICD-9 codes has been thoroughly examined in previous studies.[31–37] To ensure the validity of diagnosis, only patients having at least 3 consensus same diagnoses in the ambulatory care and/or hospitalization records during the follow-up period could be included in the study. Therefore, herpes zoster, Parkinson's disease, and comorbidities were documented for 3 or more records in the ambulatory care and/or hospitalization. Third, some risk factors for Parkinson's disease such as alcoholism, smoking, exposure to pesticides, or use of well water, were not recorded in this database due to the inherent limitation. However, alcohol-related diseases were used instead of alcoholism and chronic obstructive pulmonary diseases were used instead of smoking. These points have been described in previous studies.[37,38] Fourth, herpes zoster and Parkinson's disease are 2 common diseases found in older people. The purpose of the study was to explore this issue in older people. Younger population was not included. We noticed that age was significantly associated with Parkinson's disease (per 1 year, adjusted HR 1.03, Table 3). In addition, increasing age has been known as a risk factor for herpes zoster.[10,11] The incidence rate of Parkinson's disease in the herpes zoster group is only slightly higher than the incidence in the nonherpes zoster group. This slight difference may be due to confounders such as increasing age and decline of cell immunity which are both determinants of these diseases. Fifth, previous studies have indicated a high rate of misdiagnosis in Parkinson's disease if performed by inexperienced/nonexpert clinicians or without utility of gold-standard clinical diagnostic criteria.[39,40] In view of high quality of medical care in Taiwan, the diagnostic accuracy of Parkinson's disease can be made sure. Sixth, the analysis of comorbid conditions only spanned the 13 years of the study. Therefore, it should be emphasized that the registry search for comorbid conditions was limited to this time-frame. Seventh, As well known, once people are infected with varicella-zoster virus, this virus remains dormant in dorsal-root ganglia after an episode of chickenpox. These people become carriers of varicella-zoster virus. Herpes zoster always results from reactivation of latent varicella-zoster virus as a result of the decline of human cell-mediated immunity. [10,11] Because herpes zoster is clinically diagnosed by skin findings, if no development of skin lesions, herpes zoster cannot be diagnosed. To diminish the biased results, the control group consisted of 39,405 randomly selected subjects aged 65 years and older who had never been clinically diagnosed with herpes zoster during the study period.
There is some strength in this study. This study used a well-organized database to provide complete information. The sample size is adequate to increase its statistical power. The statistical methodology is appropriate. The findings are very intriguing. The literature review is complete. From a clinical point of view, it provides a clear message that needs to be communicated to a wider audience.
In conclusion, older people with herpes zoster confer a slightly increased hazard of developing Parkinson's disease when compared to those without herpes zoster. We think that herpes zoster correlates with increased risk of Parkinson's disease in older people. When older people with herpes zoster seek help, clinicians should pay more attention to the development of cardinal symptoms of Parkinson's disease, particularly during the first 3 months after diagnosing herpes zoster.
Footnotes
Abbreviation: ICD-9 code = International Classification of Diseases 9th Revision Clinical Modification.
S-WL and C-HL contributed equally to this study.
Authorship: S-WL planned and conducted this study. He substantially contributed to the conception of the article, initiated the draft of the article, and critically revised the article.
C-HL, H-FL, C-CL, and C-LL conducted the data analysis and critically revised the article.
K-FL planned and conducted this study. He participated in the data interpretation and also critically revised the article.
