Supplemental Digital Content is available in the text
Keywords: cost-effectiveness, drug adherence, HIV, implementation science, mHealth, mobile phone, SMS
Abstract
Background:
A surge in mobile phone availability has fueled low cost short messaging service (SMS) adherence interventions. Multiple systematic reviews have concluded that some SMS-based interventions are effective at improving antiretroviral therapy (ART) adherence, and they are hypothesized to improve retention in care. The objective of this study was to evaluate the cost-effectiveness of SMS-based adherence interventions and explore the added value of retention benefits.
Methods:
We evaluated the cost-effectiveness of weekly SMS interventions compared to standard care among HIV+ individuals initiating ART for the first time in Kenya. We used an individual level micro-simulation model populated with data from two SMS-intervention trials, an East-African HIV+ cohort and published literature. We estimated average quality adjusted life years (QALY) and lifetime HIV-related costs from a healthcare perspective. We explored a wide range of scenarios and assumptions in one-way and multivariate sensitivity analyses.
Results:
We found that SMS-based adherence interventions were cost-effective by WHO standards, with an incremental cost-effectiveness ratio (ICER) of $1,037/QALY. In the secondary analysis, potential retention benefits improved the cost-effectiveness of SMS intervention (ICER = $864/QALY). In multivariate sensitivity analyses, the interventions remained cost-effective in most analyses, but the ICER was highly sensitive to intervention costs, effectiveness and average cohort CD4 count at ART initiation. SMS interventions remained cost-effective in a test and treat scenario where individuals were assumed to initiate ART upon HIV detection.
Conclusions:
Effective SMS interventions would likely increase the efficiency of ART programs by improving HIV treatment outcomes at relatively low costs, and they could facilitate achievement of the UNAIDS goal of 90% viral suppression among those on ART by 2020.
1. Introduction
Mobile phones are a viable technology to support healthcare delivery because of their widespread global availability.[1] The global technology boom has fueled an emergence of mobile health applications delivered through text-messages, also known as short messaging service (SMS), which are now the most widely used communication technology worldwide. Several SMS-based drug adherence interventions have randomized controlled trial (RCT) evidence of effectiveness, yet have not been implemented at scale, which represents a lost opportunity for global health.[2] A promising application of such interventions is to enhance human immunodeficiency virus (HIV) treatment programs. In 2012, HIV affected 35.3 million people worldwide, including 1.6 million people in Kenya.[3] Life-saving antiretroviral therapy (ART) has become increasingly available and has improved HIV outcomes.
The World Health Organization (WHO) has recently announced that 17 million people world-wide were on ART at the end of 2015, and they are advocating for expanded testing and treatment strategies whereby everyone who tests positive for HIV is immediately eligible for treatment.[4] Additionally, the UNAIDS 90-90-90 goals aim to have 90% of global HIV cases virally suppressed by 2020.[5] Adherence above 90% to 95% is needed to achieve viral suppression for older regimens of ART that are used in many parts of the world; however, adherence has been shown to be lower in several populations.[6,7] Retention rates are also poor, as many individuals prematurely drop out of care. In African ART programs, average patient retention 3 years after ART initiation is estimated to be 65%.[7] The major consequence of both nonadherence and dropout from care is reduced viral suppression, which can accelerate progression to acquired immune deficiency syndrome (AIDS) as well as increase HIV transmission.
Systematic reviews of all interventions that target ART nonadherence suggested that SMS interventions have one of the strongest levels of supportive evidence.[6,8] Independently conducted systematic reviews of RCTs on SMS-based adherence interventions concluded that weekly delivered reminders and SMS-based interactive patient engagement improved adherence to ART (RR 1.28).[8–10] Further, 2-way SMS interventions are hypothesized to improve 1 year retention in care and showed a statistically nonsignificant increase in 1 trial (RR 1.69, P = 0.094).[11] The combined evidence supporting the use of SMS interventions led to the World Health Organization (WHO) recommending SMS to promote adherence in their consolidated guidelines on the use of ART.[12]
Despite the compelling evidence, effective SMS adherence interventions have not been implemented to sufficient scale to assist in reaching global HIV control targets. Previous studies have used computer simulation models to determine the cost-effectiveness of ART expansion.[3,13,14] However, there have been no cost-effectiveness evaluations describing the incremental value of SMS-based adherence interventions, so it is unclear how investment in them would compare to expansion of ART or to other interventions.[6,15] Thus, the objective of this study was to examine the cost-effectiveness of a weekly SMS-based adherence intervention compared to usual care in people living with HIV/AIDS initiating ART in Kenya.
2. Methods
2.1. Definitions
We define adherence as the extent to which individuals’ take daily doses of ART as prescribed and use a threshold of 90% to differentiate “highly adherent” from “sub-optimally adherent” individuals. We define adherence under standard care (ASC) as the proportion of individuals who are highly adherent under the standard of care in Kenya, which includes 1 or 2 adherence counseling sessions at ART initiation.[11] Also under standard care, peer-support, and participation in support groups were suggested, but not mandated.[11] We define retention-in-care as consistent prescription pick-up of ART, reporting for regular care as prescribed and regular CD4 testing. Dropout refers to an individual who has disengaged from care and no longer receiving regular care or medication refills.
2.2. Model structure
The target population of this analysis was Kenyan people living with HIV/AIDS initiating ART who own or have access to mobile phones. We revised a previously developed simulation model of HIV progression to estimate the long-term health and economic impacts of weekly short message service (SMS) adherence interventions compared to standard care (Fig. 1).[16,17] Ethics approval was not required for this simulation-based study. The simulation modeled relationships between multiple inputs including CD4 count, viral load, and adherence to ART and used a daily time cycle. The simulation tracked daily events including adherence to 1st or 2nd line regimens of ART, CD4 testing, and symptomatic-AIDS events (eg, hospitalization). Daily adherence was based on an individuals’ propensity to adhere, and their adherence affected their rate of drug resistance development and rate of viral suppression or rebound. The simulation reflected progression and remission of HIV through severity states based on patient-level characteristics (eg, CD4 count), which were used to calculate cost and quality-adjusted life years (QALYs) as a function of time spent in these states. A simulated individual's viral load and CD4 count were modeled as continuous variables that varied over time as a function of active ART use and daily ART adherence. The relationship between adherence and viral load suppression is described in greater detail in the technical appendix.
Figure 1.
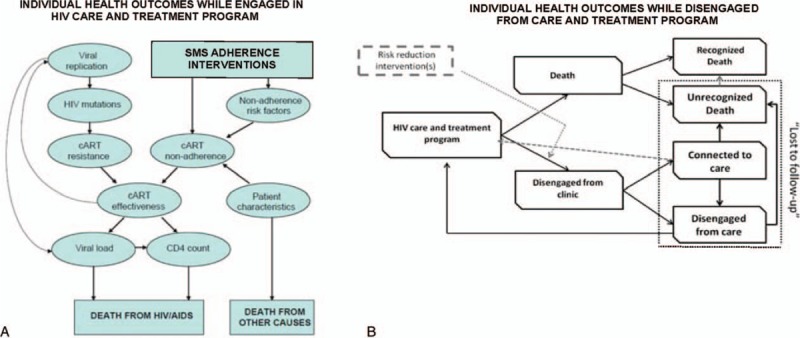
An influence diagram of the individual microsimulation model structure. (A) Adherence directly impacted the rate of viral suppression and HIV disease progression, which determined the prognosis for modeled individuals. SMS interventions improve individual adherence and thus impact health outcomes. (B) Individuals could disengage from care during the simulation with probabilities matching East African data. Once disengaged, simulated individuals could reengage with a health system or die out of care. SMS interventions were simulated to reduce the probability of disengagement. HIV = human immunodeficiency virus, SMS = short messaging service.
Disengagement from care assumed individuals were off drug therapy and did not attend regular appointments. Once disengaged, CD4 declined based on individual characteristics and on the natural history of disease.[18] The CD4 count continued to decline until a simulated individual either returned to care or died. The probability of returning to care or death was a function of an individual's CD4 count, and the probabilities increased as CD4 count declined. Individuals were at risk of dropout and return to care based on rates observed in a large East Africa cohort.[19] The potential for SMS intervention to reduce dropout was explored in secondary analysis by reducing the rate of dropout.
2.3. SMS interventions effectiveness
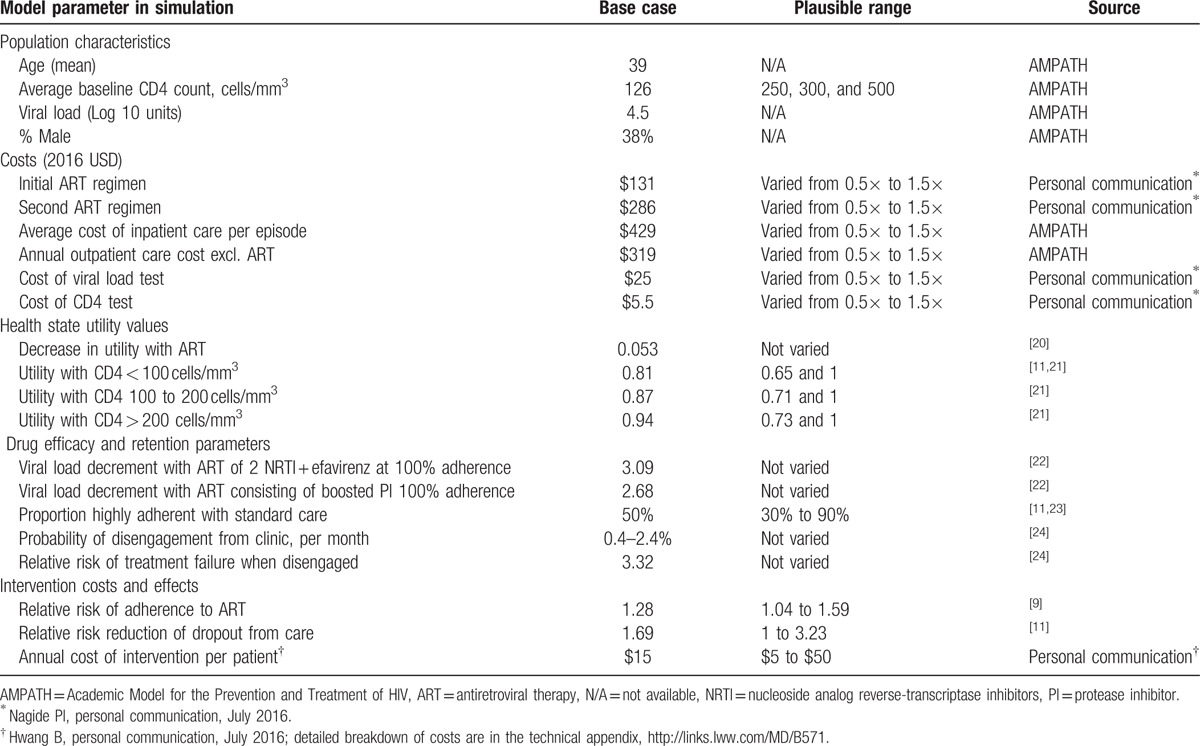
We conducted a literature review of SMS interventions and found multiple systematic reviews that summarized the adherence effects of weekly SMS (Table 1).[8–10] The review by Hovarth et al[9] incidentally only included RCTs from Kenya and presented the most conservative SMS intervention effect size. We chose to use the conservative effect size in our base case analysis to present a conservative estimate of cost-effectiveness. The published pooled effect size was used in this analysis (RR = 1.28), and the effectiveness input was varied over the bounds of the published 95% confidence interval (RR = 1.04–1.59). In Hovarth et al., 2 SMS RCTs were included in their weekly intervention analysis: one studied 1-way supportive SMS reminders, while the other studied 2-way SMS-based individual engagement. The interventions and intervention trials are described in greater detail in the technical appendix.