Funding: This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), National Research Program for Biopharmaceuticals (NRPB) Stroke Clinical Trial Consortium (MOST 105-2325-B-039 -003), Tseng-Lien Lin Foundation in Taichung in Taiwan, Taiwan Brain Disease Foundation in Taipei in Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds in Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Ziemssen T, Reichmann H. Non-motor dysfunction in Parkinson's disease. Parkinsonism Relat Disord 2007;13:323–32. [DOI] [PubMed] [Google Scholar]
- [2].Maass A, Reichmann H. Sleep and non-motor symptoms in Parkinson's disease. J Neural Transm (Vienna) 2013;120:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chaudhuri KR, Naidu Y. Early Parkinson's disease and non-motor issues. J Neurol 2008;255suppl 5:33–8. [DOI] [PubMed] [Google Scholar]
- [4].Wolters E. Non-motor extranigral signs and symptoms in Parkinson's disease. Parkinsonism Relat Disord 2009;15suppl 3:S6–12. [DOI] [PubMed] [Google Scholar]
- [5].Reichmann H, Schneider C, Lohle M. Non-motor features of Parkinson's disease: depression and dementia. Parkinsonism Relat Disord 2009;15suppl 3:S87–92. [DOI] [PubMed] [Google Scholar]
- [6].Lohle M, Storch A, Reichmann H. Beyond tremor and rigidity: non-motor features of Parkinson's disease. J Neural Transm (Vienna) 2009;116:1483–92. [DOI] [PubMed] [Google Scholar]
- [7].Lai SW, Liao KF, Lin CL, et al. Irritable bowel syndrome correlates with increased risk of Parkinson's disease in Taiwan. Eur J Epidemiol 2014;29:57–62. [DOI] [PubMed] [Google Scholar]
- [8].Lai SW, Liao KF, Lin CL, et al. Hearing loss may be a non-motor feature of Parkinson's disease in older people in Taiwan. Eur J Neurol 2014;21:752–7. [DOI] [PubMed] [Google Scholar]
- [9].Lai SW, Lin CL, Liao KF, et al. Increased risk of Parkinson's disease in cataract patients: a population-based cohort study. Parkinsonism Relat Disord 2015;21:68–71. [DOI] [PubMed] [Google Scholar]
- [10].Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis 2004;4:26–33. [DOI] [PubMed] [Google Scholar]
- [11].Weinberg JM. Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol 2007;57:S130–5. [DOI] [PubMed] [Google Scholar]
- [12].DeLaGarza VW, Arbogast JG, Podolinski CF, et al. Reactivation of herpes zoster (shingles) infection associated with an increased risk of death in immunocompetent older persons. W V Med J 2008;104:22–4. [PubMed] [Google Scholar]
- [13].Attal N, Deback C, Gavazzi G, et al. Functional decline and herpes zoster in older people: an interplay of multiple factors. Aging Clin Exp Res 2015;27:757–65. [DOI] [PubMed] [Google Scholar]
- [14].Bieganowska K, Czlonkowska A, Bidzinski A, et al. Immunological changes in the MPTP-induced Parkinson's disease mouse model. J Neuroimmunol 1993;42:33–7. [DOI] [PubMed] [Google Scholar]
- [15].Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson's disease. J Neuroimmune Pharmacol 2009;4:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson's disease and a prime target for therapy. J Neural Transm 2010;117:971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Joers V, Tansey MG, Mulas G, et al. Microglial phenotypes in Parkinson's disease and animal models of the disease. Prog Neurobiol 2016;pii: S0301-0082(15)30053-8. (doi: 10.1016/j.pneurobio.2016.04.006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ragozzino M, Kurland L, Rajput A. Investigation of the association between Herpes zoster and Parkinson's disease. Neuroepidemiology 1983;2:89–92. [Google Scholar]
- [19].National Health Insurance Research Database. Taiwan. Available at: http://nhird.nhri.org.tw/en/index.html Accessed October 1, 2016, English version. [Google Scholar]
- [20].Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine 2010;89:295–9. [DOI] [PubMed] [Google Scholar]