Table 1.
Simulation input parameters.
2.4. Simulating SMS interventions
The intervention costs consisted of initial staff training, SMS airtime, overhead and technology maintenance, and in the case of 2-way SMS-based patient engagement, labor to respond to individuals experiencing problems. Although trial data suggest the current average cost of the SMS interventions to be lower, we assumed a $15 annual cost per individual in our base case, to provide a conservative estimate of cost-effectiveness (Table 1). We conducted multivariate sensitivity analyses to show the relationship between intervention costs and other key model inputs. We applied a lifetime annual cost in most of our analyses, but tested a 1-time cost in the 1st year in sensitivity analysis. Additional details of the SMS intervention costs are discussed in the technical appendix.
In the simulation, the interventions reduced the proportion of suboptimally adherent individuals (adherence < 90%) and increased the proportion of highly adherent individuals (adherence between 90% and 100%). We modeled population adherence proportions observed in the both SMS intervention RCTs.[11,23] A key difference between the trials was the endpoint proportion of highly adherent individuals in the control group (Fig. S2). The endpoint control group adherence was used as a proxy for ASC. The Lester et al trial found 50% were highly adherent and the Pop-Eleches et al trial found that 40% of individuals were highly adherent in the control group, suggesting differences in ASC across Kenyan settings. The relationship between level of adherence and HIV progression is described in greater detail in the technical appendix.
2.5. Additional simulation settings
The remaining parameters came from literature reviews and an East African HIV cohort data (Table 1). Some characteristics of the simulated cohort, including rate of disengagement, came from the Academic Model for the Prevention and Treatment of HIV (AMPATH) cohort, a multiyear cohort in East Africa.[24,25] Costs were evaluated from a health system perspective. Drug costs for each regimen of ART were provided through personal communication with research staff in a Nairobi-based ART clinic (Nagide PI, personal communication, July 2016). ART regimens used by simulated individuals reflected current Kenyan treatment guidelines, which are based on WHO treatment guidelines.[12]
Treatment costs, including hospitalization and outpatient HIV care costs, were derived from AMPATH databases. Health state utility values (HSUVs) were based on CD4 count categories of <100 cells/mm3, 101 to 200 cells/mm3, and >200 cells/mm3 and were derived from published literature.[21,26] Base case HSUV came from a US study, but HSUV measured as part of the Lester et al trial were included in a sensitivity analysis. We assumed a lifetime adherence benefit of the SMS interventions and applied lifetime annual costs of the intervention. We discounted cost and QALY outcomes at 3% based on WHO guidelines, and we used a lifetime horizon. The WHO suggests that an intervention in select African countries, including Kenya, is cost-effective at less than $US 6461/QALY and very cost-effective at less than $US 2154/QALY.[27] We used these thresholds to interpret the final results.
The model outputs include individual level outcomes, but not secondary transmission. The model has been validated through its ability to predict clinical outcomes matching North American and East African cohort data.[13,16] We recalibrated the model to east-African data after making revisions (technical appendix: Fig. S1A to S1D). Average outcomes of a simulated cohort of 1 million individuals were reported and used to calculate incremental cost-effectiveness ratios (ICERs). Additional technical detail about the model and calibration can be found in the technical appendix, and previous publications.[13,16,17]
3. Analyses
3.1. Base case analyses
We compared SMS-based adherence interventions to standard-care and summarized results using incremental cost-effectiveness ratios (ICER). Our base case analysis focused on adherence improvements, excluding retention benefits. For the base case analysis, we conservatively chose the Lester et al control group to reflect ASC (Fig. S2), but report the results using the ASC from both trials separately. At the time of analysis, guidelines suggest initiating ART at a CD4 count of 500 cells/mm3; however, a shift is occurring in many parts of Kenya and elsewhere toward immediate ART initiation upon detection. We assumed an initiation threshold of 500 cells/mm3 in the base case, but explored no threshold in sensitivity analyses.
3.2. Secondary analyses
Retention in care may have independent benefits from adherence on HIV treatment outcomes. Based on trends in observed retention in care benefits that fell below usual statistical significance thresholds in the Lester et al trial, a secondary analysis included both adherence and retention outcomes. We varied the effectiveness input values for both adherence and retention effects to examine the change in ICER in a multivariate analysis. We repeated these analyses across a wide range of ASC assumptions (30%–90%).
3.3. Sensitivity analyses
We performed a series of multivariate and univariate sensitivity analyses show the impact of important simulation inputs and assumptions on the estimated ICER. In the 1st multivariate sensitivity analysis, we varied the ASC (40%–60%), average baseline CD4 count (126, 250, and 500), intervention costs ($5–$50), and intervention adherence effectiveness (RR = 1.04–1.59). In the 2nd multivariate sensitivity analysis, we varied the intervention costs ($5–$50), intervention adherence effectiveness (RR 1.04–1.59), costs of ART (1×, 1.5×, and 2×), and costs of HIV care (1×, 1.5×, and 2×). In the final multivariate sensitivity analysis, we varied the adherence effectiveness across the range of published effect size (RR = 1.04–1.59), the retention effectiveness (RR = 1–3.23), and ASC (30%–90%). In the remaining 2 sensitivity analyses, we explored a shorter durability of SMS intervention effects and tested alternative HSUVs for HIV health states. Details of the final 2 sensitivity analyses are in the technical appendix.
3.4. Test and treat scenario analysis
This scenario considers the value of SMS interventions under the updated WHO test and treat guidelines, which assumes immediate ART initiation upon HIV detection. Current average CD4 counts at ART initiation remain low,[28] but in time, enhanced testing and identification of HIV cases might lead to a higher average baseline CD4 count at ART initiation. We assumed that the test and treat scenario would be associated with an increase in average CD4 count at ART initiation (from the base case of 126 to 500 cells/mm3). We tested 3 values of ASC (40%–60%) in this multivariate evaluation and varied the intervention effectiveness over the uncertainty range (RR 1.04–1.59).
4. Results
4.1. Base case analyses
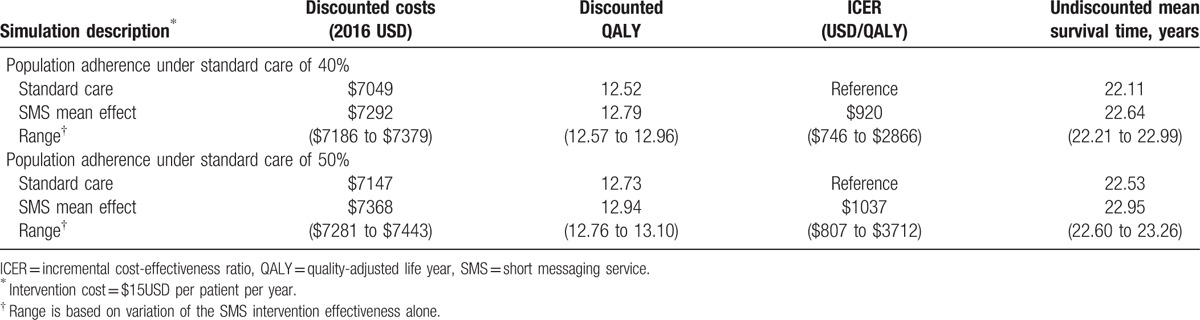
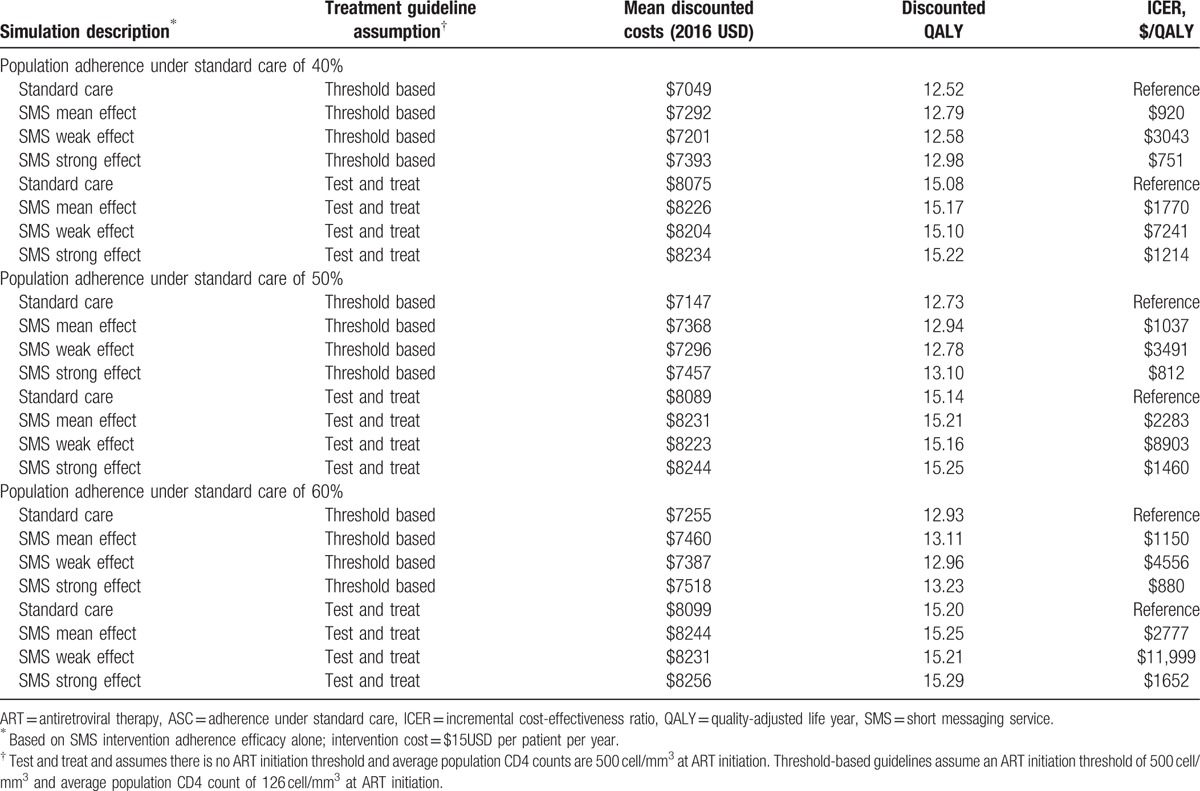
Based on the conservative ASC observed in the Lester et al trial (50%), discounted QALYs rose from 12.73 QALYs to 12.94 QALYs. Using discounted costs and QALYs, the ICER was $1037/QALY, which was below the very cost-effective threshold by WHO standards. Lifetime outcomes based on adherence benefits are presented in Table 2. Based on the ASC observed in the Pop-Eleches et al trial (40%), average survival of the cohort declined, but the difference attributable to SMS interventions increased. Discounted QALYs rose from 12.52 to 12.79 QALY. Using discounted costs and QALYs, the ICER was $920/QALY, suggesting the SMS interventions are more efficient in populations of lower average ASC.