- [21].Chen HY, Lai SW, Muo CH, et al. Ethambutol-induced optic neuropathy: a nationwide population-based study from Taiwan. Br J Ophthalmol 2012;96:1368–71. [DOI] [PubMed] [Google Scholar]
- [22].Lai HC, Chang SN, Lin CC, et al. Does diabetes mellitus with or without gallstones increase the risk of gallbladder cancer? Results from a population-based cohort study. J Gastroenterol 2013;48:856–65. [DOI] [PubMed] [Google Scholar]
- [23].Hung SC, Lai SW, Tsai PY, et al. Synergistic interaction of benign prostatic hyperplasia and prostatitis on prostate cancer risk. Br J Cancer 2013;108:1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang SP, Muo CH, Wang IK, et al. Risk of type 2 diabetes mellitus in female breast cancer patients treated with morphine: a retrospective population-based time-dependent cohort study. Diabetes Res Clin Pract 2015;110:285–90. [DOI] [PubMed] [Google Scholar]
- [25].Howard JS., 3rd Tic douloureux, Parkinson's disease and the herpes connection. Integr Physiol Behav Sci 1997;32:257–64. [DOI] [PubMed] [Google Scholar]
- [26].Vlajinac H, Dzoljic E, Maksimovic J, et al. Infections as a risk factor for Parkinson's disease: a case-control study. Int J Neurosci 2013;123:329–32. [DOI] [PubMed] [Google Scholar]
- [27].Bas J, Calopa M, Mestre M, et al. Lymphocyte populations in Parkinson's disease and in rat models of parkinsonism. J Neuroimmunol 2001;113:146–52. [DOI] [PubMed] [Google Scholar]
- [28].Baba Y, Kuroiwa A, Uitti RJ, et al. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord 2005;11:493–8. [DOI] [PubMed] [Google Scholar]
- [29].Niwa F, Kuriyama N, Nakagawa M, et al. Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson's disease. Geriatr Gerontol Int 2012;12:102–7. [DOI] [PubMed] [Google Scholar]
- [30].Stevens CH, Rowe D, Morel-Kopp MC, et al. Reduced T helper and B lymphocytes in Parkinson's disease. J Neuroimmunol 2012;252:95–9. [DOI] [PubMed] [Google Scholar]
- [31].Lai SW, Su LT, Lin CH, et al. Polypharmacy increases the risk of Parkinson's disease in older people in Taiwan: a population-based study. Psychogeriatrics 2011;11:150–6. [DOI] [PubMed] [Google Scholar]
- [32].Lai SW, Liao KF, Lin CL, et al. Association between head injury and Parkinson's disease: an observation in Taiwan. Geriatr Gerontol Int 2013;13:513–4. [DOI] [PubMed] [Google Scholar]
- [33].Lai S-W, Liu J-C, Tseng C-H, et al. No association between chronic osteomyelitis and Parkinson's disease in older people in Taiwan. J Alzheimers Dis Parkinsonism 2013;3:2161–460. 1000112. [Google Scholar]
- [34].Lai S-W, Lin C-H, Lin C-L, et al. Gout and Parkinson's disease in older people: an observation in Taiwan. Int J Gerontol 2014;8:166–7. [Google Scholar]
- [35].Liao K-F, Lin C-L, Lai S-W, et al. Parkinson's disease and risk of pancreatic cancer: a population-based case-control study in Taiwan. Neurol Asia 2015;20:251–5. [Google Scholar]
- [36].Lai S-W, Lin C-L, Liao K-F, et al. Parkinson's disease and hepatocellular carcinoma in older people: a population-based case-control study in Taiwan. Int Med J 2015;22:313–4. [Google Scholar]
- [37].Liao KF, Lin CL, Lai SW, et al. Sitagliptin use and risk of acute pancreatitis in type 2 diabetes mellitus: a population-based case-control study in Taiwan. Eur J Int Med 2016;27:76–9. [DOI] [PubMed] [Google Scholar]
- [38].Lai SW, Lin CL, Liao KF. Digoxin use may increase the relative risk of acute pancreatitis: a population-based case-control study in Taiwan. Int J Cardiol 2015;181:235–8. [DOI] [PubMed] [Google Scholar]
- [39].Newman EJ, Breen K, Patterson J, et al. Accuracy of Parkinson's disease diagnosis in 610 general practice patients in the West of Scotland. Mov Disord 2009;24:2379–85. [DOI] [PubMed] [Google Scholar]
- [40].Bajaj NP, Gontu V, Birchall J, et al. Accuracy of clinical diagnosis in tremulous parkinsonian patients: a blinded video study. J Neurol Neurosurg Psychiatry 2010;81:1223–8. [DOI] [PubMed] [Google Scholar]