Table 2.
Incremental cost-effectiveness of SMS intervention: base case with adherence effects.
4.2. Secondary analyses
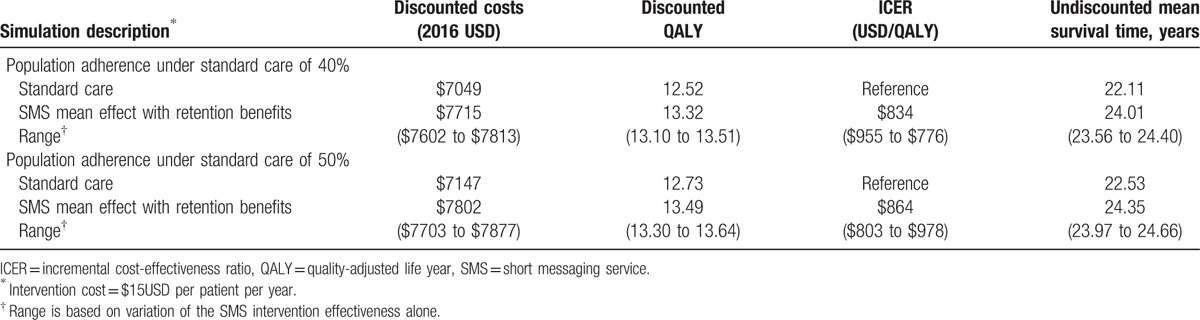
When SMS intervention retention effects were added to adherence effects, average discounted QALY of the simulated cohort improved beyond the base case analysis (13.29 vs 12.94 QALY). The ICER decreased relative to the base case analysis ($864 vs $1037/QALY), suggesting SMS interventions become more efficient if retention is also improved. Lifetime outcomes based on SMS-based adherence intervention with retention benefits are presented in Table 3.
Table 3.
Incremental cost-effectiveness of SMS intervention: secondary analyses with adherence and retention effects.
4.3. Sensitivity analyses
We tested the robustness of our results by simultaneously varying key model inputs (Fig. 2, Fig. S4 and Appendix A). In Fig. 2, threshold values are suggested for the SMS intervention costs at different levels of the other 3 variables. Under an assumption of strong intervention effectiveness within the range, the SMS interventions could cost up to $50 per patient per year and remain cost-effective in all scenarios. Conversely, at the lower end of the intervention effectiveness range, the intervention was no longer cost-effective at costs higher than $15 per patient per year in most scenarios. At an intervention cost of $5 per patient per year, the SMS interventions were cost-effective or very cost-effective in all scenarios tested. Figure S4, depicts how SMS intervention cost-effectiveness varies with overall HIV treatment and care costs. Higher treatment and care costs consistently increased the ICER of the SMS interventions, driven by increased spending on medications for individuals with higher adherence and also increased testing and care costs for individuals who were living longer. However, SMS interventions remained cost-effective despite variation in the treatment and care costs.
Figure 2.
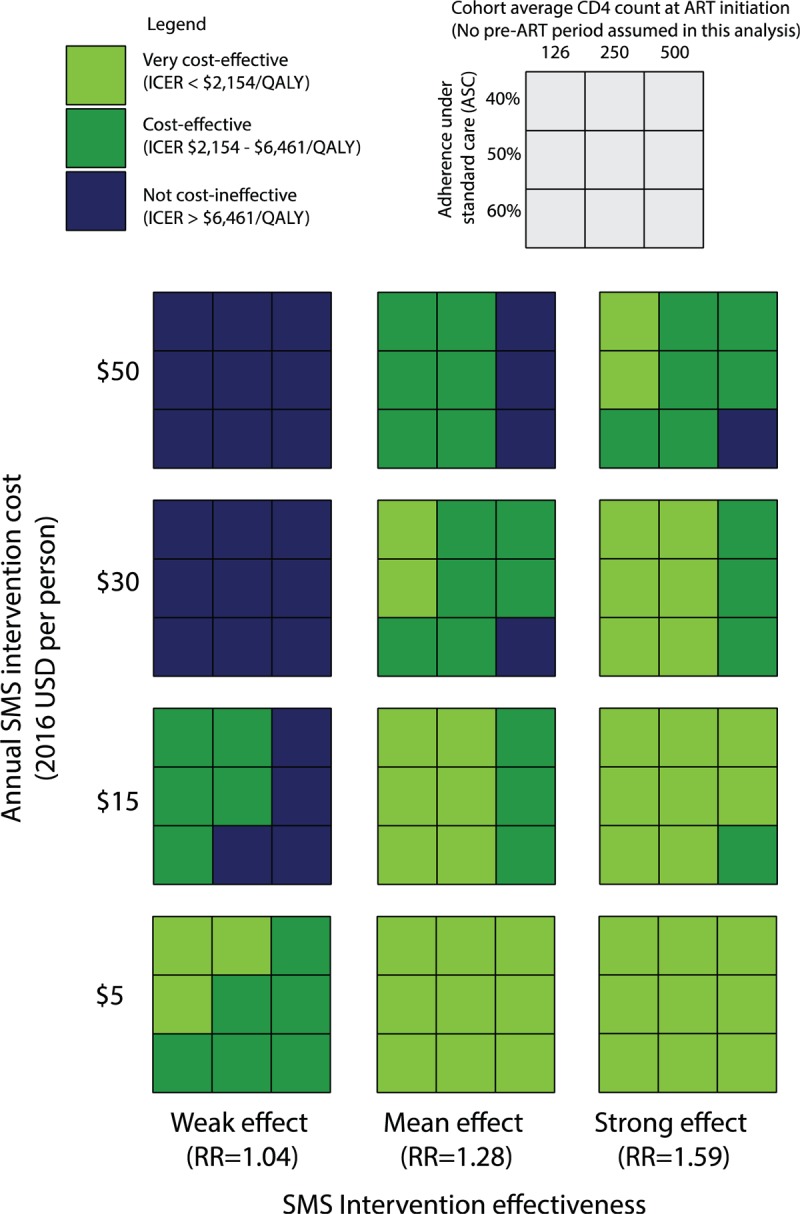
A multivariate sensitivity analysis varying intervention costs, intervention effectiveness, ASC, and average CD4 count at ART initiation. Individuals were assumed to start ART with no waiting period, consistent with the test and treat guidelines. Thresholds at which the intervention was no longer cost-effective can be seen when a variable is increased 1 level and the box turns blue. ART = antiretroviral therapy, ASC = adherence under standard care.
We also tested the value of the SMS-based adherence interventions by simultaneously varying the adherence effectiveness input, the retention effectiveness input and ASC during sensitivity analyses (Appendix A). In all analyses, retention benefits improved the ICER compared to adherence benefits alone. We also calculated the ICER using unadjusted life years and using HSUV from Kenya. Although the average discounted life years changed, the incremental difference remained similar and the SMS interventions remained cost-effective (Appendix B). We tested an attenuated efficacy of the SMS intervention over 1, 5, and 10 years. Assuming lifetime intervention costs (Appendix C), they remained cost-effective in most scenarios, but survival benefits were attenuated (ICER $897–$5924/QALY). With a 1-time intervention cost, the SMS intervention remained very cost-effective with an improved ICER ($465–$977/QALY) (Appendix D). The intervention was very cost-effective or cost-effective across most scenarios.
4.4. Test and treat scenario analyses
We evaluated how the cost-effectiveness of the SMS interventions would change under test and treat guidelines in this scenario. We made an assumption that through earlier identification of HIV cases, average CD4 counts at ART initiation would rise from the current average of 126 cells/mm3 to an average of 500 cells/mm3. Table 4 lists the outcomes of SMS interventions under the base case assumption of threshold based care (ie, initiate ART when CD4 count is <500 cells/mm3) compared to a test and treat assumption (ie, immediate ART initiation at simulation start, irrespective of CD4 count). The base case ICER of $1037/QALY increased to $2283/QALY under test and treat guidelines (Table 4). If average CD4 counts were as high as 500 cells/mm3 and SMS interventions were only weakly effective, the ICER ranged from $7241 to $11,999/QALY, which was no longer cost effective based on WHO threshold.
Table 4.
Incremental cost-effectiveness of SMS intervention under assumptions of threshold-based treatment guidelines and test and treat guidelines while varying the ASC and intervention effectiveness.
5. Discussion
Our findings suggest that weekly SMS-based interventions to support HIV treatment are very cost-effective by WHO standards in Kenya. We explored a wide range of scenarios and assumptions to strengthen our findings. The base case ICER for SMS interventions was $1037/QALY, which is below the WHO very cost-effective threshold of $US 2154/QALY. With additional retention benefits, the ICER improved to $864/QALY, making them even more efficient at extending QALYs. In addition to being an efficient use of funds, they could provide much-needed support for individuals to remain engaged with the health system. Furthermore, due to the widespread availability of cellphones, SMS interventions could be scaled up using infrastructure that has been developed as a part of an ongoing cellphone boom. These findings have important implication for ART delivery programs, which need low-cost ways to address poor adherence.
In addition to the WHO thresholds for cost-effectiveness, a 2nd way to evaluate cost-effectiveness is a comparison to past budget-constrained decisions. A previously studied budget-constrained decision was to increase the ART initiation threshold from a CD4 count of ≤200 to ≤350 cells/mm3. Two studies describe the cost-effectiveness of this change. Braithwaite et al[16] estimated the ICER of this decision was $2600/QALY. A 2nd independent study by Walensky et al[14] estimated an ICER of $1200/life year saved for the same decision. We found the ICER for the SMS interventions to be $1037/QALY with no retention benefits and $864/QALY with retention benefits. Our results suggest that investment in SMS-based adherence interventions for individuals receiving ART could have comparable or better value than the previously implemented decision of expanded ART.
Data beyond the 1-year trial period are lacking, so we tested an attenuated intervention effect and compared the results to the base case lifetime effectiveness assumption. In the most extreme scenario of attenuated intervention effect, we assumed the intervention effects were lost 1 year after the intervention, and individuals who were newly adherent returned to a previous level of nonadherence. The attenuated intervention effects reduced long-term health benefits because of an increased risk of viral rebound and resistance. The average QALY of the SMS intervention simulated cohort was reduced, thus reducing the incremental QALY difference between SMS interventions and standard care. However, the SMS-based adherence interventions remained cost-effective, because 1 year of improved adherence resulted in health improvements that meaningfully altered the average prognosis of the simulated cohort. It was clear that lifelong application of SMS interventions was less cost-effective than a 1-year application; however, the potential for added patient satisfaction and engagement might justify some of the cost.
The WHO test and treat guidelines are an important intervention to improve HIV outcomes, and assuming higher average CD4 count at ART initiation, we find it would improve health outcomes over threshold based guidelines. In these analyses, we assumed that the test and treat strategy would increase average CD4 count at ART initiation from the current 126 to 500 cells/mm3. With standard care, the discounted QALYs rose from the base case value of 12.73 to 15.14 QALYs, because of the increased average CD4 count (Table 4). We explored the value of the SMS interventions in a scenario of a fully implemented test and treat strategy. The ICER of the SMS interventions increased from $1037/QALY in the base case to $2283/QALY. Although the ICER of SMS interventions was increased, they remained cost-effective suggesting they would maintain their value under test and treat guidelines. However, the ICER crossed the cost-effective threshold under assumptions of a weak intervention effectiveness at every level of ASC tested, suggesting there is a risk of the SMS interventions not being cost-effective (ICER = $7241–$11,999/QALY).
We found that average CD4 count at the time of initiating ART influenced SMS intervention cost-effectiveness, with lower average CD4 counts at initiation being associated with a lower ICER (Fig. 2). Detection of HIV remains delayed in many parts of Kenya, and some argue that the benefits of earlier treatment initiation would not be realized without 1st addressing issues earlier in the cascade of HIV care.[28,29] If ART were initiated immediately upon detection in some Kenya cohorts today, the value of SMS interventions would be consistent with the results presented in the base case because average CD4 counts at HIV detection remain low. However, if average CD4 counts were to rise because of improvements earlier in the cascade of care, targeting of the SMS intervention to individuals with low CD4 counts could be necessary. Regardless, SMS interventions could efficiently facilitate the success of expanded treatment recommendations, since adherence will be increasingly critical within a larger population of treated individuals.
This study has several important limitations. First, the adherence data came from only 2 RCTs in Kenya. We only used the individual level adherence data from Pop-Eleches et al trial in our model. However, these studies were conducted in different settings within Kenya and included broad populations, and data may be generalizable to broader Kenyan settings.[11,23] Additionally, we accounted for these limitations by varying the ASC widely to understand the impact on the final ICER. As more refined individual level adherence data becomes available, this limitation can be further addressed in subsequent iterations of the model. A 2nd limitation is that our analysis did not include secondary transmission outcomes. Treatment as prevention, or the now accepted concept that suppressed viral load prevents further transmission, was not formally considered and would have led to greater health system savings. The cost-effectiveness of these interventions would be improved if those benefits were modeled and they might be cost-savings in the long run. Nonetheless, we were able to confirm SMS interventions are cost-effective based on individual level outcomes.
Aside from cost-effectiveness, the rationale for using public funds to support SMS interventions would be stronger if the interventions have positive health and economic externalities or if the interventions help improve outcomes for poor beneficiaries and therefore increase equity in health outcomes.[30] SMS interventions may meet both of these additional criteria. One positive externality of improved adherence is the prevention of further transmission. Low-cost ways to improve adherence are critical to prevent the spread of HIV in Kenya and beyond.[31] Additionally, SMS interventions are advantageous for reaching rural and extremely poor individuals. Cell phones are commonly available and the programmatic costs are relatively low due to automation of most tasks. The engagement SMS program may have an additional reach, in that individuals were not required to own their own phones and could access the service through a friend, family member, or treatment partner. Further research is needed to identify the most efficient ways to implement SMS programs and to investigate the relationship between retention and adherence.
Use of SMS to improve ART outcomes is cost-effective in Kenya and could be valuable in similar settings. In addition to being a cost-effective way to improve health outcomes, these programs have the opportunity to increase communication between individuals and providers in a tangible way. Introduction of these programs in extended settings would allow for further investigation of differential benefits in subgroups. Cost-effective measures are urgently needed to achieve the UNAIDS 90-90-90 targets, which aims for 90% of worldwide HIV cases to be virally suppressed by 2020.
Acknowledgments
The authors thank the funding provided by the US President's Emergency Plan for AIDS Relief grant number PHEKE.07.0045. The authors also thank National Institute of Mental Health of the National Institutes of Health under grant number R01MH097558-01 and by the Canadian HIV Trials Network (to AP), and Michael Smith Foundation award for Health Research Scholars (to RL) for the support.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AIDS = acquired immune deficiency syndrome, ART = antiretroviral therapy, ASC = adherence under standard care, HIV = human immunodeficiency virus, HSUV = health state utility value, RCT = randomized controlled trial, SMS = short messaging service, WHO = World Health Organization.
Funding/support: This study was funded by the US President's Emergency Plan for AIDS Relief grant number PHEKE.07.0045; National Institute of Mental Health of the National Institutes of Health under grant number R01MH097558-01 and by the Canadian HIV Trials Network (to AP); and Michael Smith Foundation award for Health Research Scholars (to RL).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Smith D. Internet use on mobile phones in Africa predicted to increase 20-fold. The Guardian. http://www.theguardian.com/world/2014/jun/05/internet-use-mobile-phones-africa-predicted-increase-20-fold. Published June 5, 2014 [Accessed November 10, 2014]. [Google Scholar]
- [2].Tomlinson M, Rotheram-Borus MJ, Swartz L, et al. Scaling up mHealth: where is the evidence? PLoS Med 2013;10: e1001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Eaton JW, Menzies NA, Stover J, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Global Health 2014;2:e23–34. [DOI] [PubMed] [Google Scholar]
- [4].World Health Organization. 17 million people with access to antiretroviral therapy. http://www.who.int/hiv/mediacentre/news/global-aids-update-2016-news/en/. Published May 31, 2016 [Accessed June 1, 2016]. [Google Scholar]
- [5].World Health Organization. Treat all people living with HIV, offer antiretrovirals as additional prevention choice for people at “substantial” risk. http://www.who.int/mediacentre/news/releases/2015/hiv-treat-all-recommendation/en/. Published September 30, 2015 [Accessed June 10, 2016]. [Google Scholar]
- [6].Bärnighausen T, Chaiyachati K, Chimbindi N, et al. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect Dis 2011;11:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low-and middle-income countries: systematic review and meta-analysis 2008-2013. J Acquir Immune Defic Syndr 2015;69:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mills EJ, Lester R, Thorlund K, et al. Interventions to promote adherence to antiretroviral therapy in Africa: a network meta-analysis. Lancet HIV 2014;1:e104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hovarth T, Azman H, Kennedy GE, et al. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Library 2012;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One 2014;9:e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010;376:1838–45. [DOI] [PubMed] [Google Scholar]
- [12].World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf. Published 2015 [Accessed July 11, 2016]. [PubMed] [Google Scholar]
- [13].Braithwaite RS, Roberts MS, Chang CCH, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy a decision analysis. Ann Inter Med 2008;148:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Walensky RP, Wolf LL, Wood R, et al. When to start antiretroviral therapy in resource-limited settings. Ann Inter Med 2009;151:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chaiyachati KH, Ogbuoji O, Price M, et al. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS 2014;28suppl 2:S187–204. [DOI] [PubMed] [Google Scholar]
- [16].Braithwaite RS, Nucifora KA, Yiannoutsos CT, et al. Alternative antiretroviral monitoring strategies for HIV-infected patients in east Africa: opportunities to save more lives? J Int AIDS Soc 2011;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Keebler D, Revill P, Braithwaite S, et al. Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Global Health 2014;2:e35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Inter Med 1997;126:946–54. [DOI] [PubMed] [Google Scholar]
- [19].AMPATH: Our model. http://www.ampathkenya.org/our-model [Accessed November 14, 2016]. [Google Scholar]
- [20].Braithwaite RS, Goulet J, Kudel I, et al. Quantifying the decrement in utility from perceived side effects of combination antiretroviral therapies in patients with HIV. Value Health 2008;11:975–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Freedberg KA, Scharfstein JA, Seage GRIII, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA 1998;279:130–6. [DOI] [PubMed] [Google Scholar]
- [22].Braithwaite RS, Kozal MJ, Chang CCH, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS (Lond, Engl) 2007;21:1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pop-Eleches C, Thirumurthy H, Habyarimana JP, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS 2011;25:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kessler J, Nucifora K, Li L, et al. Impact and cost-effectiveness of hypothetical strategies to enhance retention in care within HIV treatment programs in East Africa. Value Health 2015;18:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ochieng D, Ochieng V, Braitstein P, et al. Patient tracking and retention in a resource-constrained setting: the AMPATH experience in western Kenya. In: Presented at: 4th IAS Conference on HIV Pathogenesis, Treatment, and Prevention: Abstract no. CDB493, Sydney, Australia. [Google Scholar]
- [26].Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making 2002;22:475–81. [DOI] [PubMed] [Google Scholar]
- [27].World Health Organization. Choosing interventions that are cost effective (WHO-CHOICE). http://www.who.int/choice/en/. Published 2010 [Accessed November 14, 2013]. [Google Scholar]
- [28].Siedner MJ, Ng CK, Bassett IV, et al. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis 2015;60:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lahuerta M, Ue F, Hoffman S, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved 2013;24:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Musgrove P. Public spending on health care how are different criteria related. Health Policy 1999;47:207–23. [DOI] [PubMed] [Google Scholar]
- [31].Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 2006;368:531–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


